Abstract
Background:
We tested whether immune activation/inflammation markers (“immune markers”) explain differences in neurocognition among older breast cancer survivors vs. non-cancer controls.
Methods:
Women ≥60 years with primary breast cancer (stage 0-III) (n=400) were assessed pre-systemic therapy with frequency-matched controls (n=329) and followed annually to 60-months; blood was collected during annual assessments from 2016-2020. Neurocognition was measured by tests of attention, processing speed and executive function (APE). Plasma levels of interleukin (IL)-6, IL-8, IL-10, TNF-alpha and interferon (IFN)-gamma were determined using multiplex testing. Mixed linear models compared results for immune marker levels by survivor/control group by time, controlling for age, racial/ethnic group, cognitive reserve and study site. Covariate-adjusted multi-level mediation analyses tested whether survivor/control group effects on cognition were explained by immune markers; secondary analyses examined the impact of additional covariates (e.g., comorbidity, obesity) on mediation effects.
Results:
Participants were aged 60-90 years (mean 67.7 years). Most survivors had stage I (60.9%) estrogen-receptor positive (87.6%) tumors. Survivors had significantly higher IL-6 levels than controls pre-systemic therapy and at 12-, 24- and 60-months (p≤0.001-0.014), but there were no differences for other markers. Survivors had lower adjusted APE scores than controls (p<0.05). Levels of IL-6, IL-10, and TNF-alpha were related to APE, with IL-6 explaining part of the relationship between survivor/control group and APE (p=0.01). The magnitude of this mediation effect decreased but remained significant (p=0.047) after considering additional covariates.
Conclusion:
Older breast cancer survivors had worse long-term neurocognitive performance than controls and this relationship was explained in part by elevated IL-6.
Keywords: breast cancer, older survivors, cognition, inflammation, immune activation, cancer-related cognitive decline
Precis:
Mechanisms contributing to cognitive problems among cancer survivors remain unclear. This study found that one inflammatory/immune activation marker, IL-6, mediated some of the relationship between older breast cancer survivor/non-cancer control group and cognitive performance.
INTRODUCTION
As cancer becomes a chronic disease, more survivors are living with long-term treatment side-effects.1–3 Cognitive side-effects, often referred to as cancer-related cognitive decline (CRCD) include declines in performance on tests of attention, processing speed, and executive function.1,4 CRCD can have negative impacts on functioning and well-being of cancer survivors.2,5,6 Three-quarters of the nearly four million breast cancer survivors in the US are ages 60 years and older (“older”)7,8 and these older survivors are at risk to experience cognitive problems due to both CRCD and aging.2,9–11
Given the intersection of breast cancer survivorship and aging, inflammation is one candidate mechanism underlying CRCD, since inflammation is a pillar of aging and is also driven by cellular damage occurring with cancer, its surgery and systemic therapies.1,6,12–17 Several studies have examined the role of peripheral inflammatory markers in CRCD with inconsistent results. Differences in findings may be due to variations in study design, time period (usually immediately post-treatment), age groups or markers studied. Additionally, very few studies included a non-cancer control group or focused specifically on older survivors with long-term follow-up.13,18–20 These issues limit the ability to advance our understanding of potential aging-related pathways underlying CRCD in older women who make up the majority of breast cancer survivors.
In the current study, longitudinal data from the multi-site Thinking and Living with Cancer (TLC) study2 were used to test the hypothesis that worse longer-term neurocognitive performance in breast cancer survivors ages 60+ than non-cancer controls would be mediated through the effects of markers of immune activation and inflammation (“immune markers”). The results are intended to build the evidence base about mechanistic pathways involved in CRCD and suggest potential targets for intervention to reduce the risk of or mitigate cognitive problems in the aging population of cancer survivors.
METHODS
TLC enrolled participants from six cancer centers and their affiliated community hospitals and practices.2,21 We report a planned analysis included in the institutional review board-approved study protocol (NCT03451383).
Population
English-speaking women aged 60 years and older with primary breast cancer (stage 0-III) were eligible. Cancer survivor enrollment and baseline visit occurred before initiation of systemic therapy, and for survivors without neoadjuvant therapy, after surgery. We recruited controls who were friends of participating survivors; if no friend was available, we recruited age, race, education, frequency-matched community controls from the same region based on age (within 5 years), education level (average education years) and self-identified racial/ethnic group (White, non-Hispanic vs. non-White). Exclusion criteria for survivors and controls were: non-English speaking, history of stroke, head injury, major psychiatric or neurodegenerative disorder, treatment for another cancer within 5-years (except non-melanoma skin cancers) or receipt of past systemic cancer treatment at any time, Mini-Mental State Examination score <24, or less than a third-grade reading level on the Word Reading subtest of the Wide Range Achievement Test (WRAT4). All participants were required to have sufficient hearing and vision to complete neurocognitive testing.
Women were assessed at enrollment and annually thereafter for up to 60-months. Prospective blood collection was added to the protocol in 2016 and women were required to re-consent to continue follow-up. Participants enrolled in 2016 or later could have provided blood samples at enrollment and each successive study visit. Those who enrolled before 2016 only had the opportunity to provide follow-up samples.
There were 705 survivors and 569 controls ever enrolled by March 1 2020, when enrollment was halted due to the COVID-19 pandemic. Among this sample, 529 survivors and 422 controls were active in the study when blood collection began in 2016 (Figure 1). Reasons for being inactive included completing the study before 2016 or non-consent to the 2016 protocol (115 survivors and 113 controls), death (3 survivors and 2 controls) or study drop-out (58 survivors and 32 controls). Among women active in the study, 87.1% of survivors and 88.2% of controls consented to blood collection. The final analytic sample included 400 survivors and 329 non-cancer controls with one or more blood samples for immune markers and cognition data collected at the same study visit(s). Data from survivors that experienced cancer recurrence (n=6), or survivors (n=2) or controls (n=0) developing exclusion conditions during follow-up were excluded from that point forward. The analytic sample was similar to the remainder of the overall TLC sample in terms of baseline cognition and other factors, except that they had a higher percent of White vs. non-White participants (83.1% vs. 77.2%, p=0.008) and women with >2 vs. fewer comorbidities (49.1% vs. 42.3%, p=0.021). Survivors in the analytic sample had breast conserving surgery more often than those in the remainder of the survivor sample (71.5% vs. 61.7%, p<0.001).
Figure 1. CONSORT Diagram of Older Breast Cancer Survivors and Non-Cancer Controls from the TLC Study included in Analyses of the Relationships between Immune Markers and Cognition.
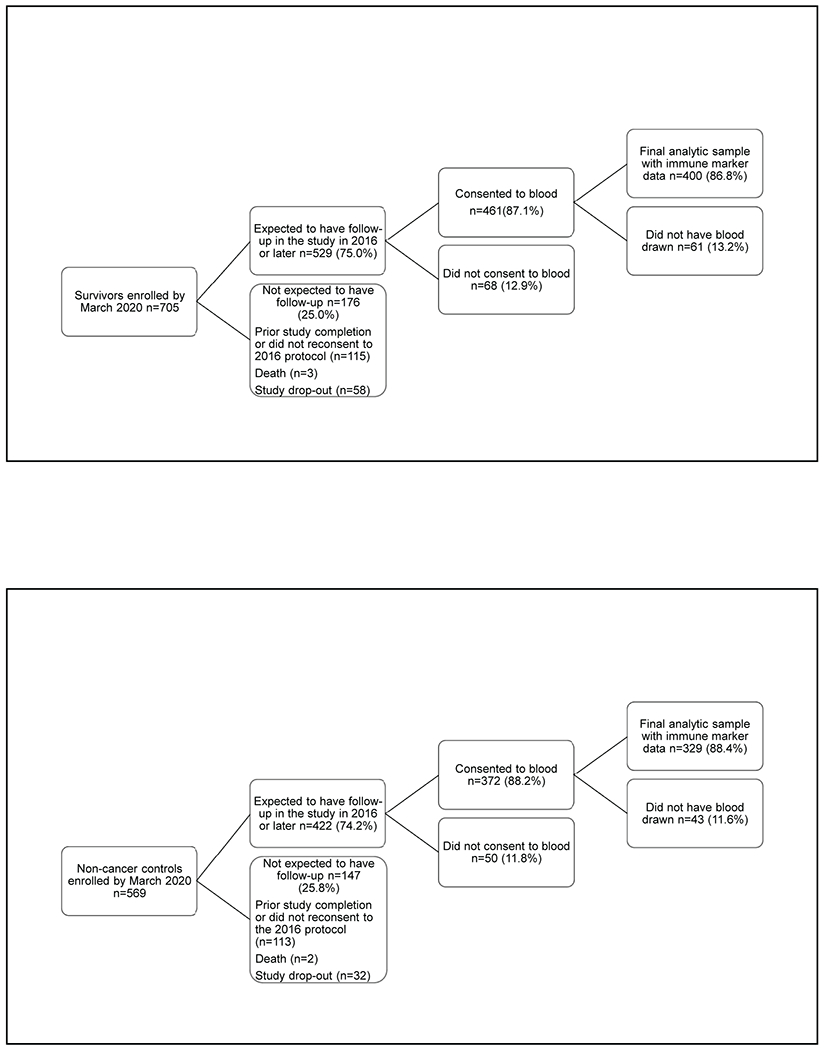
The final analytic sample had biomarker results for one or more blood draws. Reasons for not having blood drawn among those consenting to blood collection included not being able to obtain the specimen, and participant not having time or wanting to skip the blood draw. Since blood specimens were not obtained until 2016 under a protocol revision, participants enrolled from 2010 to 2015 may have already completed the study, decided not to continue or have died or dropped out prior to blood collection.
Panel A. Survivors
Panel B. Non-cancer Controls
Data and Sample Collection
Questionnaires and neurocognitive tests were completed at each visit. Questionnaires ascertained socio-demographic, clinical and psychosocial factors and self-reported cognition. Survivor treatment data were abstracted from medical records.
Blood specimens were collected on the same day or within one week of cognitive assessments. Participants did not report any active infections at the time of sample collection. Venous blood was collected in EDTA tubes and held at refrigerator temperature until processed; specimens were typically processed within two hours, and always within eight hours. Platelet-poor EDTA plasma was obtained by centrifugation at 4° C (2000xG for 15 minutes or 3000xG for 10 minutes) and aliquots were frozen immediately at −80° C. Samples were shipped on dry ice to the UCLA Cousins Center for Psychoneuroimmunology and stored at −80° C until assays were performed.
We evaluated five immune markers (interleukin [IL]-6, IL-8, IL-10, tumor necrosis factor [TNF]-alpha, interferon [IFN]-gamma) previously associated with cognition in cancer survivors.13,18–20,22–25 Marker plasma levels were determined using an electro-chemiluminescent immunoassay (MesoScale Discovery [MSD] Custom Human Cytokine Proinflammatory Panel, MSD, Rockville, MD).26 Assays were performed in duplicate at a 2-fold sample dilution with an extended 8-point standard curve of serial 3-fold dilutions. Marker-specific lower limits of detection (LLD) were calculated for each assay plate. Typical lower limits were 0.2 pg/mL (IL-6, IL-8), 0.1 pg/mL (IL-10) and 0.6 pg/mL or less (TNF-alpha, IFN-gamma). Samples with concentrations below the LLD for a given marker (1.0% of samples for IL-6, 9.5% for IL-10, 0.1% for IFN-gamma, none for IL-8 or TNF-alpha) were assigned values equal to half the plate-specific LLD per laboratory protocols.26 All samples from a single participant were assayed on the same plate, with a balance of survivor and control participants from 3 or more different study sites per plate. All plates were from the same kit lot. An internal quality control sample was included on every plate to monitor assay reproducibility. Inter-assay coefficients of variation were less than 12% for IL-6, IL-8 and IFN-gamma and ≤ 18% and 19% for TNF-alpha and IL-10, respectively. Mean intra-assay coefficients of variation were <10% for all markers.
Measures
Survivor vs. control group was the primary predictor of cognition and the mediators were the individual immune markers. Cognitive outcomes were based on performance in two domains: attention, processing speed, and executive function (APE, six neurocognitive tests) and learning and memory (LM, five neurocognitive tests) based on past factor analyses.2,21 The TLC protocol-specified primary outcome was APE since this domain is affected most often in CRCD.1,4 Scores were standardized (z-scores) to the control means at baseline by age-group and education level. The Functional Assessment of Cancer Therapy-Cognitive Function (FACT-Cog) version 327 Perceived Cognitive Impairments (PCI) sub-scale measured self-reported cognition.
Analyses
All immune marker values were natural log-transformed for analyses since the distributions were highly skewed. T-tests, ANOVA and chi-square tests were used to test bivariate differences in characteristics of survivors and controls. Among survivors, the association of baseline immune marker levels and time from cancer surgery were evaluated by Pearson’s correlation.
Separate linear mixed-effects models were used to test for differences in adjusted natural log-transformed levels of each immune marker by survivor/control group at each study visit, including an interaction term for group by time and covariates (baseline age, self-identified racial/ethnic group [collapsed as White, non-Hispanic vs. non-White], cognitive reserve (WRAT4 Word Reading score) and study site. Adjusted mean ± standard error (SE) natural log-transformed levels of each marker at each time-point by survivor/control group were plotted; corresponding non-transformed values were also provided. Box [mean, median, interquartile range (IQR, 25th-75th percentile)] and whisker (±1.5 IQR) plots were used to graphically represent the distribution of adjusted natural log-transformed immune marker levels by group and time-point.
Separate multi-level mediation analyses for each immune marker tested pathways between survivor/control group, immune markers and cognition (APE).28 Pair-wise Pearson’s correlations between immune markers were significant and small/moderate, ranging from 0.14 to 0.36, so we did not include multiple markers in mediation models. The models consider repeated data for immune markers and cognition at each of multiple visits, with each woman having different numbers of visits and different assessment times depending on where they were in study follow-up when blood collection was added to the protocol. We conducted additional analyses of individual APE domain test scores to explore components of the domain that might be driving any observed associations. Secondary multi-level mediation analyses examined the learning and memory domain (LM) and self-reported cognitive problems. In follow-up analyses, we considered the effects of individual added covariates that might affect immune markers and risk of or reaction to cancer: comorbidities (≤2 vs. >2), obesity (body mass index ≥30 vs <30 kg/m2), cardiovascular disease (yes/no), diabetes (yes/no), depressive symptoms (measured with continuous CES-D scores)29 and state anxiety (based on continuous STAI scores); all models controlled for age, racial/ethnic group, cognitive reserve and study site.30
Since our objective was discovery of potential CRCD pathways, the main and secondary analyses considered a two-sided alpha of 0.05 for statistical significance. A Bonferroni adjusted alpha of 0.01 (0.05/5) due to testing of five immune markers is noted for the primary mediation analysis. Descriptive and linear mixed-model mixed analyses were performed in SAS Version 9.4 (SAS Institute Inc., Cary, NC, USA) and multilevel mediation analyses were conducted using Mplus v8.31
RESULTS
Participants ranged in age from 60-90 years (average 67.7) and were largely White and well-educated (Table 1). The survivors were comparable to frequency-matched non-cancer controls at enrollment in demographic characteristics, except that survivors were more significantly more likely to have >2 comorbidities (56.2% vs 40.7%, p=0.001), including more cardiovascular disease (p=0.004) and diabetes (p=0.021) and a BMI ≥ 30 kg/m2 (p<0.001). Most survivors had stage I disease (60.9%), had estrogen receptor positive (87.6%) and HER2 negative (88.0%) tumors, and were an average of 49.5 (SD 29.8) days from surgery at baseline, with no significant correlations between time from cancer surgery and baseline immune marker levels (Table 1 and Supplemental Figure 1).
Table 1.
Characteristics of Older Breast Cancer Survivors Prior to Systemic Therapy, and Older Non-cancer Controls, at Study Enrollment (Baseline)
| All (n=729) | Survivors (n=400) | Controls (n=329) | ||
|---|---|---|---|---|
|
| ||||
| Mean (SD) or % (n) | p value a | |||
|
| ||||
| Sociodemographic | ||||
|
| ||||
| Age, years | 0.539 | |||
| Mean (SD) | 67.7 (5.7) | 67.8 (5.3) | 67.6 (6.2) | |
| [Range], [Median] | [60-90], [67] | [60-85], [67] | [60- 90], [66] | |
|
| ||||
| Racial/ethnic group | 0.764 | |||
| Non-White (Black, Hispanic, Asian, other) | 16.9% (123) | 17.3% (69) | 16.4% (54) | |
| White, non-Hispanic | 83.1% (606) | 82.8% (331) | 83.6% (275) | |
|
| ||||
| Education, years | 15.5 (2.2) | 15.5 (2.1) | 15.6 (2.2) | 0.317 |
|
| ||||
| Cognitive reserve, WRAT4 reading score | 110.8 (15.7) | 109.8 (14.8) | 111.9 (16.7) | 0.072 |
|
| ||||
| Clinical | ||||
|
| ||||
| Number of comorbidities | 2.7 (2.0) | 3.0 (2.0) | 2.5 (2.0) | 0.001 |
|
| ||||
| High comorbidity, >2 | 49.1% (345) | 56.2% (214) | 40.7% (131) | <0.001 |
|
| ||||
| Diabetes | 10.0% (70) | 12.4% (47) | 7.1% (23) | 0.021 |
|
| ||||
| Cardiovascular disease (with hypertension) | 50.4% (354) | 55.4% (211) | 44.5% (143) | 0.004 |
|
| ||||
| Obesity, BMI ≥30 kg/m2 | 33.6% (243) | 39.3% (157) | 26.5% (86) | <0.001 |
|
| ||||
| Psychosocial | ||||
|
| ||||
| Depressive symptoms, CES-D score b | 5.7 (6.7) | 6.7 (7.2) | 4.5 (5.8) | <0.001 |
|
| ||||
| Anxiety, STAI State score c | 27.9 (6.9) | 28.8 (7.6) | 26.9 (5.9) | <0.001 |
|
| ||||
| Cognition | ||||
|
| ||||
| FACT-PCI score d | 61.1 (10.2) | 60.7 (10.8) | 61.5 (9.5) | 0.289 |
|
| ||||
| Attention, Processing speed, Executive function (APE) z-score e | −0.01 (0.62) | −0.07 (0.61) | 0.06 (0.62) | 0.007 |
|
| ||||
| Learning and Memory (LM) z-score e | 0.03 (0.77) | 0.00 (0.77) | 0.06 (0.78) | 0.357 |
|
| ||||
| Breast cancer characteristics in Survivors | ||||
|
| ||||
| AJCC v.6 Tumor Stage | ||||
|
| ||||
| 0 | - | 17.4% (68) | - | |
|
| ||||
| I | - | 60.9% (238) | - | |
|
| ||||
| II | - | 18.2% (71) | - | |
|
| ||||
| III | - | 3.6% (14) | - | |
|
| ||||
| Tumor markers | ||||
|
| ||||
| ER positive | - | 18.2% (71) | - | |
|
| ||||
| HER2 positive | - | 3.6% (14) | - | |
|
| ||||
| Surgery | ||||
| Breast conservation alone | - | 52.4% (208) | - | |
| Breast conservation with radiotherapy | - | 19.1% (76) | - | |
| Mastectomy | - | 28.5% (113) | - | |
|
| ||||
| Time between surgery and baseline pre-systemic therapy assessment, days f | ||||
| Mean (SD) | - | 49.5 (SD 29.8), | ||
| [Range], [Median] | - | [4-169], [42] | ||
|
| ||||
| Systemic Therapy g | - | |||
|
| ||||
| Chemotherapy (+/− hormonal therapy) | - | 25.8% (92) | - | |
|
| ||||
| Hormonal therapy, no chemotherapy | - | 74.2% (264) | - | |
p values from t-tests, ANOVA or chi-square tests comparing survivors vs. controls
Based on CES-D continuous scores (range 0-60); scores of 16+ are considered clinical depression. Mean score differences are statistically, but not clinically meaningfully different.
Based on STAI State continuous scores (range 20-80); scores of 54+ are considered clinical anxiety in older adults. Mean score differences are statistically, but not clinically meaningfully different.
FACT-PCI scores for 18 items (range 0-72); higher scores indicate better self-reported cognition.
Z-scores for neurocognitive test performance are age and education standardized to the overall TLC control sample mean at baseline. Scores range from −1 to +1, where zero indicates having the same score as the control group average, and scores from >0 to 1 are better than the average, and scores from <0 to −1 are worse than the average.
N=375; survivors who had neoadjuvant therapy (n=23), did not receive surgery (n=1) and failed to return for surgery for ~9 months (n=1) were not included in calculation of time from surgery to baseline immune marker sample.
89.2% of hormonal therapy was with aromatase inhibitors
Participants provided a total of 1550 blood specimens for immune assays. From baseline through 60-month follow-up, there were 819 specimens from 400 survivors and 731 specimens from 329 controls; 62.5% and 70.5% of survivors and controls, respectively, provided two or more specimens (Supplemental Table 1).
Immune Marker Levels
Survivors had higher adjusted IL-6 levels than controls and these differences were significant for most (baseline, 12-, 24- and 60-months, p-values between < 0.001 and 0.014), but not all timepoints (Figure 2A; Supplemental Figure 2A). While survivors tended to have higher IL-8 and IL-10 levels than controls, the differences were not statistically significant; there were no survivor-control differences for TNF-alpha or IFN-gamma (Figure 2B–E; Supplemental Figure 2B–E).
Figure 2. Adjusted Natural Log-transformed Immune Marker Levels by Timepoint for Older Breast Cancer Survivors vs. Older Non-Cancer Controls.
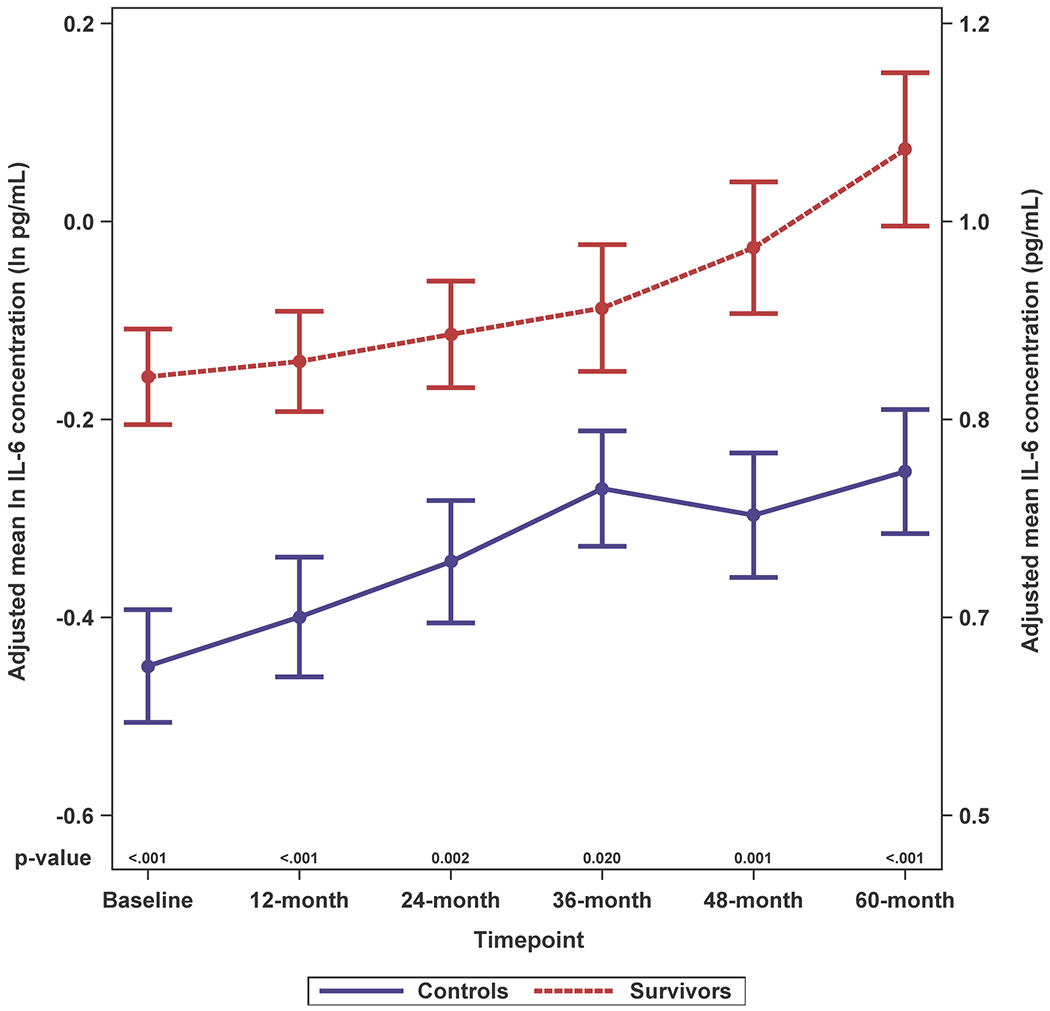
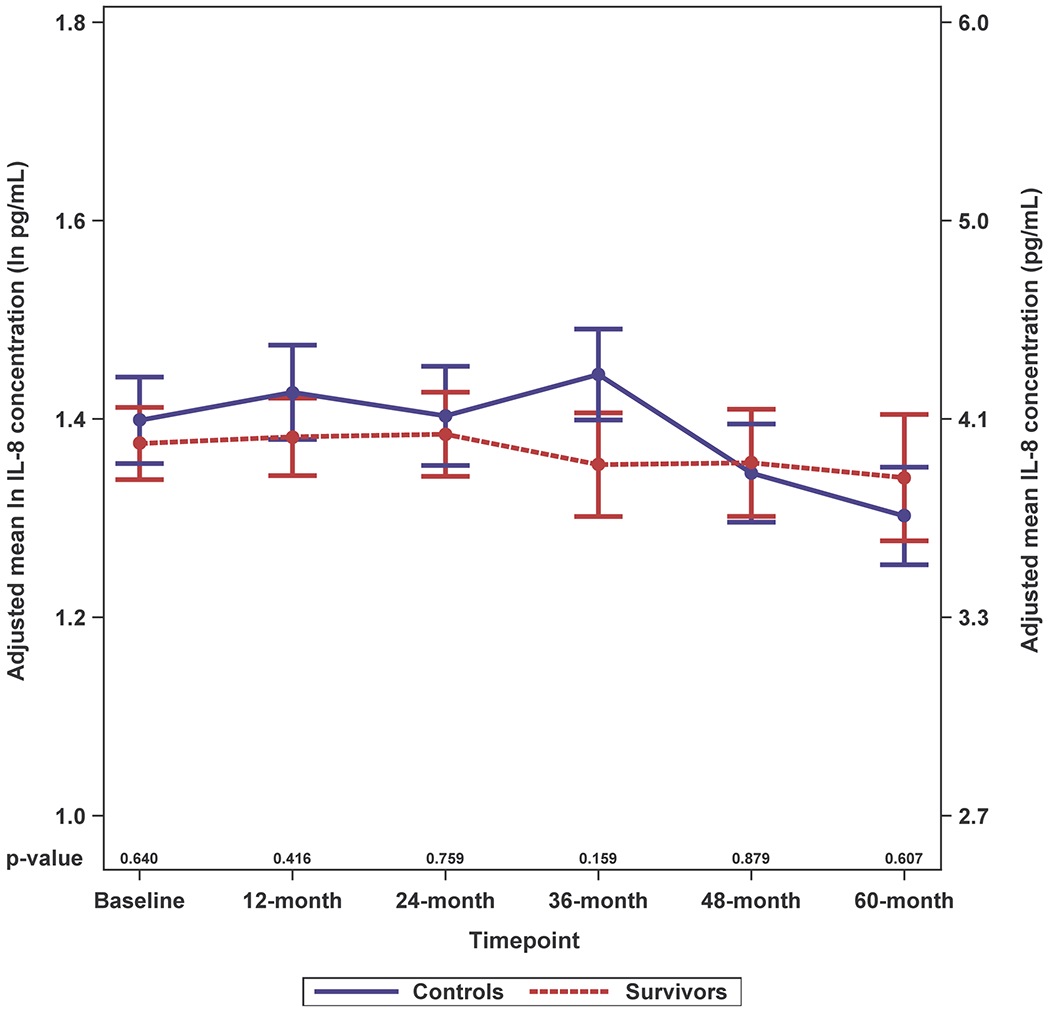
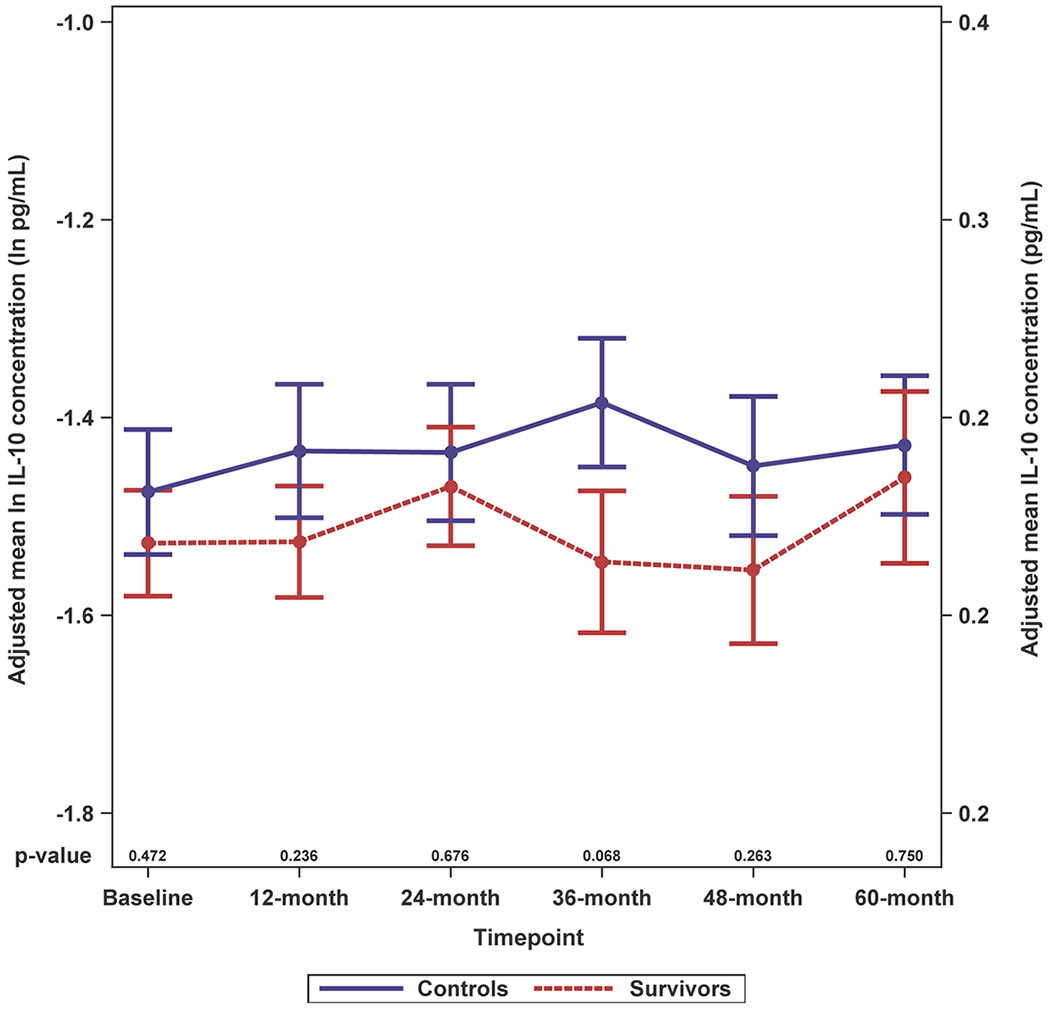
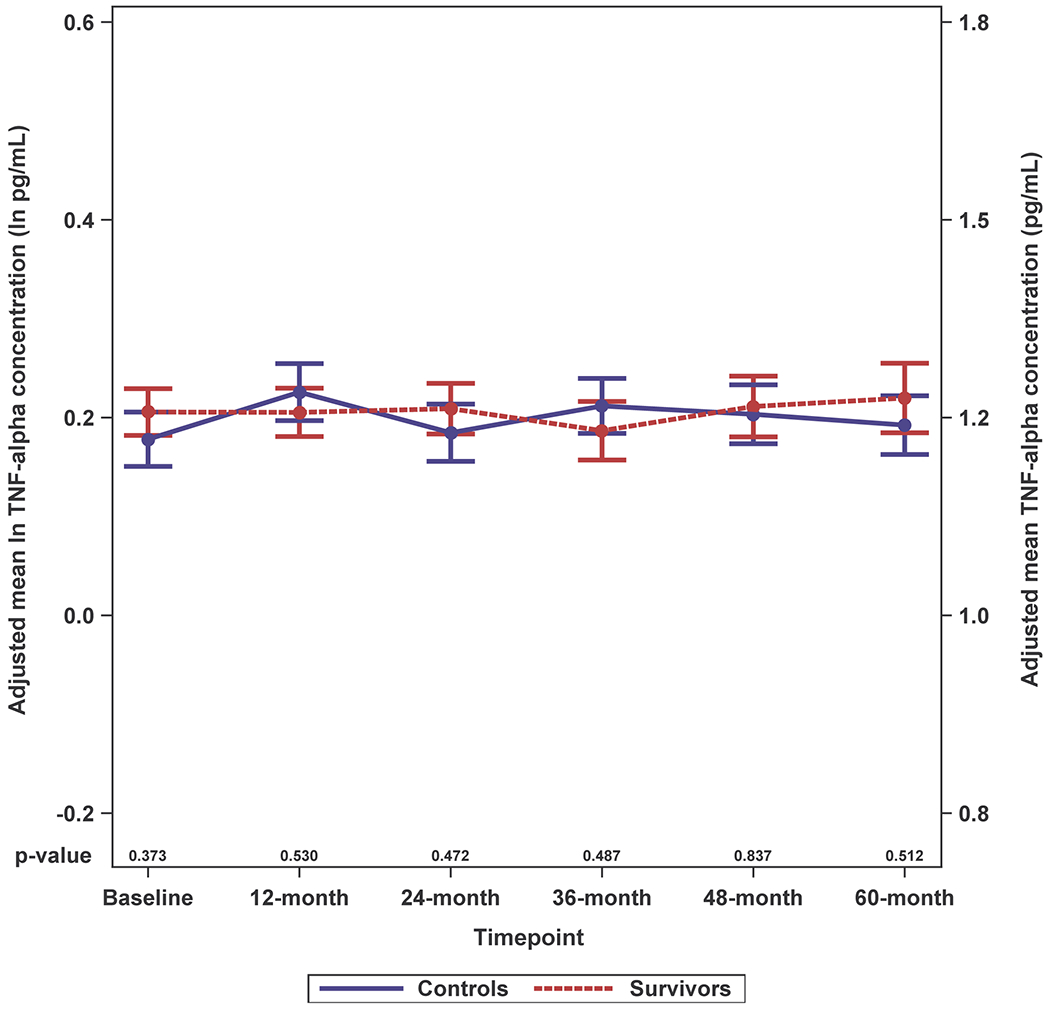
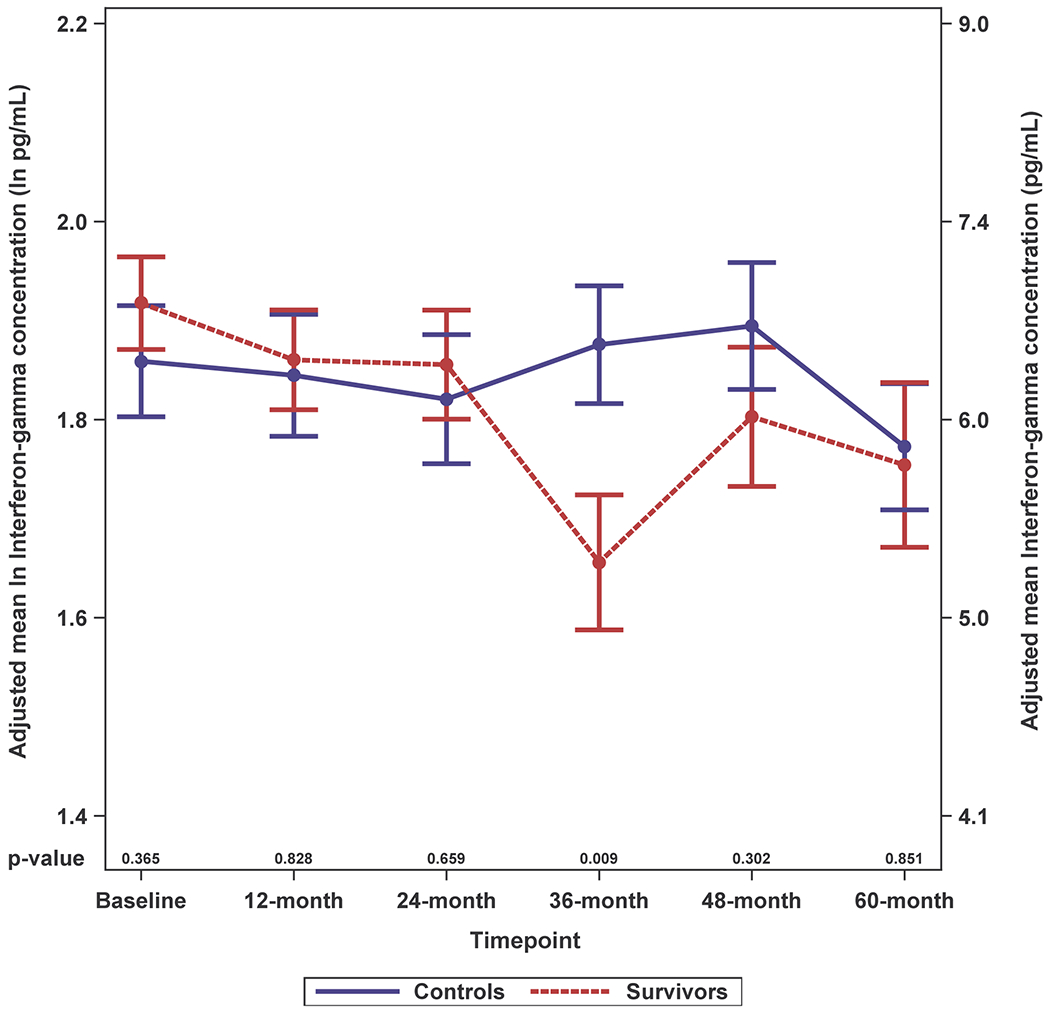
Results of linear mixed effects model analyses in survivors (red, n=399 total due to one missing WRAT4 score) and non-cancer controls (blue, n=328 or 329 total due to missing values for IL-10 or TNF-alpha) at each study visit (baseline, 12-, 24-, 36-, 48- and 60-months), including an interaction of survivor/control group by time and adjusted for age, race (White vs. non-White), cognitive reserve (WRAT4 Word Reading score), and study site. Mean adjusted immune marker levels at each timepoint are plotted on the natural log scale (ln pg/mL) as indicated on the left y-axis, and error bars show the standard error (SE). For ease of interpretation, equivalent non-transformed pg/mL values are shown on the right y-axis. P values from the mixed models for survivor vs. control differences in adjusted immune marker levels at each study visit are shown along the x-axis. Please see Supplemental Figure 2 for box plots, and Supplemental Table 1 for sample sizes at each timepoint.
Panel A. IL-6
Panel B. IL-8
Panel C. IL-10
Panel D. TNF-alpha
Panel E. Interferon (IFN)-gamma
Immune Mediation
Survivors had significantly lower adjusted APE scores than controls in all models (p≤ 0.05) (Figure 3; Table 2, column βc). Higher levels of IL-6, IL-10 and TNF-alpha were also associated with lower APE scores (p-values <0.01 to <0.05; Table 2, column βb), but only IL-6 was higher in survivors vs. controls (p<0.001, Table 2 column βa).
Figure 3. Schematic Representation of Models Testing Whether Survivor/Control Group Differences in Cognitive Outcomes are Mediated by the Effects of Group on Immune Markers.
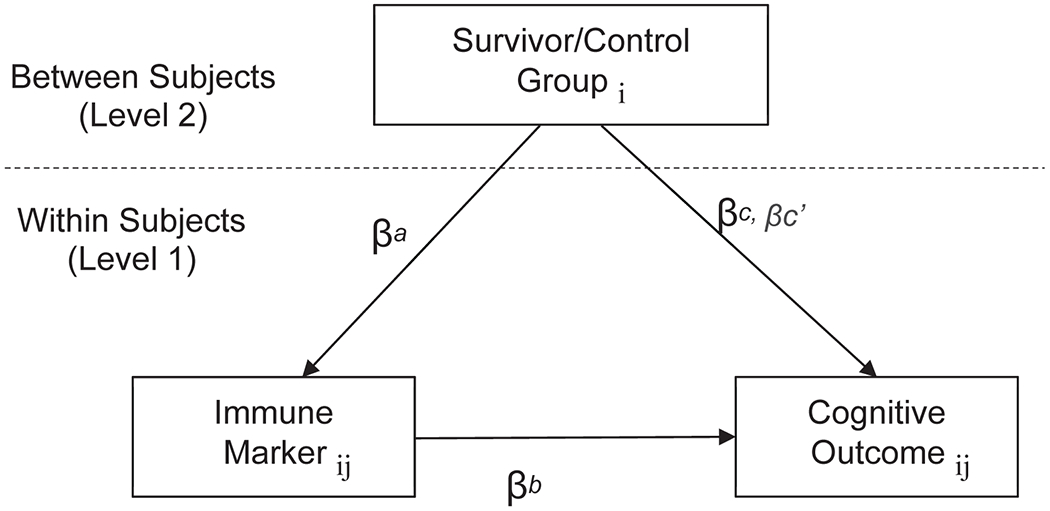
The figure illustrates the 2–1-1 multi-level mediation models28 that evaluate between group differences (Level 2) on time-varying changes in immune markers (Level 1) and cognitive outcomes (Level 1). These models test the total effect of survivor vs. control group on cognition scores (line βc), which combine independent effects of the immune marker effects (line βc’) and the product of the indirect effects of survivor/control group on the immune marker (line βa) and the effects of the marker on cognitive outcomes (line βb). The remaining effects of group on cognition after considering indirect effects of the pathway through inflammation are noted as βc’. Significant indirect effects (βab, a product of paths a and b) and indicates that the direct relationship between survivor vs. control group and cognition scores is due in part to the effects of cancer and its treatment on immune activation and/or inflammation. Finally, the subscripts denote differences between persons (i) and those that vary across time (j).
Table 2.
Effects of Being an Older Breast Cancer Survivor vs. Older Non-Cancer Control on Neurocognitive Tests of the Attention, Processing Speed and Executive Functioning (APE) Domain, and Mediation of Effects through Immune Activation/Inflammatory Pathways 1
| Immune Marker (per one unit change in natural log transformed pg/mL) | Survivor/control group effect on APE, independent of indirect effect on immune markers | Survivor/control group effect on immune marker | Immune marker effect on APE | Indirect effect of group on APE via immune marker (Mediation effect) | Survivor/control group effect on APE, controlling for indirect effect on immune markers |
|---|---|---|---|---|---|
| βc (SE)2 | βa (SE) | βb (SE) | βab (SE) | βc’ (SE) | |
|
| |||||
| IL-63 | −.097 (.038)* | .218 (.049)*** | −.106 (.036)** | −.023 (.009)** | −.074 (.039)+ |
|
| |||||
| IL-8 | −.096 (.038)* | −.038 (.036) | −.063 (.068) | .002 (.003) | −.099 (.038)* |
|
| |||||
| IL-10 | −.095 (.038)* | −.107 (.055)* | −.100 (.034)** | .011 (.007)+ | −.106 (.038)** |
|
| |||||
| TNF-alpha | −.095 (.038)* | −.005 (.026) | −.171 (.063)** | .001 (.004) | −.096 (.038)* |
|
| |||||
| IFN-gamma | −.097 (.038)* | −.013 (.042) | −.041 (.058) | .001 (.002) | −.097 (.038)* |
SE=standard error; P values are indicated as follows:
0.10> p >0.05,
p <0.05,
p <0.01,
p < 0.001.
Results from 399 survivors and 328 (IL-10, TNF-alpha) or 329 (IL-6, IL-8, IFN-gamma) controls in multi-level mediation analyses controlling for age, racial/ethnic group (White, non-Hispanic vs. non-White), WRAT4 reading score, study site.
Each β (SE) is labeled with a, b, ab, c or c’ based on the pathways shown on Figure 3.
Note that result for the IL-6 mediation effect (βab) remains significant (p <0.01) if a Bonferroni correction for testing 5 markers (0.05/5=0.01) is applied. If cardiovascular comorbidities are added to the model, the beta for IL-6 mediation changes from 0.023 (SD 0.00) to 0.025 (SD 0.009) and remains significant at p=<0.01.
Lower adjusted APE scores among survivors vs. controls were mediated by the effects of being a cancer survivor vs. control on elevated levels of IL-6 (p=0.01, Table 2, column βab). There was a similar pattern of results for IL-10, but the mediation effect was not statistically significant (p = 0.10). Among the tests included in the APE domain, there was a statistically significant mediated effect between IL-6 and the Trail-Making A test (p<0.05), and trends for mediated effects for Trail-making B and Digit Symbol for IL-6 (p < 0.10) (Supplemental Table 2).
In follow-up APE analyses that considered additional covariates (obesity and comorbidity plus anxiety and depression, or cardiovascular disease or diabetes plus anxiety and depression), the mediation effect of IL-6 on the relationship between survivor/control group and APE score was unchanged or only slightly attenuated and remained significant (Supplemental Table 3).
Models for learning and memory (LM) did not reveal any associations between survivor/control group and cognitive performance, so there were no significant mediation results (Supplemental Table 4). Self-reported impairment was significantly greater for survivors than controls in all models (except IL-10), but this effect was not mediated by immune markers and was not maintained after considering anxiety and depression (Supplemental Table 4).
DISCUSSION
This is the largest, long-term study of older breast cancer survivors and non-cancer controls examining whether immune markers mediate the relationship between cancer survivorship and neurocognitive performance. We found that differences in cognitive performance on tests of attention, processing speed and executive function (APE) between survivors and controls were, in part, mediated through IL-6. Among the individual APE tests, Trail-making A appeared to be most sensitive to this mediation effect. We also found that multiple immune markers (IL-6, IL-10,TNF-alpha) had a direct relationship with APE. There were no or only limited relationships observed for learning and memory and self-reported cognition.
This study provides novel data showing that worse long-term cognitive performance in older breast cancer survivors vs. controls is mediated through effects of immune markers. Specifically, we found that IL-6 mediated the relationship between being a survivor (vs. control) and poorer neurocognitive performance in the APE domain, the most commonly reported domain in CRCD.2,6,19 Interestingly, APE performance also corresponds to areas of the brain, such as the prefrontal cortex, which shows neuroimaging abnormalities in cancer survivors, including a subsample of this cohort.32,33
IL-6 is a unique pleiotropic cytokine, with pro-inflammatory and stimulatory activity on a wide variety of cell types, whose expression can be increased in cancer cells via p53 mutations, with resultant increased STAT3 transcriptional signaling.34 IL-6 can be upregulated by tissue damage and/or be a secondary response to pro-inflammatory cytokines like TNF-alpha and is thought to contribute to chronic inflammatory activation via the production of C-reactive protein (CRP).35 Research has also suggested that only very low circulating levels of IL-6 are needed to affect inflammatory cascades,36 which may be one reason this immune marker had the strongest association in our study. Additionally, there is some evidence that IL-6 receptors in the brain may contribute to neuroinflammation and microglia activation arising from neurotoxicity.35 The pre-systemic therapy differences in IL-6 levels between survivors and controls also add to the body of evidence that cancer may have important effects on inflammation and cognition even before systemic therapy begins.21,22,37,38 The finding that survivor/control differences in factors including comorbidity and obesity slightly attenuated IL-6 mediation effects on cognition suggests that a pre-existing pro-inflammatory state may contribute to the risk of developing breast cancer and/or cognitive decline.21 Prospective studies with assessments before and after cancer would be necessary to fully explore this possibility.
We found that IL-6, IL-10, and TNF-alpha had a direct relationship with cognition in survivors and controls, findings that parallel prior work by others.13,18–20,22–25,39,40 The modest but non-significant mediation effect of IL-10, which has regulatory anti-inflammatory and B cell stimulatory activity, may reflect the dynamic nature of its secondary responses to increased pro-inflammatory markers.41 IFN-gamma, which is produced during immune activation and anti-viral responses, and IL-8, which is a chemoattractant for neutrophils, were unrelated to cognition. Other studies reporting on the period from pre- to immediately post-chemotherapy in mainly younger samples have found relationships of cognition with other markers that were not tested in our study, such as soluble TNF-alpha receptor 1 and 2 (markers for TNF-alpha activity), IL-7, MCP-1 and MIP-1β18,19,40,42 and relationships of other markers with verbal fluency.39 Interestingly, one small cross-sectional study of breast cancer survivors also found that plasma biomarkers of Alzheimer’s disease-related pathology and several cytokines were associated with cognition.43
This study has many strengths, including the large cohort focused on older women who are already at risk for aging-related cognitive decline at the time of cancer diagnosis, baseline data prior to the initiation of systemic therapies, as well as data from a five-year follow-up period and inclusion of matched controls. However, there are several limitations that should be considered in evaluating our findings. First, due to the TLC annual visit schedule, we did not have data on immune marker levels during or immediately following systemic therapy, so our results are most applicable to longer-term cognition. Second, despite significant findings for APE, especially with the Trailmaking B test, there were no differences in learning and memory or self-reported cognition between survivors and controls, limiting ability to test pathways for these aspects of CRCD. In our past studies with the full TLC sample,2 our LM battery has not been as sensitive as APE tests to detect differences in groups, suggesting that more sensitive neurocognitive testing approaches may be needed to better understand the nature of CRCD.44,45 Third, while women in our sample were representative of the communities served by our tertiary academic medical centers and their community affiliates, they were predominantly White, non-Hispanic and well-educated, limiting external generalizability. It will be critical to replicate our results in more diverse samples, especially groups with lifetime experiences associated with increased chronic inflammation.9 However, since the mean age of survivors in TLC is close to the median age at diagnosis of US breast cancer survivors, our results may have broad generalizability. Fourth, we had an insufficient number of survivors with aggressive tumor markers or receiving chemotherapy and limited variability in types of hormonal regimens to determine if the mediation of the relationship between survivor/control group and cognition by IL-6 markers varies by tumor type or specific treatment regimens. This will be important to examine further, since others have found that radiotherapy and/or chemotherapy induce cellular damage that increases peripheral inflammation and cognitive problems and that HER2-positive cancers are associated with cognitive problems even prior to treatment.19,24,38 Fifth, since we added blood collection to an already established cohort, not all survivors had plasma data prior to systemic therapy.
Taken together, our results suggest that immune activation and inflammation may be involved in mechanistic pathways leading to CRCD. This idea is biologically plausible since peripheral inflammation results in subsequent impairments in cognitive performance in preclinical models, has a known relationship with cognitive disorders, can increase brain inflammation seen with neurodegeneration, and can further promote inflammation in a feed-forward loop.46,47,48 The role of inflammation may also be clinically important because it may signal risk of increasing aging and frailty49,50 since cognitive issues are often interwoven with frailty.51
It will be useful to develop transdisciplinary teams to replicate our findings using pre-clinical models and back-translation of findings to human cohorts. This approach is especially important given the multi-directional relationships between inflammation, aging, and cancer, and complex interrelated cascades of immune signaling, inflammatory pathways, and feedback loops in cognitive aging and neurodegeneration.9 A transdisciplinary approach could be especially useful to determine leverage points for development of pharmaceutical or behavioral interventions designed to mitigate the effects of cancer and its treatments on cognition,9 since cognitive issues can have a pervasive impact on multiple aspects of survivors’ daily lives.2,5,6
Supplementary Material
Acknowledgements:
The work of Paul Jacobsen was done while he was at Moffitt Cancer Center. We would like to thank the participants in the TLC study for their sharing of their time and experiences; without their generosity, this study would not have been possible. We are also indebted to Sherri Stahl, Naomi Greenwood, Margery London, and Sue Winarsky, patient advocates from the Georgetown Breast Cancer Advocates, for their insights and suggestions on study design and methods to recruit and retain participants. We thank the TLC study staff who contributed by ascertaining, enrolling, interviewing, and collecting blood samples from participants, and Miguel A. Guzman and Chaoyi He of the Cousins Center for Psychoneuroimmunology at UCLA, for sample management and performance of immunoassays.
Funding
This research was supported by the National Cancer Institute of the National Institutes of Health through grants R01CA129769 and R35CA197289 to JM. This study was also supported in part by the National Institutes of Health through grants P30CA51008 to Georgetown Lombardi Comprehensive Cancer Center for support of the Biostatistics and Bioinformatics Resource and the Non-Therapeutic Shared Resource, R56AG068086 (JC, JM), R01AG068193 (JM, AJS), R01CA237535 (JEC), K01AG065485 (KER), P30AG028716 (HJC), K01CA212056 (TNB), K08CA241337 (KVD), R01CA172119 (TA, JR), U54CA137788 (TA), P30CA008748 (TA), K99CA270294 (DBT), R01CA244673 (BCM), and P30AG010133 and P30AG072976 (AJS), RO1CA261793 (S.K.P.) and UCLA Cousins Center for Psychoneuroimmunology (KER, JEC).
Role of the Funder:
The study sponsor did not have any role in the design of the study, the collection, analysis, and interpretation of the data, the writing of the manuscript, or the decision to submit the manuscript for publication.
Disclosures:
Dr Extermann served as a consultant for Aileron and Alnylam. She received honoraria from OncLive.
Dr. Isaacs’ disclosures are available at: https://coi.asco.org/share/KZZ-5URC/Claudine Isaacs
Dr. Saykin has received support from Avid Radiopharmaceuticals, a subsidiary of Eli Lilly (in kind contribution of PET tracer precursor); Bayer Oncology (Scientific Advisory Board); Eisai (Scientific Advisory Board); Siemens Medical Solutions USA, Inc. (Dementia Advisory Board); and Springer-Nature Publishing (Editorial Office Support as Editor-in-Chief, Brain Imaging and Behavior).
All other authors have declared no conflicts of interest.
Footnotes
An earlier version of this research was presented at the American Association of Cancer Research Special Conference on Aging and Cancer, November 17-20, 2022, San Diego, California
Disclaimer: The content is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health.
CRediT (Contributor Roles Taxonomy) Author Contributions.
Jeanne S. Mandelblatt was responsible for conceptualization, investigation, resources, writing and editing, supervision, project administration and funding acquisition.
Brent J. Small was responsible for supervision, methodology, formal analysis, data curation and writing.
Xingtao Zhou was responsible for formal analysis, data curation and writing.
Zev M. Nakamura was responsible for investigation, writing and editing.
Tim Ahles was responsible for conceptualization, investigation, resources, writing, project administration and funding acquisition.
Jaeil Ahn was responsible for supervision, methodology, formal analysis, data curation and writing.
Traci Bethea was responsible for investigation, writing and editing.
Harvey Jay Cohen was responsible for conceptualization and writing and editing.
Martine Extermann was responsible for investigation, writing and editing.
Deena Graham was responsible for investigation, resources, writing and editing.
Paul B. Jacobsen was responsible for investigation, writing and editing.
Claudine Isaacs was responsible for investigation, resources, writing and editing.
Heather Jim was responsible for investigation, resources, writing and editing, project administration and funding acquisition.
Brenna C. McDonald was responsible for investigation, resources, writing, editing, project administration and funding acquisition.
Sunita J. Patel was responsible for investigation, resources, writing, editing and project administration.
Kelly E. Rentscher was responsible for investigation, writing and editing.
James C. Root was responsible for investigation, supervision, writing and editing.
Andrew J. Saykin was responsible for investigation, resources, writing, editing, project administration and funding acquisition.
Danielle B. Tometich was responsible for investigation, writing and editing.
Kathleen Van Dyk, PhD was responsible for investigation, writing and editing.
Wanting Zhai was responsible for formal analysis, data curation and writing.
Elizabeth C. Breen was responsible for conceptualization, investigation, data curation, writing and editing, and laboratory supervision and administration.
Judith E. Carroll was responsible for conceptualization, project administration and writing and editing.
Data Sharing:
The Thinking and Living with Cancer data are available for sharing following the NIH requirements and FAIR principles (Findability, Accessibility, Interoperability, Reproducibility) for data access. Data access is via requests to the first author. The SAS or Mplus code and data for the analyses included in the paper are available on request within the constraints of the TLC IRB requirements.
REFERENCES
- 1.Magnuson A, Ahles T, Chen BT, Mandelblatt J, Janelsins MC. Cognitive Function in Older Adults With Cancer: Assessment, Management, and Research Opportunities. Journal of Clinical Oncology. 2021:JCO.21.00239. doi: 10.1200/JCO.21.00239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mandelblatt JS, Small BJ, Luta G, et al. Cancer-Related Cognitive Outcomes Among Older Breast Cancer Survivors in the Thinking and Living With Cancer Study. J Clin Oncol. Oct 3 2018;36(32):Jco1800140. doi: 10.1200/jco.18.00140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mandelblatt JS, Zhai W, Ahn J, et al. Symptom burden among older breast cancer survivors: The Thinking and Living With Cancer (TLC) study. Cancer. Mar 15 2020;126(6):1183–1192. doi: 10.1002/cncr.32663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahles TA, Saykin AJ, McDonald BC, et al. Longitudinal assessment of cognitive changes associated with adjuvant treatment for breast cancer: impact of age and cognitive reserve. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28(29):4434–4440. doi: 10.1200/JCO.2009.27.0827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kobayashi LC, Cohen HJ, Zhai W, et al. Cognitive function prior to systemic therapy and subsequent well-being in older breast cancer survivors: Longitudinal findings from the Thinking and Living with Cancer Study. Psychooncology. Jun 2020;29(6):1051–1059. doi: 10.1002/pon.5376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Janelsins MC, Heckler CE, Peppone LJ, et al. Cognitive Complaints in Survivors of Breast Cancer After Chemotherapy Compared With Age-Matched Controls: An Analysis From a Nationwide, Multicenter, Prospective Longitudinal Study. J Clin Oncol. Feb 10 2017;35(5):506–514. doi: 10.1200/jco.2016.68.5826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bluethmann S, Mariotto A, Rowland J. Anticipating the “Silver Tsunami”: Prevalence trajectories and comorbidity burden among older cancer survivors in the United States. Cancer Epidemiol Biomarkers Prev. Jul 2016;25(7):1029–36. doi: 10.1158/1055-9965.Epi-16-0133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Howlader NNA, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER Cancer Statistics Review, 1975–2017. . 2019. [Google Scholar]
- 9.Mandelblatt JS, Ahles TA, Lippman ME, et al. Applying a Life Course Biological Age Framework to Improving the Care of Individuals With Adult Cancers: Review and Research Recommendations. JAMA Oncology. 2021;doi: 10.1001/jamaoncol.2021.116010.1001/jamaoncol.2021.1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pergolotti M, Battisti NML, Padgett L, et al. Embracing the complexity: Older adults with cancer-related cognitive decline-A Young International Society of Geriatric Oncology position paper. J Geriatr Oncol. Mar 2020;11(2):237–243. doi: 10.1016/j.jgo.2019.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crouch A, Champion V, Von Ah D. Cognitive Dysfunction in Older Breast Cancer Survivors: An Integrative Review. Cancer Nurs. Jan-Feb 01 2022;45(1):E162–e178. doi: 10.1097/ncc.0000000000000896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carroll JE NZ, Small BJ, Zhou X, Cohen HJ, Ahles TA, Ahn J, Bethea TN, Extermann M, Graham D, Isaacs C, Jim HSL, Jacobsen PB, McDonald BC, Patel SK, Rentscher K, Root J, Saykin AJ, Tometich DB, Van Dyk K, Zhai W, Breen EC, Mandelblatt J. . Elevated C-Reactive Protein and Subsequent Patient-Reported Cognitive Problems in Older Breast Cancer Survivors: The Thinking and Living with Cancer (TLC) Study. JCO. 2022; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams AM, Shah R, Shayne M, et al. Associations between inflammatory markers and cognitive function in breast cancer patients receiving chemotherapy. J Neuroimmunol. Jan 15 2018;314:17–23. doi: 10.1016/j.jneuroim.2017.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hodes RJ, Sierra F, Austad SN, et al. Disease drivers of aging. Ann N Y Acad Sci. Dec 2016;1386(1):45–68. doi: 10.1111/nyas.13299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahles TA, Saykin AJ. Candidate mechanisms for chemotherapy-induced cognitive changes. Nat Rev Cancer. Mar 2007;7(3):192–201. doi: 10.1038/nrc2073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Janelsins MC, Kesler SR, Ahles TA, Morrow GR. Prevalence, mechanisms, and management of cancer-related cognitive impairment. International Review of Psychiatry. 2014/02/January 2014;26(1):102–113. doi: 10.3109/09540261.2013.864260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oppegaard K, Harris CS, Shin J, et al. Cancer-related cognitive impairment is associated with perturbations in inflammatory pathways. Cytokine. 2021/12/January/ 2021;148:155653. doi: 10.1016/j.cyto.2021.155653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Belcher EK, Culakova E, Gilmore NJ, et al. Inflammation, Attention, and Processing Speed in Patients with Breast Cancer before and after Chemotherapy. J Natl Cancer Inst. Feb 4 2022;doi: 10.1093/jnci/djac022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Janelsins MC, Lei L, Netherby-Winslow C, et al. Relationships between cytokines and cognitive function from pre- to post-chemotherapy in patients with breast cancer. J Neuroimmunol. Jan 15 2022;362:577769. doi: 10.1016/j.jneuroim.2021.577769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pomykala KL, Ganz PA, Bower JE, et al. The association between pro-inflammatory cytokines, regional cerebral metabolism, and cognitive complaints following adjuvant chemotherapy for breast cancer. 10.1007/s11682-013-9243-2 doi. Brain Imaging Behav. 2013;7(4):511–523. NOT IN FILE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mandelblatt JS, Stern RA, Luta G, et al. Cognitive impairment in older patients with breast cancer before systemic therapy: is there an interaction between cancer and comorbidity? J Clin Oncol. Jun 20 2014;32(18):1909–18. doi: 10.1200/jco.2013.54.2050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patel SK, Wong AL, Wong FL, et al. Inflammatory Biomarkers, Comorbidity, and Neurocognition in Women With Newly Diagnosed Breast Cancer. J Natl Cancer Inst. Aug 2015;107(8)doi: 10.1093/jnci/djv131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Radin AS, Bower JE, Irwin MR, et al. Acute health-related quality of life outcomes and systemic inflammatory markers following contemporary breast cancer surgery. NPJ Breast Cancer. Aug 8 2022;8(1):91. doi: 10.1038/s41523-022-00456-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bower JE, Ganz PA, Irwin MR, et al. Acute and chronic effects of adjuvant therapy on inflammatory markers in breast cancer patients. JNCI Cancer Spectr. Jul 1 2022;6(4)doi: 10.1093/jncics/pkac052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ganz PA, Castellon SA, Silverman DHS, et al. Does circulating tumor necrosis factor (TNF) play a role in post-chemotherapy cerebral dysfunction in breast cancer survivors (BCS)? Abstract presented at Annual Meeting of the American Society of Clinical Oncology, Chicago, June 3, 2011. 2013. [Google Scholar]
- 26.Piber D, Eisenberger NI, Olmstead R, et al. Sleep, inflammation, and perception of sad facial emotion: A laboratory-based study in older adults. Brain, behavior, and immunity. 2020/10/January/ 2020;89:159–167. doi: 10.1016/j.bbi.2020.06.011 [DOI] [PubMed] [Google Scholar]
- 27.Wagner LI, Sweet J, Butt Z, Lai JS, Cella D. Measuring patient self-reported cognitive function: Development of the Functional Assessment of Cancer Therapy–Cognitive Function Instrument. J Support Oncol. January/01 2009;7:W32–W39. [Google Scholar]
- 28.Preacher KJ ZM, Zhang ZA general multilevel SEM framework for assessing multilevel mediation. Psychol Methods 15:209–33, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Radloff L The CES-D Scale: A self-report depression scale for research in the general popualtion. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 30.Spielberger C Manual for the State-Trait Inventory. Consulting Psychologisits Press; 1983. [Google Scholar]
- 31.Muthén BO MLMUGv. Mplus Users Guide (v. 8). 2022.
- 32.McDonald BC, Conroy SK, Smith DJ, West JD, Saykin AJ. Frontal gray matter reduction after breast cancer chemotherapy and association with executive symptoms: a replication and extension study. Brain, behavior, and immunity. Mar 2013;30 Suppl:S117–25. doi: 10.1016/j.bbi.2012.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McDonald BC, Van Dyk K, Deardorff RL, et al. Multimodal MRI examination of structural and functional brain changes in older women with breast cancer in the first year of antiestrogen hormonal therapy. Breast Cancer Res Treat. Jul 2022;194(1):113–126. doi: 10.1007/s10549-022-06597-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sehgal PB. Interleukin-6 at the Host-Tumor Interface: STAT3 in Biomolecular Condensates in Cancer Cells. Cells. Mar 30 2022;11(7)doi: 10.3390/cells11071164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trovato M, Sciacchitano S, Facciolà A, Valenti A, Visalli G, Di Pietro A. Interleukin‑6 signalling as a valuable cornerstone for molecular medicine (Review). Int J Mol Med. Jun 2021;47(6)doi: 10.3892/ijmm.2021.4940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hansen MB. Interleukin-6 signaling requires only few IL-6 molecules: Relation to physiological concentrations of extracellular IL-6. Immun Inflamm Dis. Jun 2020;8(2):170–180. doi: 10.1002/iid3.292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hanalis-Miller T, Nudelman G, Ben-Eliyahu S, Jacoby R. The Effect of Pre-operative Psychological Interventions on Psychological, Physiological, and Immunological Indices in Oncology Patients: A Scoping Review. Front Psychol. 2022;13:839065. doi: 10.3389/fpsyg.2022.839065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Root JC, Zhou X, Ahn J, et al. Association of markers of tumor aggressivity and cognition in women with breast cancer before adjuvant treatment: The Thinking and Living with Cancer Study. Breast cancer research and treatment. 2022;194(2):413–422. doi: 10.1007/s10549-022-06623-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen VC, Lin CK, Hsiao HP, et al. Effects of Cancer, Chemotherapy, and Cytokines on Subjective and Objective Cognitive Functioning Among Patients with Breast Cancer. Cancers (Basel). May 24 2021;13(11)doi: 10.3390/cancers13112576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lyon DE, Cohen R, Chen H, et al. Relationship of systemic cytokine concentrations to cognitive function over two years in women with early stage breast cancer. J Neuroimmunol. Dec 15 2016;301:74–82. doi: 10.1016/j.jneuroim.2016.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saraiva M, Vieira P, O’Garra A. Biology and therapeutic potential of interleukin-10. J Exp Med. Jan 6 2020;217(1)doi: 10.1084/jem.20190418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ganz PA, Bower JE, Kwan L, et al. Does tumor necrosis factor-alpha (TNF-alpha) play a role in post-chemotherapy cerebral dysfunction? S0889-1591(12)00198-5 pii ; 10.1016/j.bbi.2012.07.015 doi. Brain Behav Immun. 2013:(Suppl:S99-108). NOT IN FILE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Henneghan A, Haley AP, Kesler S. Exploring Relationships Among Peripheral Amyloid Beta, Tau, Cytokines, Cognitive Function, and Psychosomatic Symptoms in Breast Cancer Survivors. Biol Res Nurs. Jan 2020;22(1):126–138. doi: 10.1177/1099800419887230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Horowitz TS, Suls J, Treviño M. A Call for a Neuroscience Approach to Cancer-Related Cognitive Impairment. Trends Neurosci. Aug 2018;41(8):493–496. doi: 10.1016/j.tins.2018.05.001 [DOI] [PubMed] [Google Scholar]
- 45.Gaynor AM, Ahsan A, Jung D, et al. Novel computerized neurocognitive test battery is sensitive to cancer-related cognitive deficits in survivors. J Cancer Surviv. Aug 8 2022;doi: 10.1007/s11764-022-01232-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chitnis T, Weiner HL. CNS inflammation and neurodegeneration. J Clin Invest. Oct 2 2017;127(10):3577–3587. doi: 10.1172/jci90609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schroyen G, Vissers J, Smeets A, et al. Blood and neuroimaging biomarkers of cognitive sequelae in breast cancer patients throughout chemotherapy: A systematic review. Transl Oncol. Feb 2022;16:101297. doi: 10.1016/j.tranon.2021.101297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fernandez HR, Varma A, Flowers SA, Rebeck GW. Cancer Chemotherapy Related Cognitive Impairment and the Impact of the Alzheimer’s Disease Risk Factor APOE. Cancers (Basel). Dec 19 2020;12(12)doi: 10.3390/cancers12123842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gilmore N, Kadambi S, Lei L, et al. Associations of inflammation with frailty in patients with breast cancer aged 50 and over receiving chemotherapy. J Geriatr Oncol. Apr 2020;11(3):423–430. doi: 10.1016/j.jgo.2019.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cupit-Link MC, Kirkland JL, Ness KK, et al. Biology of premature ageing in survivors of cancer. ESMO Open. 2017;2(5):e000250. doi: 10.1136/esmoopen-2017-000250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ahles TA, Schofield E, Li Y, et al. Relationship between cognitive functioning and frailty in older breast cancer survivors. J Geriatr Oncol. Jan 2022;13(1):27–32. doi: 10.1016/j.jgo.2021.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The Thinking and Living with Cancer data are available for sharing following the NIH requirements and FAIR principles (Findability, Accessibility, Interoperability, Reproducibility) for data access. Data access is via requests to the first author. The SAS or Mplus code and data for the analyses included in the paper are available on request within the constraints of the TLC IRB requirements.


