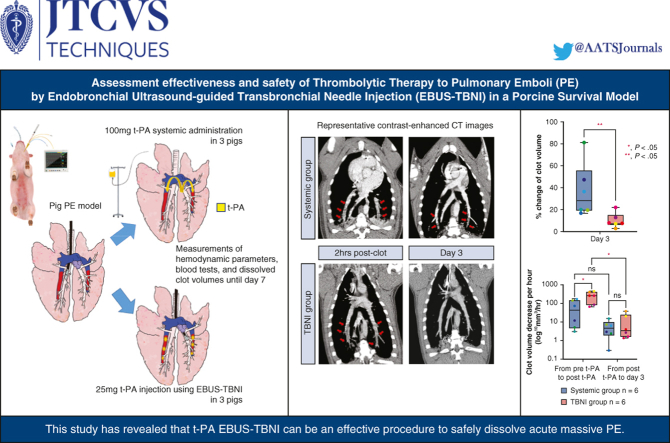
Abstract
Objective
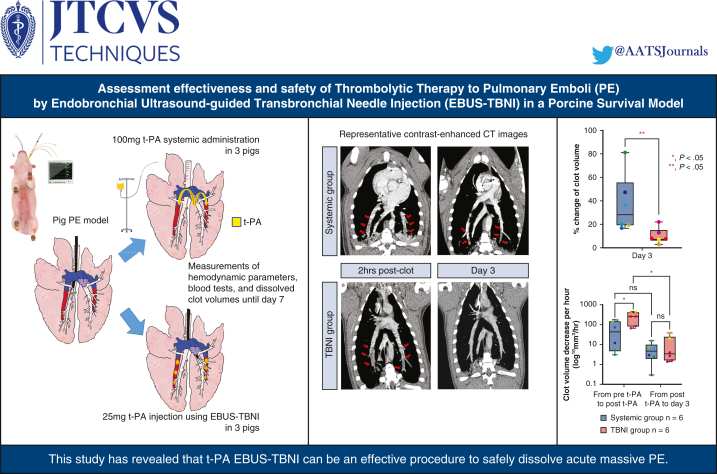
Endobronchial ultrasound–guided transbronchial needle injection (EBUS-TBNI) may effectively treat acute pulmonary embolisms (PEs). Here, we assessed the effectiveness of clot dissolution and safety of tissue plasminogen activator (t-PA) injection using EBUS-TBNI in a 1-week survival study of a porcine PE model.
Methods
Six pigs with bilateral PEs were used: 3 for t-PA injection using EBUS-TBNI (TBNI group) and 3 for systemic administration of t-PA (systemic group). Once bilateral PEs were created, each 25 mg of t-PA injection using EBUS-TBNI for bilateral PEs (a total of 50 mg t-PA) and 100 mg of t-PA systemic administration was performed on day 1. Hemodynamic parameters, blood tests, and contrast-enhanced computed tomography scans were carried out at several time points. On day 7, pigs were humanely killed to evaluate the residual clot volume in the pulmonary arteries.
Results
The average of percent change of residual clot volumes was significantly lower in the TBNI group than in the systemic group (%: systemic group 36.6 ± 22.6 vs TBNI group 9.6 ± 6.1, P < .01) on day 3. Considering the elapsed time, the average decrease of clot volume per hour at pre-t-PA to post t-PA was significantly greater in the TBNI group than in the systemic group (mm3/hour: systemic 68.1 ± 68.1 vs TBNI 256.8 ± 148.1, P < .05). No hemorrhage was observed intracranially, intrathoracically, or intraperitoneally on any contrast-enhanced computed tomography images.
Conclusions
This study revealed that t-PA injection using EBUS-TBNI is an effective and safe way to dissolve clots.
Key Words: endobronchial ultrasound, transbronchial needle injection, preclinical animal model, pulmonary embolism, thrombolysis
Graphical abstract
Injection of t-PA using endobronchial ultrasound–guided transbronchial needle injection.
Central Message.
Injection of t-PA using the endobronchial ultrasound-guided transbronchial needle injection for pulmonary embolism can safely dissolve clots better than t-PA systemic administration.
Perspective.
Acute pulmonary embolism at high risk remains a highly fatal disease. No major advances or changes have been made in the last decade. Our proposed method, using the endobronchial ultrasound-guided transbronchial needle injection, may be a major step toward improvement of treatment for the acute pulmonary embolism at high risk.
See Discussion on page 305.
Acute pulmonary embolism (PE) at high risk is associated with a high mortality rate; for patients with systolic blood pressure lower than 90 mm Hg and/or hemodynamic instability lasting more than 15 minutes, the risk of 30-day mortality is approximately 20%. Currently, systemic thrombolytic therapy, rather than catheter-directed thrombolysis, is the suggested treatment for patients with acute PE at high risk.1, 2, 3 Ultrasound-accelerated thrombolysis therapy (USAT) was reported as an improved therapy for PE4 and even though some meta-analyses reported its usefulness,5, 6, 7 the evidence was insufficient to change the guidelines.3 There is room to increase the effectiveness of treatment of tissue plasminogen activator (t-PA) therapy as a treatment strategy for acute PE. In this study, we have examined the safety and the feasibility of endobronchial ultrasound (EBUS)-guided transbronchial needle injection (TBNI) as a means of improved t-PA delivery. EBUS is a well-established modality for the diagnosis of peribronchial lesions8 and is also an approach that enables the identification of PE, as bronchi are always accompanied by a pulmonary artery.9, 10, 11, 12 Therefore, we considered that the EBUS-guided transbronchial needle aspiration (TBNA) needle could be an effective means of reaching PE transbronchially and devised a novel therapeutic approach for PE using EBUS-TBNI.13,14 EBUS-TBNI can be a novel therapeutic approach for more accurate drug delivery by selecting the injection site within the thrombus under real-time ultrasound guidance in order to dissolve clot effectively.
The safety of performing EBUS-TBNA through the pulmonary artery has previously been reported.15, 16, 17, 18, 19 Regarding pulmonary artery punctures using EBUS-TBNA needle based on skilled technique and adequate anatomical knowledge, the possibility of bleeding is considered to be low. Accidental puncture of the pulmonary artery by a trainee can occurs in practice, but major bleeding is extremely uncommon, as evidenced by reports that bleeding is a rare complication associated with EBUS-TBNA.20, 21, 22 Therefore, the approach of puncturing the pulmonary artery itself may be considered as an acceptable technique, but it is unclear whether t-PA injection using EBUS-TBNI is safe. To clarify this point, we have reported on t-PA injection using EBUS-TBNI in the porcine PE model. No complications were observed for 1 week after t-PA injection using EBUS-TBNI.13 This was evaluated by bronchoscopy and examination of the resected lungs, not by computed tomography (CT) scans.
In this study, using contrast-enhanced CT scans, hemodynamic parameters, and blood test (including coagulation over time), we evaluated the effectiveness in clot dissolution by t-PA injection using EBUS-TBNI and assessed the safety of this technique.
Methods
All experiments were approved by the University Health Network Animal Care Committee (Animal Use Protocol 5928.5.2) on August 10, 2021. Six Yorkshire pigs (mean weight 31.9 ± 2.9 kg; Caughell Farms Ltd) were used as the porcine PE model. EBUS and EBUS-TBNI were performed with a convex probe EBUS bronchoscope (BF-UC180F; Olympus Corporation) with a dedicated ultrasound processor (EU-ME1; Olympus Corporation). A 21- or 22-gauge ViziShot 2 EBUS-TBNA needle (NA-U401SX-4021-A/NA-U401SX-4022-A; Olympus Corporation) was used for clot injection, and a 25-gauge ViziShot 2 EBUS-TBNA needle (NA-U401SX-4025N; Olympus Corporation) was used for t-PA injection.
Animal Model
Methods of anesthesia and monitoring during the procedures were used as previously described.13,14,23 Under general anesthesia, the pig underwent intubation with an 8.5-mm endotracheal tube and then was set on a pressure-controlled ventilator. An intravenous injection catheter was placed at the pig ear and a Swan-Ganz catheter (Synthetic ControlCath TD catheter C146F7; Edwards Lifesciences Corporation) was placed into the jugular vein via percutaneous sheath introducer kit (Arrow Percutaneous Sheath Introducer Kits 9 Fr AK-09903-CDC; Teleflex Incorporated). Maintenance of anesthesia took place with monitoring of blood pressure, oxygen saturation, end-tidal carbon dioxide, electrocardiogram, and rectal temperature using a multiparameter monitor (BM3Vet touch; Bionet Co). Pulmonary artery pressure (PAP) was measured using a pressure transducer (TruWave Disposable Pressure Transducer Standard Kits PX284; Edwards Lifesciences).
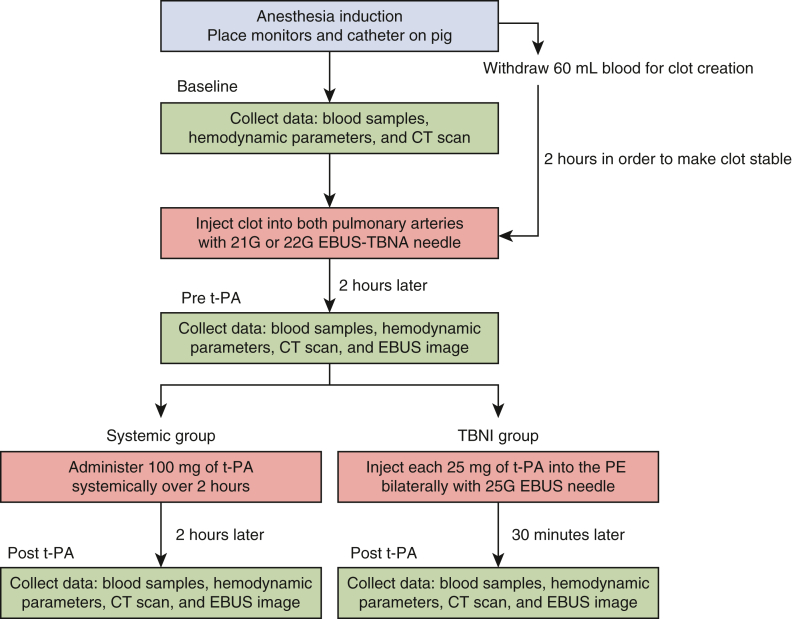
The experimental schedule on day 1 is described in Figure 1. Sixty milliliters of autologous blood was withdrawn and maintained at room temperature for 2 hours in order to create stable clots contained in syringes. After 2 hours, the serum was discarded and a total of 30 mL of the clots was dispensed to 1-mL syringes for clot injection. Fifteen milliliters of clot was injected into bilateral pulmonary arteries. The first 15 mL of clot was injected into the right pulmonary artery and the second 15 mL of clot was injected into left pulmonary artery, through the EBUS-TBNA needle (Video 1). Since our previous results showed that the amount of thrombus formation in the pulmonary artery varied among individuals, we decided to conduct the experiment with an aligned volume of clots in order to create PE. Clot injection started from the most distal main pulmonary artery, limited only by the reach of EBUS scope, since the clot is easily dispersed throughout the segmental pulmonary arteries when clot injection is performed in the main pulmonary artery starting from the central side. Once the injected clot reached the tip of EBUS-TBNA needle on EBUS, the puncture site was moved proximal to the pulmonary artery, and the clot was injected at 3 points in total to create a continuous thrombus. Thrombolytic agent, t-PA was administered 2 hours’ post-clot injection.
Figure 1.
This illustration shows the procedures on day 1. The green frame shows the collection time points for the hemodynamic parameter, the blood test, the CT scans, and the EBUS images. The red frame shows the time points for clot injection or t-PA administration. Note that the systemic group hemodynamic parameters were collected 30 minutes and 2 hours after t-PA administration. The other data: the blood tests, the EBUS images, and the CT scans were collected 2 hours' post-t-PA administration. Systemic group: t-PA systemic administration; TBNI group: t-PA injection using EBUS-TBNI. CT, Computed tomography; EBUS, endobronchial ultrasound; EBUS-TBNA, endobronchial ultrasound-guided transbronchial needle aspiration; t-PA, tissue plasminogen activator; EBUS-TBNI, endobronchial ultrasound-guided transbronchial needle injection; PE, pulmonary embolisms.
We designed 2 types of thrombolytics treatment method for the porcine PE model. The first treatment is an injection of 25 mg of t-PA, dissolved in 25 mL of distilled water, into a PE using EBUS-TBNI (TBNI group). Fifty milligrams of t-PA was used for bilateral PEs in a pig in total. As we reported previously, t-PA was then injected directly into the clots using EBUS-TBNI by a 25-gauge needle. Clots were punctured in 3 places sequentially from distal to proximal, with one-third of 25 mg t-PA administered at each puncture site (Video 2). The second treatment is a systemic administration of 100 mg of t-PA, delivered intravenously over 2 hours (systemic group as a control) conforming to the suggested treatment in the ACCP and the ESC guidelines.1, 2, 3 Hemodynamic parameters, blood collection, and contrast-enhanced CT scans underwent at the time of (1) baseline as control, (2) pre t-PA, (3a) 30 minutes' post t-PA in TBNI group, and (3b) 2 hours' post t-PA in Systemic group on day 1. Only the hemodynamic parameters at 30 minutes’ post t-PA systemic administration in the systemic group were additionally collected. Those data were collected on (4) day 3 and (5) day 7. On day 7, the pigs were humanely killed 5 minutes after 10,000 U (10 mL) heparin administration and underwent bilateral pneumonectomy to measure the residual clots in both of pulmonary arteries.
Measurement of Clot Volume
Regarding the analysis of the residual clot volume, we collected the clot volumes separately from each pulmonary artery in 1 pig, so we used 3 pigs for each group, meaning 6 residual clot volumes were evaluated from each group. The clot volumes in pulmonary arteries were measured using the 3-dimensional (3D) slicer software (version 5.0.3 r30893/7ea0f43).24 This is an open-source software application for medical image computing. The 3D Slicer application framework provides standard Digital Imaging and Communications in Medicine import and export, 2-dimensional image slicing and 3D visualization and quantification. In this study, Segment Editor and Quantification module were conducted to measure the clot volumes. The clots were traced on each slice of CT images. The 3D slicer software automatically generates a 3D model using these traced areas and calculates the amount of clot volumes. Regarding the residual clots on day 7 confirmed by pneumonectomy, the volume was calculated using the truncated cone formula we previously reported23 because we were not able to clearly determine the clot volume based on the CT due to the lack of sensitivity in this modality.
Statistical Analysis
Summary statistics are expressed herein as mean ± standard error of the mean. A 2-factor repeated-measures analysis of variance (ANOVA) was conducted to compare the hemodynamic parameters and the blood test between the groups over time. P values are indicated for pairwise comparisons of interest and were computed by using Sidak's multiple comparison test between treatment groups and by using Tukey's multiple comparisons test between time points when a significant difference was shown in the result of the ANOVA. The residual clot volume and percent change of clot volume were also conducted by a 2-factor repeated-measures ANOVA. Clot volume decrease per hour was compared between treatment groups using the Mann–Whitney U test. The Wilcoxon signed-rank test was used for comparison between each time point in one treatment group.
Results
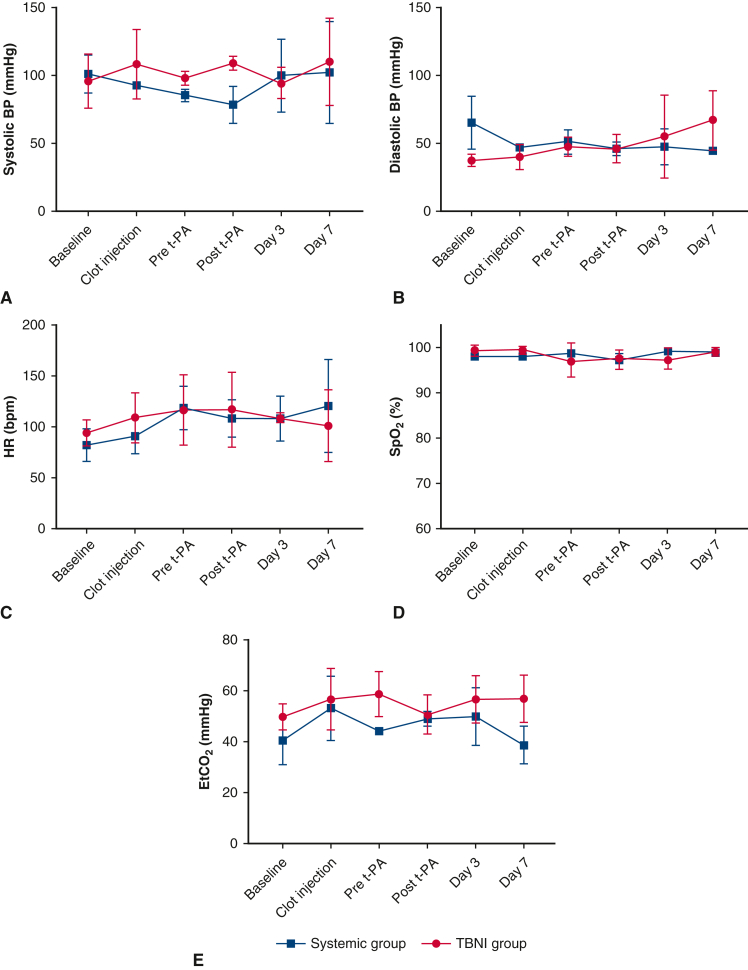
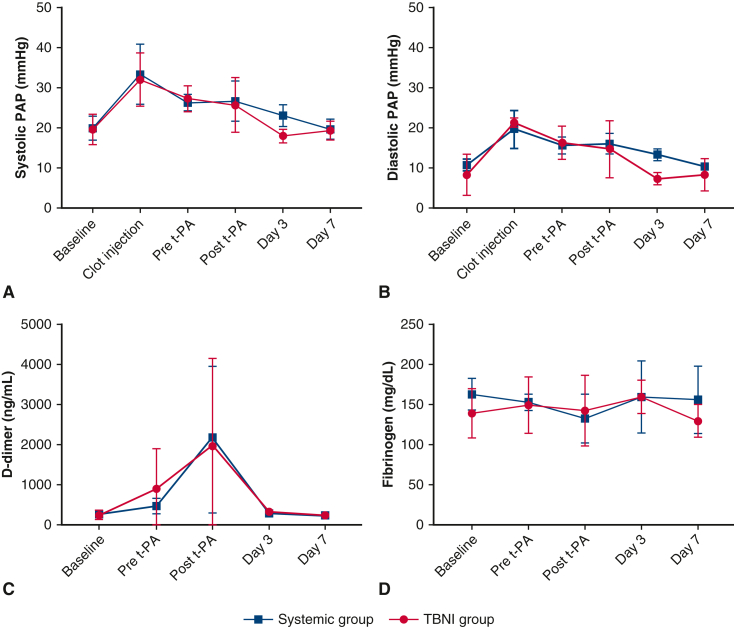
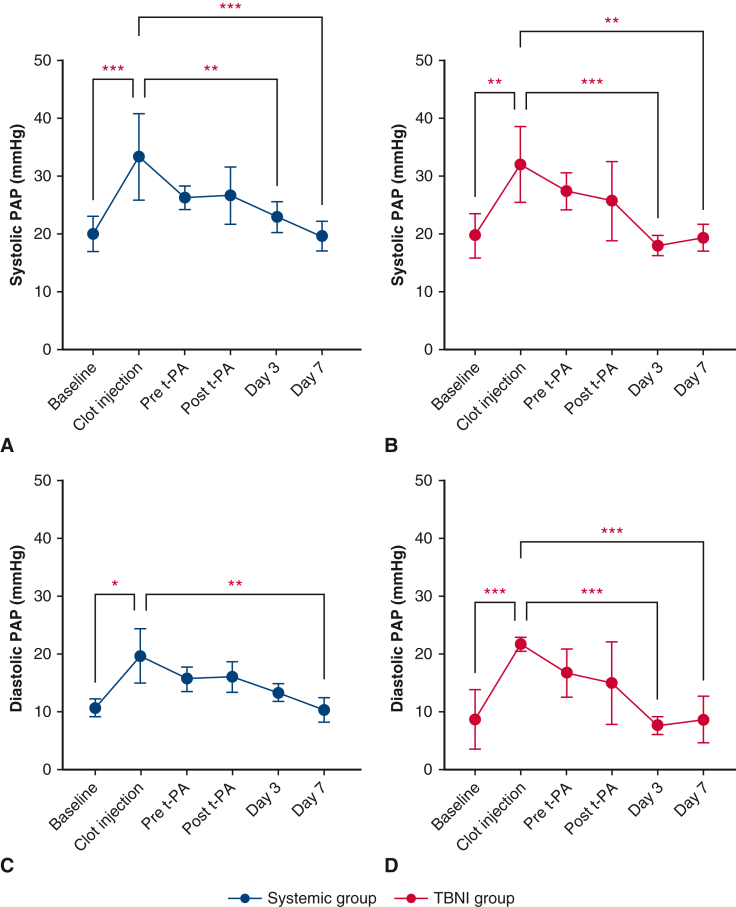
Bilateral clot injections were performed on 6 pigs: 3 for the systemic group; 3 for the TBNI group. In all cases, thrombus formation was successful, and we were able to measure the hemodynamics parameters, blood tests, and acquire the enhanced-contrast CT scans. All pigs survived until being humanely killed on day 7. The results of the hemodynamic parameters and the blood tests for both groups are summarized in Figure 2. In both groups, PAP increased immediately after clot injection and decreased gradually over 2 hours. The results of systolic PAP and diastolic PAP showed significant differences in the time factor by 2-factor repeated-measures ANOVA, respectively (systolic PAP, F = 13.67, P < .0001; diastolic PAP F = 11.9, P < .0001). However, the result between the systemic group and the TBNI group did not show significant differences (Figure 3, A and B). For pairwise comparisons of sPAP between each time point in the TBNI group, there were some significant differences between the time points; in the systemic group, there were also some significant differences between the points (Figure E1, A and B). The pairwise comparisons of dPAP between each time point showed similar results as well (Figure E1, C and D).
Figure 2.
The changes in each hemodynamic parameter at each time point in the systemic group and TBNI group. The average changes of (A) systolic BP, (B) diastolic BP, (C) HR, (D) SpO2, and (E) EtCO2 were shown at each time point for each group. BP, Blood pressure; t-PA, tissue plasminogen activator; TBNI, transbronchial needle injection; HR, heart rate; SpO2, oxygen saturation; EtCO2, end-tidal carbon dioxide.
Figure 3.
The changes in pulmonary artery pressure (PAP), d-dimer, and fibrinogen at each time point in the systemic group and TBNI group. The average changes of (A) systolic PAP, (B) diastolic PAP, (C) d-dimers, and (D) fibrinogen are shown at each time point for each group. t-PA, Tissue plasminogen activator; TBNI, transbronchial needle injection.
Figure E1.
The changes in systolic PAP and diastolic PAP in the systemic group and TBNI group. The average changes of (A) systolic PAP in the systemic group, (B) systolic PAP in TBNI group, (C) diastolic PAP in the systemic group, and (D) diastolic PAP in TBNI group. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. PAP, Pulmonary artery pressure; t-PA, tissue plasminogen activator; TBNI, transbronchial needle injection.
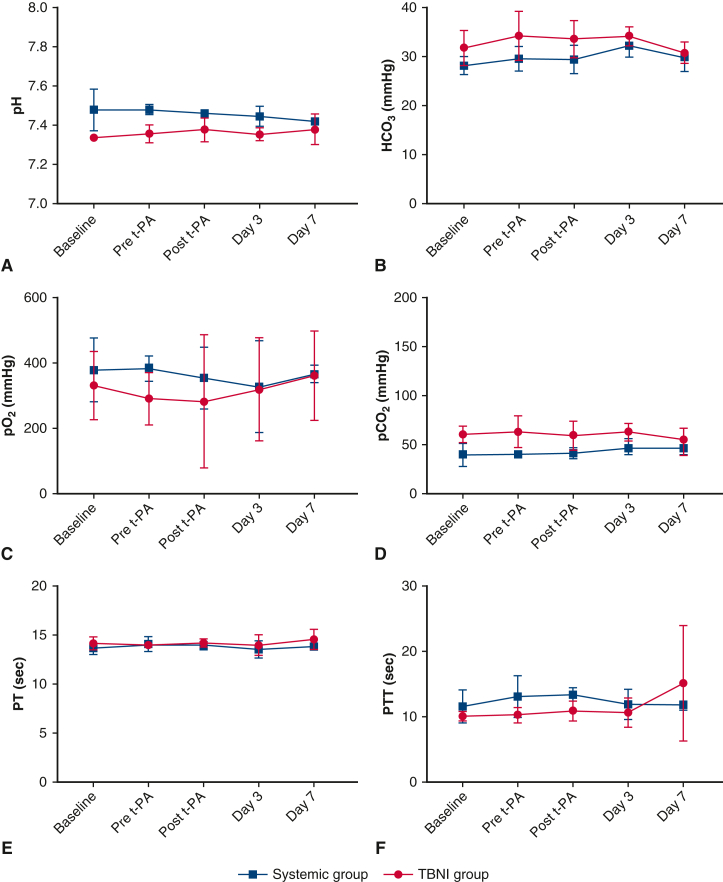
The average of the d-dimer plateaued at a maximum at the post–t-PA time point and then decreased and returned to baseline in both groups on day 3. The average of d-dimer showed significant differences in the time factor by 2-factor repeated-measures ANOVA (F = 4.84, P < .01). There was no significant difference between the groups (Figure 3, C). The average of the fibrinogen also didn't show any significant differences in the time factor and in the group factor (Figure 3, D). The other hemodynamic parameters and blood tests were also measured. Only the average of pH showed significant differences in the group factor by 2-factor repeated-measures ANOVA (F = 11.05, P < .05) (Figure 4).
Figure 4.
The changes in each blood test at each time point in the systemic group and TBNI group. The average changes of (A) pH, (B) HCO3, (C) pO2, (D) pCO2, (E) PT, and (F) PTT were shown at each time point for each group. t-PA, Tissue plasminogen activator; HCO3, bicarbonate; TBNI, transbronchial needle injection; po2, oxygen tension; pco2, carbon dioxide tension; PT, prothrombin time; PTT, partial thromboplastin time.
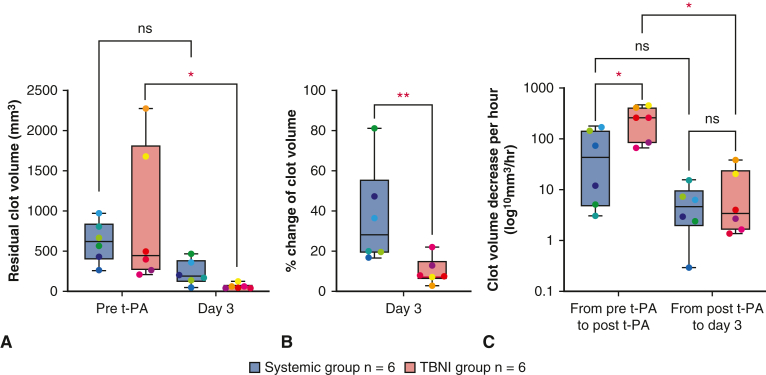
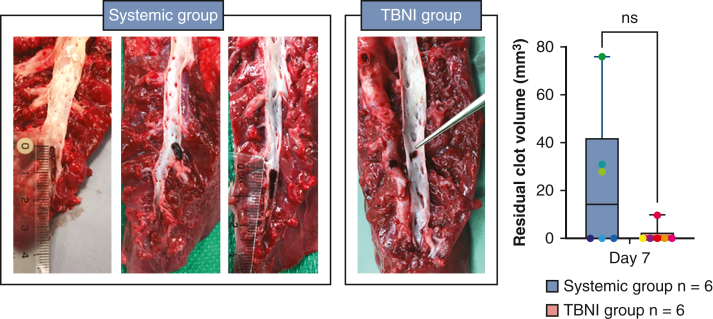
The evaluations of residual clot volumes are shown in Figure 5, A (mm3: systemic group 611.0 ± 234.4 vs TBNI group 880.1 ± 799.2 at pre-t-PA; systemic group 221.8 ± 139.6 vs TBNI group 50.3 ± 28.4 at day 3). For the residual clot volume, a 2-way repeated-measures ANOVA was statistically significant for differences in the time factor (F = 11.3, P < .01). For pairwise comparisons of the residual clot volume between each time point in TBNI group, there were significant differences between pre-t-PA/day 3 (P < .05). There was no significant difference between both groups. Looking at the percent change of clot volumes, normalizing each residual clot volume at the time point of post clot as 100%, the average of % change of the residual clot volumes also showed significantly differences in the time factor (F = 214.2, P < .0001) and in the group factor (F = 6.609, P < .05) by a 2-factor repeated-measures ANOVA (Figure 5, B). For pairwise comparisons of the percent change of clot volumes between the systemic group and the TBNI group, there was a significant difference (%: systemic group 36.6 ± 22.6 vs TBNI group 9.6 ± 6.1, P < .01). Measuring the elapsed time from the time record of the contrast-enhanced CT scan, this measured time was used to calculate the clot volume decrease volume per hour. Considering the elapsed time, the average decrease of clot volume per hour at pre-t-PA to post-t-PA was greater in TBNI group than in the systemic group (mm3/hour: systemic group 68.1 ± 68.1 vs TBNI group 256.8 ± 148.1, P < .05) (Figure 5, C). The speed of clot dissolution was about 3.8 times faster in TBNI group than in the systemic group at t-PA to post-t-PA. Comparing 2 terms in each group separately, only the TBNI group showed a significant decrease between 2 terms (mm3/hour: in TBNI group, from pre-t-PA to post t-PA 256.8 ± 162.2 vs from post t-PA to day 3 11.3 ± 15.0, P < .05). On day 7, there was one residual clot in TBNI group, and 3 clots in the systemic group, respectively. There was no significant difference in the average of residual clot volumes on day 7 between the systemic group and TBNI group (mm3: systemic group 22.4 ± 29.9 vs TBNI group 1.6 ± 3.9, P = .18) (Figure E2).
Figure 5.
Analysis of the residual clot volumes. A, The comparison of the residual clot volumes between the systemic group and TBNI group at pre-t-PA and on day 3. B, The percentage change of clot volumes on day 3 compared with pre-t-PA. C, Amount of clot volume decrease per an hour from pre-t-PA to post-t-PA and from post-t-PA to day 3. ∗P < .05; ∗∗P < .01. The lower and upper borders of the box represent the lower and upper quartiles. The middle horizontal line represents the median. The lower and upper whiskers represent the minimum and maximum values of nonoutliers. Each value is plotted in the same color. t-PA, Tissue plasminogen activator; TBNI, transbronchial needle injection.
Figure E2.
The comparison of the residual clot volumes between the systemic group and TBNI group on day 7. TBNI, Transbronchial needle injection; ns, not significant.
Regarding complications, one pig in TBNI group developed stridor on postoperatively day 1. We believe this is due to failed intubation. Therefore, it was determined to be laryngeal edema caused by the damage of failed intubation. The stridor improved after we administered dexamethasone (0.1 mg/kg) intramuscularly, but it lasted until day 7. No hemorrhage caused by t-PA administration and EBUS-TBNI was observed intracranially, intrathoracically, or intraperitoneally on any contrast-enhanced CT images in either group. Bronchoscopy views did not show any bleeding during procedures (Video 3). In the systemic group, one pig had a large consolidation in right lower lobe, which spread between right main bronchus and dorsal lower lobe. These radiologic findings revealed atelectasis by pathologic assessment.
Discussion
This study showed the effectiveness in clot dissolution and the safety of t-PA injection using EBUS-TBNI for the porcine PE model (Figure 6). Regarding the dissolving of the clot via t-PA injection using EBUS-TBNI, this treatment was effective immediately after t-PA administration, correlating with results of previous studies. In particular, as shown in Figure 5, C, the clot volume decrease per hour clearly shows the immediate effect after t-PA administration in TBNI group, with a dissolution rate of 256.8 mm3/h (compared with 68.1 mm3/h in the systemic group). This result suggests that this treatment method can be applied to massive PE in emergency situations. However, t-PA injection using EBUS-TBNI was not sufficiently effective to reduce the average PAP at 30 minutes’ post-t-PA (Figure 3, A and B). There were no significant differences in PAP between both groups; however, when we focused on day 3, we found there were differences between both groups. This result becomes interesting when considering clinical trials using this treatment. In our model, the porcine thrombus filled the pulmonary artery in the caudal lung lobe sequentially from the periphery, which suggests that the clot must be dissolved all the way down to the periphery to release the increased PAP. This may explain why the results of PAP were not as effective as the rate of thrombus dissolution. In the case of normal acute PE at high risk, the thrombus is often stuck in the central side of pulmonary artery, and it is important to dissolve the thrombus in the central pulmonary artery or dislodge it quickly into the peripheral pulmonary artery, where it would pose a smaller risk. In this respect, the superiority of t-PA injection using EBUS-TBNI is evident in the thrombus volume data, so we do not believe that the fact there were no significant differences in PAP is a problem.
Figure 6.
Summary of this study on the safety and effectiveness of endobronchial ultrasound–guided transbronchial needle injection (EBUS-TBNI) in a porcine survival model. A pig model of pulmonary embolism (PE) was created by injecting clots into the pulmonary artery of healthy pigs under EBUS guidance. One group received 100 mg of t-PA systemic administration to treat PE (systemic group); the other group received each 25 mg of t-PA injection into bilateral PEs using EBUS-TBNI (total of 50 mg t-PA). Hemodynamic parameters, blood tests, and CT scans were collected to evaluate the clot dissolving. In TBNI group, the speed of dissolving clot was significantly greater than in the systemic group. There were no complications in both groups. Twenty-five milligrams of t-PA injection using EBUS-TBNI can be a safe and effective procedure to treat acute massive PE. The promising efficacy data suggest a potential role for clinical practice. t-PA, Tissue plasminogen activator; CT, computed tomography.
It has been reported that a decrease of fibrinogen is associated with bleeding during t-PA treatment.25 No significant differences between the 2 groups were observed, nor were there any significant differences from baseline. It could be considered a reasonable result as no bleeding complications occurred. The values of d-dimer also showed no significant differences between 2 groups; however, the d-dimer increased immediately after clot injections, so that this result was also reasonable as d-dimer is used to exclude the diagnosis of PE. Other than PAP, the hemodynamic monitoring was not significantly different between the 2 groups. This might be due to the pigs being under general anesthesia and ventilator management with tracheal intubation, and therefore, the systemic management was adjusted as needed.
We previously reported that a minimum of 10 mg of t-PA injection using EBUS-TBNI can provide maximum efficacy on PE as well as 25 mg of t-PA injection using EBUS-TBNI.23 Administration of t-PA has dose-dependent bleeding risk in general.26 Since one of the purposes in this study was to confirm the safety, experiments were conducted using a greater dose of 25 mg t-PA injection EBUS-TBNI. Three pigs were used for each group in this study; therefore, the conclusion on the safety of t-PA injection using EBUS-TBNI is limited by the small sample size. In our previous experiments, no intra-airway bleeding was observed in 6 pigs during a 1-week survival model13 and in the 8 pigs with nonsurvival model.23 Combining the previous results with the current experiment, a total of 75 punctures of t-PA injection using EBUS-TBNI have been performed on 26 pulmonary arteries in 17 pigs. We have never observed any active bleeding from pulmonary artery by bronchoscopy (Table 1),13,23 although there were no CT scans and hemodynamic parameters collected at that time. Taking this into account, we presume that major bleeding in the airway is unlikely to occur in t-PA injection using EBUS-TBNI.
Table 1.
The total number of pigs used, PAs used, and punctures done to evaluate t-PA injection using EBUS-TBNI over 3 studies
| Authors | Number of pigs used | Number of PAs used | Number of punctures | Duration of observation |
|---|---|---|---|---|
| Inage et al, 202113 | 6 | 6 | 18 | 1 wk |
| Aragaki et al, 202323 | 8 | 14 | 39 | 2 h |
| This study | 3 | 6 | 18 | 1 wk |
| Total | 17 | 26 | 75 |
PA, Pulmonary artery; t-PA, tissue plasminogen activator; EBUS-TBNI, endobronchial ultrasound–guided transbronchial needle injection.
The first key advantage of t-PA injection using EBUS-TBNI for PE is that EBUS can evaluate the location of PE in real time, and unlike the t-PA systemic administration or USAT, t-PA using EBUS-TBNI can be administered at a pinpoint within the thrombus. t-PA is only effective at dissolving clots when it is present with the clots at the same time. Therefore, by injecting t-PA directly into the thrombus, it might be possible to achieve its effect efficiently and to minimize the total dosage of t-PA administered. Although direct placement of a catheter in the pulmonary artery to inject t-PA should be considered to increase the effect of t-PA, guidelines point out that there may be greater complications than systemic administration. Therefore, that technique was not considered in this study. The second key advantage of t-PA injection using EBUS-TBNI is its ease of adoption into current practice. EBUS-TBNA and USAT both require training for proficiency before implementation, but unlike USAT, the EBUS system is usually already equipped in core hospitals that can treat acute PE.
This study has several important limitations. First, the number of animals used in this study was small. However, when we looked at the difference in treatment efficacy between t-PA systemic administration and t-PA injection using EBUS-TBNI, we found there was a significant difference in the speed of clot dissolution, which suggests that even with only 3 cases, sufficient data were obtained as described previously. In contrast, to the point of safety, no conclusions could be drawn from this study alone without considering the previous studies. Since this was an animal study, it is difficult to guarantee the safety, so the decision should take past studies into consideration. Second, since this experiment was conducted in a porcine model, it is important to note the difference in anticoagulation between humans and pigs. Although reported in vitro, porcine blood has similar maximum coagulation firmness27 and has prolonged clot lysis by t-PA compared with humans.28 However, as our previous13 and current studies have shown, the response of t-PA to PE is still effective in vivo. This suggests the possibility of further reduction of the t-PA dose when t-PA injection using EBUS-TBNI is adapted to humans. Regarding intubation complication, although intubation was performed for the pig experiment, complications due to intubation are not expected to cause any issues in a clinical setting, as this procedure would be carried out under conscious sedation without intubation. Third, this study did not evaluate the safety of using parenteral anticoagulation concurrently or after t-PA injection using EBUS-TBNI. It is recommended that the acute PE at high risk receives a parenteral anticoagulation during or after t-PA administration.1, 2, 3 Since it has been noted that t-PA administration during systemic heparinization is likely to increase the risk of bleeding, careful consideration should be given regarding the timing of heparin initiation when this technique is actually used for treatment. However, this technique is apparently less invasive than the pulmonary embolectomy. Therefore, we assume this technique followed by the systemic heparinization could be acceptable in case heparinization starts 4 to 6 hours later because theoretically t-PA is rapidly metabolized. Fourth, this experiment is only effective in quickly dissolving thrombus, but it is not clear whether it truly improves cardiac burden such as RV dysfunction and prognosis. However, since it is difficult to evaluate this in animal studies, it will become clear in future clinical trials.
Conclusions
This study has revealed that t-PA EBUS-TBNI can be an effective procedure to safely dissolve acute massive PE. Its dissolving effect is more immediate than the systemic administration of t-PA. These efficacy data suggest a potential role for a clinical trial.
Webcast
You can watch a Webcast of this AATS meeting presentation by going to: https://www.aats.org/resources/assessment-of-safety-and-feasibility-of-thrombolytic-therapy-to-pulmonary-emboli-by-endobronchial-ultrasound-guided-transbronchial-needle-injection-in-a-porcine-survival-model.

Conflict of Interest Statement
K.Y. received industry sponsored grants from the Olympus Corporation, Johnson & Johnson, and ODS Medical Inc. K.Y. is a consultant for Olympus Medical Corporation, Johnson & Johnson, and Medtronic. K.Y. has research collaborations with Siemens, Zidan Medical Inc, and OKF Technology. K.Y. is on the advisory board for Olympus American Inc, Medtronic, and Johnson & Johnson. All other authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Footnotes
H.O. received a research grant from Uehara Memorial Foundation.
Supplementary Data
Clot injection into the pulmonary artery by EBUS-TBNA needle. EBUS-TBNI, Endobronchial ultrasound–guided transbronchial needle injection. Video available at: https://www.jtcvs.org/article/S2666-2507(23)00307-3/fulltext.
Administration of t-PA using EBUS-TBNI. t-PA, Tissue plasminogen activator; EBUS-TBNI, endobronchial ultrasound–guided transbronchial needle injection. Video available at: https://www.jtcvs.org/article/S2666-2507(23)00307-3/fulltext.
Bronchial view 30 minutes after t-PA injection using EBUS-TBNI. t-PA, Tissue plasminogen activator; EBUS-TBNI, endobronchial ultrasound–guided transbronchial needle injection. Video available at: https://www.jtcvs.org/article/S2666-2507(23)00307-3/fulltext.
Appendix 1
References
- 1.Kearon C., Akl E.A., Comerota A.J., Prandoni P., Bounameaux H., Goldhaber S.Z., et al. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest physicians evidence-based clinical practice guidelines. Chest. 2012;141:e419S–e496S. doi: 10.1378/chest.11-2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Konstantinides S.V., Meyer G., Becattini C., Bueno H., Geersing G.J., Harjola V.P., et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS) Eur Heart J. 2020;41:543–603. doi: 10.1093/eurheartj/ehz405. [DOI] [PubMed] [Google Scholar]
- 3.Stevens S.M., Woller S.C., Kreuziger L.B., Bounameaux H., Doerschug K., Geersing G.J., et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. Chest. 2021;160:e545–e608. doi: 10.1016/j.chest.2021.07.055. [DOI] [PubMed] [Google Scholar]
- 4.Engelhardt T.C., Taylor A.J., Simprini L.A., Kucher N. Catheter-directed ultrasound-accelerated thrombolysis for the treatment of acute pulmonary embolism. Thromb Res. 2011;128:149–154. doi: 10.1016/j.thromres.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 5.Pei D.T., Liu J., Yaqoob M., Ahmad W., Bandeali S.S., Hamzeh I.R., et al. Meta-analysis of catheter directed ultrasound-assisted thrombolysis in pulmonary embolism. Am J Cardiol. 2019;124:1470–1477. doi: 10.1016/j.amjcard.2019.07.040. [DOI] [PubMed] [Google Scholar]
- 6.Sun B., Yang J.X., Wang Z.K., Zhou H.J., Chu Y., Li Y., et al. Clinical efficacy and safety of ultrasound-assisted thrombolysis vs. standard catheter-directed thrombolysis in patients with acute pulmonary embolism: a study level meta-analysis of clinical trials. Front Cardiovasc Med. 2022;9 doi: 10.3389/fcvm.2022.967786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pietrasik A., Gąsecka A., Szarpak Ł., Pruc M., Kopiec T., Darocha S., et al. Catheter-based therapies decrease mortality in patients with intermediate and high-risk pulmonary embolism: evidence from meta-analysis of 65,589 patients. Front Cardiovasc Med. 2022;9 doi: 10.3389/fcvm.2022.861307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yasufuku K., Chiyo M., Sekine Y., Chhajed P.N., Shibuya K., Iizasa T., et al. Real-time endobronchial ultrasound-guided transbronchial needle aspiration of mediastinal and hilar lymph nodes. Chest. 2004;126:122–128. doi: 10.1378/chest.126.1.122. [DOI] [PubMed] [Google Scholar]
- 9.Aumiller J., Herth F.J., Krasnik M., Eberhardt R. Endobronchial ultrasound for detecting central pulmonary emboli: a pilot study. Respiration. 2009;77:298–302. doi: 10.1159/000183197. [DOI] [PubMed] [Google Scholar]
- 10.Li P., Wu C., Zheng W., Zhao L. Pathway and application value of exploration of the pulmonary artery by endobronchial ultrasound. J Thorac Dis. 2017;9:5345–5351. doi: 10.21037/jtd.2017.12.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harris K., Chalhoub M. Endobronchial ultrasound as a confirmatory tool for the diagnosis of pulmonary embolism. Ann Thorac Med. 2014;9:127–128. doi: 10.4103/1817-1737.128863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dhillon S.S., Harris K. Endobronchial ultrasound for the detection of chronic pulmonary artery thrombus. Endosc Ultrasound. 2016;5:272–273. doi: 10.4103/2303-9027.187893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inage T., Fujino K., Motooka Y., Ishiwata T., Ujiie H., Bernards N., et al. Thrombolysis of pulmonary emboli via endobronchial ultrasound-guided transbronchial needle injection. Ann Thorac Surg. 2021;112:395–404. doi: 10.1016/j.athoracsur.2020.08.043. [DOI] [PubMed] [Google Scholar]
- 14.Inage T., Fujino K., Motooka Y., Ishiwata T., Ujiie H., Gregor A., et al. Development of a minimally invasive pulmonary porcine embolism model via endobronchial ultrasound. J Thorac Dis. 2022;14:238–246. doi: 10.21037/jtd-21-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Panchabhai T.S., Machuzak M.S., Sethi S., Vijhani P., Gildea T.R., Mehta A.C., et al. Endobronchial ultrasound-guided transvascular needle aspiration: a single-center experience. J Bronchology Interv Pulmonol. 2015;22:306–311. doi: 10.1097/LBR.0000000000000227. [DOI] [PubMed] [Google Scholar]
- 16.Mehta R.M., Biraris P.R., Pattabhiraman V., Srinivasan A., Singla A., Kumar S., et al. Defining expanded areas in EBUS sampling: EBUS guided trans- and intra-pulmonary artery needle aspiration, with review of transvascular EBUS. Clin Respir J. 2018;12:1958–1963. doi: 10.1111/crj.12764. [DOI] [PubMed] [Google Scholar]
- 17.Folch E., Santacruz J.F., Fernandez-Bussy S., Gangadharan S., Kent M.S., Jantz M., et al. The feasibility of EBUS-guided TBNA through the pulmonary artery in highly selected patients. J Bronchology Interv Pulmonol. 2016;23:7–13. doi: 10.1097/LBR.0000000000000245. [DOI] [PubMed] [Google Scholar]
- 18.Kazakov J., Hegde P., Tahiri M., Thiffault V., Ferraro P., Liberman M. Endobronchial and endoscopic ultrasound-guided transvascular biopsy of mediastinal, hilar, and lung lesions. Ann Thorac Surg. 2017;103:951–955. doi: 10.1016/j.athoracsur.2016.08.111. [DOI] [PubMed] [Google Scholar]
- 19.Naaman R., Lautenschlaeger T., Diab K. Feasibility of performing transvascular endobronchial ultrasound-guided transbronchial needle aspiration. Clin Lung Cancer. 2021;22:e595–e601. doi: 10.1016/j.cllc.2020.10.020. [DOI] [PubMed] [Google Scholar]
- 20.Asano F., Aoe M., Ohsaki Y., Okada Y., Sasada S., Sato S., et al. Complications associated with endobronchial ultrasound-guided transbronchial needle aspiration: a nationwide survey by the Japan Society for Respiratory Endoscopy. Respir Res. 2013;14:50. doi: 10.1186/1465-9921-14-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vaidya P.J., Munavvar M., Leuppi J.D., Mehta A.C., Chhajed P.N. Endobronchial ultrasound-guided transbronchial needle aspiration: safe as it sounds. Respirology. 2017;22:1093–1101. doi: 10.1111/resp.13094. [DOI] [PubMed] [Google Scholar]
- 22.Gürün Kaya A., Çiledağ A., Erol S., Öz M., Doğan Mülazımoğlu D., Işık Ö., et al. Is endobronchial ultrasound-transbronchial needle aspiration (EBUS-TBNA) reliable and safe procedure in geriatric patients? Aging Clin Exp Res. 2022;34:913–925. doi: 10.1007/s40520-021-02012-9. [DOI] [PubMed] [Google Scholar]
- 23.Aragaki M., Inage T., Ishiwata T., Gregor A., Bernards N., Kato T., et al. Optimization of thrombolytic dose for treatment of pulmonary emboli using endobronchial ultrasound-guided transbronchial needle injection. J Thorac Cardiovasc Surg. 2023;165:e210–e221. doi: 10.1016/j.jtcvs.2022.08.044. [DOI] [PubMed] [Google Scholar]
- 24.Pinter C., Lasso A., Fichtinger G. Polymorph segmentation representation for medical image computing. Comput Methods Programs Biomed. 2019;171:19–26. doi: 10.1016/j.cmpb.2019.02.011. [DOI] [PubMed] [Google Scholar]
- 25.Kaufman C., Kinney T., Quencer K. Practice trends of fibrinogen monitoring in thrombolysis. J Clin Med. 2018;7:111. doi: 10.3390/jcm7050111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stewart D., Kong M., Novokhatny V., Jesmok G., Marder V.J. Distinct dose-dependent effects of plasmin and TPA on coagulation and hemorrhage. Blood. 2003;101:3002–3007. doi: 10.1182/blood-2002-08-2546. [DOI] [PubMed] [Google Scholar]
- 27.Kessler U., Grau T., Gronchi F., Berger S., Brandt S., Bracht H., et al. Comparison of porcine and human coagulation by thrombelastometry. Thromb Res. 2011;128:477–482. doi: 10.1016/j.thromres.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 28.Flight S.M., Masci P.P., Lavin M.F., Gaffney P.J. Resistance of porcine blood clots to lysis relates to poor activation of porcine plasminogen by tissue plasminogen activator. Blood Coagul Fibrinolysis. 2006;17:417–420. doi: 10.1097/01.mbc.0000233374.79593.57. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Clot injection into the pulmonary artery by EBUS-TBNA needle. EBUS-TBNI, Endobronchial ultrasound–guided transbronchial needle injection. Video available at: https://www.jtcvs.org/article/S2666-2507(23)00307-3/fulltext.
Administration of t-PA using EBUS-TBNI. t-PA, Tissue plasminogen activator; EBUS-TBNI, endobronchial ultrasound–guided transbronchial needle injection. Video available at: https://www.jtcvs.org/article/S2666-2507(23)00307-3/fulltext.
Bronchial view 30 minutes after t-PA injection using EBUS-TBNI. t-PA, Tissue plasminogen activator; EBUS-TBNI, endobronchial ultrasound–guided transbronchial needle injection. Video available at: https://www.jtcvs.org/article/S2666-2507(23)00307-3/fulltext.