Abstract
We assessed the distribution of alien fishes in the Karun River Basin, Iran. Fish were collected from 39 sites during the November–December 2018 low-flow period. In total, 39 fish species from nine orders and 14 families were documented. Among these, 10 species were alien to the basin (986 individuals; 15.7%). Four species were the most abundant alien species and primarily in impounded, downstream reaches. Redundancy analysis (RDA) was conducted to identify the extent of changes in alien fish assemblages with environmental parameters. RDA1 and RDA2 accounted for 36.24% and 25.33% of the variation of alien species, respectively. Altitude, depth, electrical conductivity, water temperature, turbidity, dissolved oxygen, and river width were the most significant parameters affecting alien species distributions. We present a dual-pathway cause-and-effect hypothesis proposing that alien fish species presence causes declines in the ecological status of native fish communities. We then explore how human-induced aquatic ecosystem degradation creates opportunities for alien species to invade new ecosystems, further impacting native fish communities. Our study contributes insight into the cause and effect of the presence of alien fish species in the Karun River Basin and emphasizes the urgency of conservation measures to protect this critically endangered watershed.
Keywords: Karun Basin, fish, human impact, alien species, redundancy analysis, habitat quality
1. Introduction
A large number of stressors induce major threats and risks to aquatic habitats and their living and non-living elements and structures globally [1–5]. Consequently, fishes, as a vital group of organisms in aquatic ecosystems [6–8], are in decline. They suffer secondary losses due to the diverse impacts of human activities [9–22]. Global concern is growing as biodiversity plays a crucial role in ecosystem function and resilience [1,3,23]. Over the last 200 years, alien species have increased their range by 37% and this expansion shows no signs of stopping [24]. Seebens et al. [25] predict a further 36% increase in the number of alien species established globally by 2050. This is concerning as the dynamic equilibrium of an ecosystem can be disrupted by alien species introduction [26,27]. Consequently, alien species are widely considered to be one of the main threats to biodiversity and the second leading cause of animal extinctions [28]. Declines are particularly noteworthy in freshwater ecosystems, primarily due to habitat destruction, pollution, overexploitation, and the introduction of alien species [3,29]. Trends in socio-economic development suggest that the introduction of alien fish will persist, along with the associated environmental risks and biodiversity losses [30].
By definition, “an alien species is any species intentionally or accidentally transported and released by humans outside its native current range” [31]. The introduction of alien freshwater fish species has been practiced since the late 1800s [32,33] to support aquaculture for food and aquarium fish production, stocking of ecosystems to control disease vectors, and to support recreational fishing [34,35]. Once introduced, alien species easily spread to neighboring systems, including those of adjacent countries [35]. The success of alien species in freshwater ecosystems is often attributed to their broader environmental and physiological tolerance [36,37]. However, the specific mechanisms and impacts of alien species differ across ecosystems, species, and spatial scales [30,38–46]. As a result of these impacts on native populations, the presence and relative abundance of alien fish species can function as an indicator of biological integrity [47,48].
In recent decades, several alien fish species have been introduced in Iranian waters [49]. Among these are the blue tilapia, Oreochromis aureus (Steindachner 1864), and redbelly Tilapia, Coptodon zillii (Gervais 1848), [50] that entered the waters of Iran via transboundary waters due to inadequate precautions to prevent their spread [51–55]. An additional 23 to 32 alien fish species have been documented in Iranian freshwater ecosystems [56–58].
Focusing specifically on the Karun River Basin, a comprehensive analysis of the status of all native and endemic fish species has been previously provided [48]. This paper focuses specifically on the distribution and factors influencing the expansion of alien fish species in the same study area. This is relevant as the Karun River Basin provides crucial ecosystem services (e.g., drinking water, irrigation for agriculture, support for industries and hydropower plants) [58] to those in the basin and beyond. Subsistence fishing, which can be significantly affected by the presence of alien fish species, is of special concern as it is an integral part of rural and urban livelihood systems in the Karun Basin. It is easy to conceive how the presence of alien fish species contribute to the decline in the ecological status of native fish communities, but the human-induced degradation of aquatic ecosystems that enhances opportunities for these aliens to penetrate new ecosystems must also be considered. This dual-way cause-and-effect working hypothesis forms the foundation of this research [54,55]. We examine the relationship between alien fish data and environmental variables at sampling sites. We then discuss the potential negative impacts of invasive alien species on native fish communities of the basin which include many endemic species. This information is essential for informed decision making and effective environmental management of the Karun River.
2. Materials and Methods
2.1. Study Area
The Karun River Basin is located in southwestern Iran and encompasses seven provinces (Chaharmahal-va-Bakhtiari, Fars, Isfahan, Khuzestan, Kohgiluyeh-va-Boyer-Ahmad, Lorestan, and Markazi) [59] (Figure 1).
Figure 1.

Map of the study area and sampling site locations.
It is Iran’s largest river system and covers 67,000 km2, i.e., 4.2% of Iran’s area [60,61]. Its main stream is more than 950 km long with an average annual flow of about 575 m3/s in Ahvaz city which is located in the downstream extent of the system. The headwaters of the Karun are in the Zagros mountains in the north and flow into the Arvandrud River (Tigris–Euphrates Basin) before discharging into the Persian Gulf. The river system has a slope of 0–8.5% in the low- and high-altitude areas. Water abstraction is used for irrigation of agricultural lands that covers more than 380,000 ha [62]. The altitude ranges from sea level to >4000 m above the sea level (m.a.s.l.), which, along with its topography, creates diverse climatic conditions. According to the Koppen–Geiger climate classification, the Karun River has four different sub-type climatic conditions including arid, semi-arid, Mediterranean, and humid continental climatic conditions [63]. The catchment has an average annual rainfall of 600 mm and an air temperature that ranges from 20 to more than 50 °C in summer and from <0 to 30 °C in winter [64].
2.2. Fish Sampling
Fish were collected in the Karun River Basin at 35 sites (Figure 1) during the low-flow period in November and December 2018. Sites 1 to 26 were positioned in wadable river sections and were sampled using backpack electrofishing equipment (model: Samus 1000) and a dip net. Sites 27 to 35 (non-wadable sites) were positioned in downstream basin areas and sampled using a boat electrofishing system and a dip net (hand-made for electrofishing with a power input of 220 V and output power of 100–500 W). At each site, the reach sampled was approximately 200 m in length and included available mesohabitats (e.g., riffles, runs, and pools). We standardized the catch per unit effort (CPUE) on distance rather than time because the effort (i.e., time) required to safely navigate the stream habitats varied greatly among sites, prohibiting the use of time as a standardizing factor. We used species detection curves to affirm the adequacy of sampling (Appendix A) [65]. The fish sampling effort at each site was approximately 90 ± 20 min [48,64]. Although this standardization does not prevent methodological differences in fishing efficiency at different sites with consequences on the dataset, we chose this procedure as the best compromise. Additionally, we retained a certain number of individuals per species in formaldehyde 10% and transferred them to the laboratory at Isfahan University of Technology for further examination and verification of the field identification. Subsequently, the remaining fish were released back into the stream at the original capture site. With the assistance of locally relevant identification guides, and expert ichthyologists, we successfully identified all fish species based on morphological characteristics [49,66–68]. At each site, the biological data collected included the occurrence (presence or absence) and abundance of alien, native (species that evolved in the Karun and other rivers), and endemic fish species (species that are restricted in distribution to the Karun Basin).
2.3. Water Sampling
We collected triplicate surface water samples at 10–15 cm depths at all the 35 sites with pre-washed (HCl 2%) plastic containers. Samples were transported to the laboratory at Isfahan University of Technology (IUT) for water chemistry analysis. A portable multiparameter probe (Oxi, 3205, WTWWeilheim, Germany) was used for in situ measurements of dissolved oxygen concentrations and water temperature. Other variables such as pH, electrical conductivity (μmho/cm), hardness (mg/L CaCo3), total suspended solids (mg/L), total dissolved solids (mg/L), nitrate (mg/L), nitrite (mg/L), phosphate (mg/L), alkalinity (mg/L CaCo3), biological oxygen demand (mg/L), and chemical oxygen demand (mg O2/L) were analyzed in the lab using standard methods. Data were also collected on general habitat features such as river width and depth. Altitude was also recorded at all sampling sites from GPS information (Garmin GPSMAP 64X).
2.4. Data Analysis
Detrended Canonical Correspondence Analysis (DCCA) was run to identify the most relevant response model (between linear or unimodal) for gradient analysis [47,69–71]. A linear model, redundancy analysis (RDA), was selected rather than a unimodal method (e.g., Canonical Correspondence Analysis—CCA) because the dominant gradient length was less than 3 [70,72]. Redundancy analysis (RDA) was performed as a direct gradient method to define the amount of variation in alien fish communities that could be described by environmental parameters [73,74]. Based on the “forward selection method” and ordiR2step function, the most important variables were selected based on significance and adjusted squared R. Of the ten alien fish species collected, only five species (with a frequency of occurrence higher than 5% [72]) and 12 environmental variables were selected (after a forward stepwise selection) for inclusion in the RDA.
To determine the optimal and less favorable ecological conditions in the Karun River, we utilized the Least-Disturbed method, which characterizes the “best-available physical, chemical, and biological habitat conditions given the current state of the waterbody”. Essentially, the least-disturbed sites represent areas where biota experience minimal exposure to the prevailing stressor gradients [75]. Therefore, we performed Principal Component Analysis (PCA) on 16 standardized and centered variables including physicochemical and habitat characteristics. PCA is a valuable tool for identifying factors and sources that may affect water systems and cause changes in water quality. The first axis (PC1) derived from the PCA was identified as the primary stressor gradient, following the approach by Blocksom and Johnson [75]. By observing the stressor’s direction along PC1, sites falling within the 25th quartile of the PC1 were categorized as least disturbed, those within the 75th quartile as most disturbed, and sites between the two quartiles as moderately disturbed sites [65,76]. Afterwards, we generated box plots to compare the biomass and abundance values of native, endemic, and alien fish species across the least, moderate, and most disturbed sites of the Karun Basin. To examine significant differences, we conducted an analysis of variance (ANOVA) using the Tukey HSD method. All statistical analyses were conducted utilizing Microsoft Excel 2016 and R software (v. 4.0.4) [77]. The vegan (2.5–6) [78] and ggplot2 (v. 2.2.0) [79] packages were used for analysis and graphics. The study area map was generated using ArcGIS 10.2 software [80–82].
3. Results
3.1. Alien Fish Species Composition
A total of 6272 fish representing nine orders, 14 families, 30 genera, and 39 species of bony fishes were collected (Table 1). Of these, 29 were native species (12 endemic to the basin) and 10 were alien species (Table 1). Appendix B provides a comprehensive record of all observed fish species, indicating their presence or absence. Among these, 3387 (54%) were native, 1899 (30.3%) were endemic, and 986 (15.7%) were alien fish species. Proportions of these groups varied among sampling sites (Figure 2). The PCA results revealed that the majority of the downstream sites were categorized as the most disturbed locations (Figure 3). As a result, the biomass and abundance of alien fish species were significantly higher in the most disturbed sites in comparison to native and endemic fish species (Figure 4). Alien fish species occurred at 19 of 35 sites, particularly in the downstream sites, whereas they were absent from most headwaters (Figures 2 and 4). Among the alien species, Gambusia holbrooki and Pseudrasbora parva were found only at site 10 (i.e., Tireh), while Carassius gibelio was present in the upper and lower sections of the Karun River Basin. Some alien species such as Oreochromis aureus, Hemiculter leucisculus, and Coptodon zillii were present only in downstream areas of the system. Appendix C provides photos of all observed alien fish species in the Karun River Basin.
Table 1.
Abundance (N) of all recorded fish species in the Karun River Basin, Iran, and IUCN red list status.
| Family | Species | IUCN Status | Status | Relative Abundance |
|---|---|---|---|---|
| Xenocyprididae | Hemiculter leucisculus (Basilewsky, 1855) | Least Concern | Alien | 2.18 |
| Ctenopharyngodon idella (Valenciennes, 1844) | Least Concern | Alien | 0.02 | |
| Danionidae | Barilius mesopotamicus (Berg, 1932) | Least Concern | Native | 0.57 |
| Gobionidae | Pseudorasbora parva (Temminck & Schlegel, 1846) | Least Concern | Alien | 0.05 |
| Cyprinidae | Capoeta coadi (Alwan, Zareian & Esmaeili, 2016) | Not Evaluated | Endemic | 13.78 |
| Capoeta aculeata (Valenciennes, 1844) | Not Evaluated | Endemic | 7.32 | |
| Capoeta trutta (Heckel, 1843) | Least Concern | Native | 6.79 | |
| Carassius gibelio (Bloch, 1782) | Not Evaluated | Alien | 5.29 | |
| Arabibarbus grypus (Heckel, 1843) | Vulnerable/Decreasing | Native | 0.14 | |
| Cyprinus carpio (Linnaeus, 1758) | Vulnerable | Alien | 0.08 | |
| Carasobarbus luteus (Heckel, 1843) | Least Concern | Native | 0.14 | |
| Barbus lacerta (Heckel, 1843) | Least Concern | Native | 0.26 | |
| Barbus karunensis (Khaefi, Esmaeili, Geiger & Eagderi, 2017) | Not Evaluated | Endemic | 0.24 | |
| Cyprinion macrostomus (Heckel, 1843) | Least Concern | Native | 3.84 | |
| Luciobarbus barbulus (Heckel, 1847) | Not Evaluated | Native | 0.29 | |
| Carasobarbus kosswigi (Ladiges, 1960) | Vulnerable/Decreasing | Native | 0.08 | |
| Garra rufa (Heckel, 1843) | Least Concern | Native | 1.16 | |
| Garra gymnothorax (Berg, 1949) | Not Evaluated | Endemic | 0.8 | |
| Leuciscidae | Alburnus caeruleus (Heckel, 1843) | Least Concern | Native | 0.13 |
| Alburnus sellal (Heckel, 1843) | Least Concern | Native | 19.66 | |
| Alburnus doriae (De Filippi, 1865) | Not Evaluated | Endemic | 2.65 | |
| Alburnoides idignensis (Bogutskaya & Coad, 2009) | Not Evaluated | Endemic | 2.10 | |
| Chondrostoma regium (Heckel, 1843) | Least Concern | Native | 13.95 | |
| Squalius berak (Heckel, 1843) | Least Concern | Native | 0.92 | |
| Squalius lepidus (Heckel, 1843) | Least Concern | Native | 0.51 | |
| Acanthobrama marmid (Heckel, 1843) | Least Concern | Native | 4.94 | |
| Nemacheilidae | Turcinoemacheilus saadii (Esmaeili, Sayyadzadeh, Özulug, Geiger & Freyhof, 2014) | Not Evaluated | Endemic | 0.51 |
| Turcinoemacheilus hafezi (Golzarianpour, Abdoli, Patimar & Freyhof, 2013) | Not Evaluated | Endemic | 0.05 | |
| Oxynoemacheilus euphraticus (Bănărescu & Nalbant, 1964) | Not Evaluated | Endemic | 0.37 | |
| Cichlidae | Oreochromis aureus (Steindachner, 1864) | Not Evaluated | Alien | 4.02 |
| Coptodon zillii (Gervais, 1848) | Least Concern | Alien | 2.98 | |
| Sisoridae | Glyptothorax galaxias (Mousavi-Sabet & Eagderi & Vatandoust & Freyhof, 2021) | Not Evaluated | Endemic | 0.62 |
| Glyptothorax alidaeii (Mousavi-Sabet & Eagderi & Vatandoust & Freyhof, 2021) | Not Evaluated | Endemic | 0.62 | |
| Aphanidae | Esmaeilius vladykovi (Coad, 1988) | Not Evaluated | Endemic | 1.24 |
| Poeciliidae | Gambusia holbrooki (Girard, 1859) | Least Concern | Alien | 0.14 |
| Mugilidae | Planiliza abu (Heckel, 1843) | Least Concern | Native | 0.54 |
| Salmonidae | Oncorhynchus mykiss (Walbaum, 1792) | Not Evaluated | Alien | 0.03 |
| Gobiidae | Rhinogobius lindbergi (Berg, 1933) | Not Evaluated | Alien | 0.92 |
| Mastacembelidae | Mastacembelus mastacembelus (Banks & Solander, 1794) | Least Concern | Native | 0.05 |
Figure 2.

The relative abundance of individuals (%) of endemic, native, and alien species at different sampling sites in the Karun River Basin, Iran.
Figure 3.

Principal Component Analysis (PCA) plot in the Karun River Basin, Iran. (Abbreviation: DO: dissolved oxygen; BOD: biological oxygen demand; COD: chemical oxygen demand; NO3: nitrate; TDS: total dissolved solids; WT: water temperature; EC: electrical conductivity; NO2: nitrite, instream, morphological, and total habitat scores.)
Figure 4.

(a) The biomass (in grams of fresh weight) and (b) relative abundance of alien, native, and endemic fish species at the least, moderate, and most disturbed sites of Karun River Basin, Iran. Letters above boxplots show statistically different groups.
3.2. Relationships between Alien Fish Assemblages and Environmental Variables
The average values of the environmental variables measured in the Karun River Basin are presented in Table 2. The first two axes (RDA1 and RDA2) accounted for 36.24% and 25.33% of the variation for five alien fish species, respectively (Figure 5). Altitude, depth (D), electrical conductivity (EC), water temperature (WT), turbidity, dissolved oxygen (DO), and width (W) were the most influential and significant variables affecting the distribution of alien fish species in the study area (Table 3). Different fish species preferred different environmental conditions. For example, the presence of O. aureus, C. zillii, and H. leucisculus was positively correlated with EC, turbidity, water temperature, width, and depth of the river, but it was negatively correlated with altitude. The dominate substrate at sites varied from mud to small boulders (Appendix D).
Table 2.
The results of environmental and physico-chemical variables (Mean ± SD) in the Karun River Basin, Iran.
| Variable | Unit | Mean ± SD | Range (Min–Max) |
|---|---|---|---|
| Altitude | Meter above sea level | 1061 ± 681 | 1–1961 |
| Depth (D) | Cm | 58 ± 26 | 25–120 |
| Water temperature (WT) | °C | 13.5 ± 3.2 | 7.2–19.6 |
| Electrical conductivity (EC) | (μmho/cm) | 740.7 ± 541.3 | 259–2186 |
| Turbidity | (mg/L) | 68 ± 187 | 16.8–1149 |
| Width (W) | M | 52 ± 48 | 5–170 |
| Dissolved Oxygen (DO) | (mg/L) | 8.4 ± 1.3 | 5.3–12.6 |
| Alkalinity | (mg/L CaCO3) | 220 ± 13 | 201–274 |
| Biological Oxygen Demand (BOD) | (mg/L) | 2.19 ± 0.99 | 0.56–4.7 |
| Nitrate (NO3) | (mg/L) | 8.55 ± 5.4 | 3.7–37 |
| Chemical Oxygen demand (COD) | (mgO2/L) | 14 ± 9.4 | 0.02–41.7 |
| Phosphate (PO4) | (mg/L) | 0.52 ± 0.33 | 0.1–1.89 |
Figure 5.
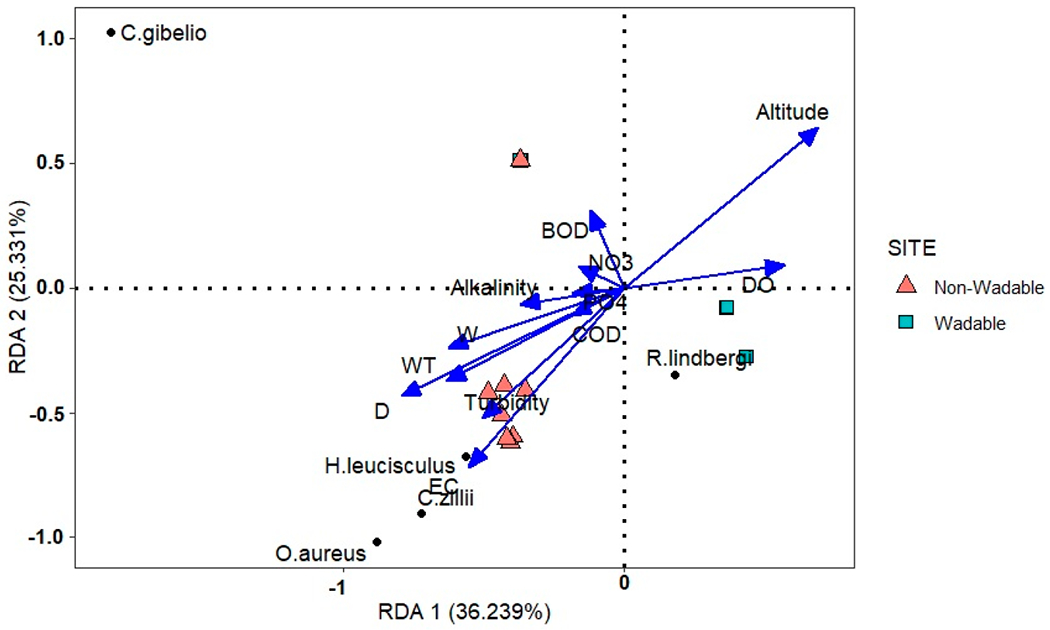
Redundancy analysis ordination between alien fish assemblages and environmental variables in the Karun River Basin, Iran (abbreviations are explained in Table 3).
Table 3.
Results of the redundancy analysis for the occurrence of alien fish species and environmental variables in the Karun River Basin, Iran. Bold variables are influential in the distribution of alien fish species.
| Variable | Axis1 | Axis 2 | F-Ratio | p-Value |
|---|---|---|---|---|
| Altitude | 0.70 | 0.64 | 12.77 | 0.005 ** |
| Depth (D) | −0.80 | −0.43 | 12.56 | 0.005 ** |
| Electrical conductivity (EC) | −0.55 | −0.72 | 10.79 | 0.005 ** |
| Water temperature (WT) | −0.63 | −0.37 | 7.52 | 0.005 ** |
| Turbidity | −0.50 | −0.52 | 6.74 | 0.005 ** |
| Width (W) | −0.63 | −0.24 | 6.32 | 0.005 ** |
| Dissolved Oxygen (DO) | 0.57 | 0.09 | 4.50 | 0.02 * |
| Alkalinity | −0.37 | −0.06 | 1.78 | 0.165 |
| Biological Oxygen Demand (BOD) | −0.11 | 0.30 | 1.21 | 0.295 |
| Nitrate (NO3) | −0.16 | 0.08 | 0.74 | 0.505 |
| Chemical Oxygen Demand (COD) | −0.18 | −0.10 | 0.56 | 0.69 |
| Phosphate (PO4) | −0.18 | −0.02 | 0.41 | 0.735 |
| Cumulative percentage of the variance of the species abundance | 36.24 | 25.33 | ||
| Cumulative percentage of the relation of species abundance and environmental variables | 53.14 | 37.14 |
Note:
= significant at α = 0.01;
significant at α = 0.05. Bold rows indicate the most influential variables on distribution of alien fish species.
4. Discussion
Recent decreases in water quality in the downstream sections of the Karun River Basin due to reduced water flow and pollution by urban sewage, in conjunction with spawning habitat degradation, have resulted in the decreased survival of native and endemic fish species such as Luciobarbus esocinus, Luciobarbus barbulus, Barbus lacerta, and Barbus karunensis [83]. Current research documented the presence of ten alien species from seven families in the Karun River Basin. Most of these species are considered to be relatively tolerant to river impairment, which also makes them useful candidates as indicators of the declining river health [47,84].
Water temperature was among the environmental factors revealed as contributing to the distribution of alien species in the Karun River. For instance, some alien species (e.g., O. aureus and C. zillii) may not be as competitive at cooler temperatures, which may explain why their presence was limited to the downstream parts of the catchment having higher temperatures [85–87]. The distributions of O. aureus, C. zillii, and H. leucisculus populations were correlated with high turbidity and decreasing water transparency. This finding corroborates those of other studies showing the presence of alien species in waters with poor quality [42,86–88]. Site 10 (Tireh) in the upstream section of the study area (sites 1–25) had substantially more alien species than other upstream sites. At this sampling site, it is believed that the presence of certain alien species (e.g., R. lindbergi, G. holbrooki, and P. parva) can be attributed to organizations introducing commercial carp into dams or wetlands as part of their ranching program. Small ponds of different sizes with obstacles limiting access to the main river, and the existence of physical barriers in the main channel of the river, have been put in place to limit further distribution of these species (Figure 6). Taylor et al. [89–91] suggested habitat alteration to help control the spread of non-native species in North America.
Figure 6.

The presence of small ponds and physical barriers at site 10 in the Karun River Basin (red symbol shows the barrier at this sampling site).
Four species (O. aureus, C. zillii, H. leucisculus, and C. carpio) were only found in lower altitudes in urbanized areas. In these downstream sections, there are many hydroelectronic power plants with dams that control water level and alter flow velocity and habitat types which modify the spatial structure of the fish community structure in an indirect way [92]. The expansion of these species into shallower upstream sections of the basin has likely not occurred for two reasons. First, these species prefer slow flow velocities and warmer temperatures like those that prevail in the impounded deep-water habitats of the downstream parts of the basin [67]. Second, reduced hydrological connectivity due to dams and other structures likely impedes the spread of alien species in the basin, as suggested by only sporadic occurrence in upstream reaches (e.g., site 10); although in this case, they were probably actively introduced [93]. If anthropogenic impacts creating these habitat conditions (e.g., climate change, water abstraction) extend further upstream, these species will likely extend their occupancy to those areas. Additional human disturbances (e.g., contamination, river modifications, and flow regulation) could likewise contribute to upstream expansion as they can lead to higher conductivity, muddy substrates, lower riparian vegetation cover, lower dissolved oxygen concentrations, and the presence of aquatic macrophytes and filamentous algae [47,53,82,94].
Potential Origin, Possible Destructive Effects, and Management of Alien Fish Species Observed in the Karun River Basin
In the downstream sections (sites 27 to 35) of the Karun Basin, the high relative abundance and biomass of alien species have contributed to decreases in the relative abundance of native species, including many endemics (Figures 4 and 5). In recent years, tilapia (C. zillii and O. aureus), which are native to Africa, entered Iranian waters from Iraq and/or through accidental or intentional introductions [51]. Tilapia are omnivorous fish that feed at lower trophic levels, which makes them much less expensive to feed and breed than other fish species and explains their widespread use in aquaculture [95]. In the Khuzestan Province of Iran, as well as in other countries, tilapia have also been actively introduced to control aquatic plants in sugarcane effluent drainage channels [96,97]. The species, once escaped from their point of initial introduction, might affect native aquatic plants and cause structural impacts on entire ecosystems [97]. According to anecdotal information from local fishermen, tilapia now dominate the catch in downstream sections of the Karun Basin. Their adaptation to high temperature, low dissolved oxygen, and salinity fluctuations [46,51,98,99], combined with reproduction strategies which include paternal care, rapid growth rate, high fertility, and omnivorous feeding habits, allows tilapia to efficiently colonize areas outside their native range [72,100–103]. Due to their competitive advantages, tilapia can then outcompete native and endemic species and cause fish community structure changes as shown in this study and other studies [47,104]. Sing et al. [46] reported that Nile tilapia (Oreochromis niloticus) reduced the catch of native carp species in the Ghana River while increasing overall fish production in the system. Due to the negative impact upon native and endemic species [46,105], the status and range of these invasive species should be monitored in Iranian waters.
The spread of tilapia can be controlled using biological methods, i.e., by supporting other organisms, ideally endemic and other native fish species. For example, predatory fishes such as the native Silurus triostegus and Leuciscus vorax have contributed to reductions in tilapia populations (especially juvenile tilapia) in the Khuzestan Province [97,106,107]. Management practices supporting their presence in the system would help address the issue. This is important because fishing nets used to catch adult tilapia are not effective for capturing juvenile tilapia [97]. Without such management measures, it is likely that tilapia populations would further increase and expand into the upstream sections of the Karun River Basin.
Prussian carp (Carassius gibelio), originally from Siberia, is an alien species that was caught at different sampling sites throughout the Karun Basin. It is reported from rivers and ponds in neighboring countries like Iraq [52] but also in Europe [89,108]. Based on its biological features and requirements, it outcompetes many cyprinid species for food and habitat. This can reduce population sizes and promote the local extinction of some native/endemic fish species [34,109]. Prussian carp have unique reproductive traits partly explaining their competitive advantage. They have eggs that can be induced by sperms of other cyprinid species, allowing the production of offspring in the absence of conspecific males [34,108,110,111]. Given that a substantial trade of this species as a decorative fish is ongoing, this species is still being traded in Iran, so managers and authorities in Iran should be mindful of its potential environmental impacts [34]. For instance, Azevedo-Santos et al. [112] highlighted a potentially effective method to prevent fish introductions in Brazil: promoting educational opportunities that foster a change in human behavior.
The native range of Sharpbelly (Hemiculter leucisculus) extends from southern Russia to southern Korea via China and Vietnam [113], and it is likely to have been accidentally introduced to Iran from Central Asia with commercial shipping. This species was detected only in downstream sections of the Karun Basin and may be related to higher water temperature and lower water velocity. It is noted for having a strong dorsal fin spine which serves as a deterrent to predators. It may compete with native species and may also feed on their eggs and fry [36]. Due to its greater resistance to predation, high fertility, and omnivorous feeding preferences, it has replaced native fish in the Aral Sea Basin [114] and likely presents a risk to native and endemic species in the Karun River Basin.
The common carp (Cyprinus carpio), native to Eurasia, is a farmed fish and is widely found in all freshwater resources of Iran [115]. In our study, its occurrence was fewer than five individuals at one site and we did not consider it in our data analyses. However, according to the local fishermen, it is abundant in their catches from this basin. This discrepancy is likely due to common carp being more susceptible to the fishing nets used by local fisherman than the electrofishing approaches used in this study [116–118]. The species is an omnivorous bottom feeder in rivers and lakes and its presence is noted for resulting in increased siltation, decreased water quality, and influences on native flora and fauna [119]. It is associated with the decline and local disappearance of native and endemic species in Argentina, Australia, Venezuela, Mexico, Kenya, India, etc. [120], and it should be monitored in the Karun River Basin.
The Amur goby (Rhinogobius lindbergi) was described from the Amur and Ussuri rivers, Russia [121]. It was probably accidentally introduced to Iranian inland waters along with non-native Cyprinids for the Iranian aquaculture industry [122]. In the upstream part of sites 26 and 27 are the Masjed Soleiman and Gotvand hydroelectric power plant, which are opened and closed daily. One could argue that the lack of stable water flow and substrate has produced environmental conditions undesirable for most fish species yet adequate for the presence of this species.
The eastern mosquitofish (Gambusia holbrooki), which is native to the United States [123], has been introduced to Iran, Iraq, Türkiye, and Syria to control larval mosquitoes and reduce malaria outbreaks [52,124]. In our study, it was found only in one site (site 10) with shallow and slow water. Individuals of this species are fertile breeders, are able to enter into the microhabitats of rare and native species, and are often reported as predators [125,126]. Eastern mosquitofish feed on eggs of fish including those of economically valuable species but also those of endangered native species, amphibians, and invertebrates [126]. Taybi et al. [126] further reported that in disturbed areas, G. holbrooki is often abundant because of a wide tolerance to unfavorable abiotic conditions. Considering the negative impacts (aggressive and predatory behavior) of this species, the famous ichthyologist Myers (1965) called it the “fish destroyer”.
The stone moroko (Pseudorasbora parva), which is an East Asian cyprinid species, was found in only one shallow site (site 10, Figure 6) with moderate water quality (Figure 3). Ekmekçï and Kirankaya [125] described it as an opportunistic species with great ecological and physiological tolerance, also tolerant to moderate contamination, high temperature, and low water levels. An important factor contributing to the rapid distribution of this species can be related to the spawning ability on different soft substrates and competition for food with native and endemic species [36,125]. Furthermore, it is considered a serious threat due to risk for disease transmission and reproduction inhibition of Leucaspius delineates (endangered species) in Europe [30,43]
Grass carp (Ctenopharyngodon idella), a species native to East Asia [108], were found at a sampling site covered by macrophytes in the littoral zone (site 28). It can damage the spawning substrate of phytophilous fish species by feeding on macrophytes and thereby affecting some native and endemic species in the Karun River Basin such as Capoeta aculeata, Squalius berak, Squalius Lepidus, and Alburnoides idignensis [127–129].
Rainbow trout (Oncorhynchus mykiss) were introduced as a relevant species for aquaculture and recreational angling, and they are noted as being one of the main predators of eggs and small individuals of native species [130,131]. The species was present at Kata (site 16) which is close to a rainbow trout aquaculture facility. Oncorhynchus mykiss, a salmonid native to the North American west coast, is one of the first species considered to be almost globally invasive. It currently exists in more than 90 countries [108,132]. It is a very common alien species in Iranian freshwaters which it colonized after escaping from aquaculture facilities [131].
5. Conclusions
The main goal of the current study was to update the status of alien fish species distribution in the Karun River Basin in support of improved biodiversity conservation. Invasive species are a major biodiversity threat due to their extensive tolerance to unfavorable conditions and ability to replace native species. Ecological and biological threats are mainly caused by invasive species that are either generalists or sufficiently adapted to the prevailing natural conditions of non-native ecosystems and show high reproductive rates. Under the appropriate situations, some alien species such as C. gibelio, O. aureus, and C. zillii produce large populations and exert significant pressure on populations of native and endemic fish species in the Karun River Basin, including Carasobarbus kosswigi, Arabibarbus grypus, Barbus karunensis, Capoeta coadi, and Luciobarbus barbulus.
The drastic decline in important native fish species, concomitant with the rapid invasion and establishment of alien fish, most notably in downstream sections of the Karun Basin, is receiving increased attention from scientists, conservation entities, and the Iranian government. The collection of scientifically robust data on the occurrence and extent of alien species in the basin is an important indicator for understanding the drivers of impairment and is critical to monitoring efforts intended to support the protection and recovery of populations of native and endemic species. Habitat restoration activities (e.g., flow modification, woody debris introductions, or bank vegetation restoration) could further improve the ecological conditions required for native fish reproduction and may reduce the competitive advantage of alien fish populations. An increased understanding of the temporal and spatial changes in the fish community structure, and the effects of human and ecological processes that drive these changes, is essential for the development of management policies that will support the protection and recovery of native and endemic fish biodiversity in the downstream sections of the Karun River Basin.
In conclusion, this research has documented the prevalence of alien species in the lower sections of the Karun River which have unquestionably contributed to the declines in the ecological status of local and regional native fish associations and communities. Additionally, it highlights that human activities leading to the deterioration of aquatic ecosystems have a direct impact on native fish associations and communities, thereby facilitating the intrusion of alien species into new ecosystems.
To protect what remains of the native and endemic fishes of the Karun River Basin, urgent steps must be taken to mitigate the degradation of existing conditions and implement preventive measures to prevent the unintentional introduction of additional alien fish species. Some introductions of alien fish species in Iran can be attributed to a lack of awareness among the general public and individuals in the fishery and aquaculture sectors regarding its associated risks. To prevent such further introductions, we suggest the development of educational and promotional programs designed to inform relevant stakeholders and enhance public awareness about the risk posed by introduced species. Furthermore, future research should focus on understanding the specific mechanisms leading to declines in native fish species (e.g., competitive advantage in accessing food resources and habitats, habitat loss) to inform of possible management strategies that may aid in their recovery and mitigate future losses.
Key Contribution:
An original dual-pathway working hypothesis was proposed, suggesting that alien fish species contribute to the decline in the ecological status of local and regional native fish associations and communities. Additionally, it was argued that human-induced degradation of aquatic ecosystems facilitates the invasion of alien species into new ecosystems further affecting native fish associations and communities.
Acknowledgments:
We thank the Iranian Ministry of Energy, Iran, Water and Power Resources Development Company, and the Department of Environment for their in-kind support. We also acknowledge Ebrahim Mottaghi and Saeid Asadollah for field work assistance. We appreciate early review comments from Brent Johnson and Walsh Brannon (U.S. Environmental Protection Agency (EPA)) that enhanced this paper. The research presented was not performed or funded by EPA and was not subject to EPA’s quality system requirements. The views expressed in this article are those of the author(s) and do not necessarily represent the views or the policies of the U.S. EPA.
Funding:
This study was financially supported by the Swiss Leading House for South Asia and Iran (ZHAW) and Isfahan University of Technology, Iran(funding number: 500/97/13283), as a joint program between Isfahan University of Technology and the University of Applied Sciences and Arts of Southern Switzerland. The APC was funded by Ecotur Sibiu (CUI 5841004).
Appendix A
Figure A1.

The curve of the number of species in the sampling reach over 90 minutes.
Appendix B
Table A1.
Presence and absence of recorded fish species in the Karun River Basin, Iran.
| Family | Cyprinidae | Leuciscidae | Xenocyprididae | Nemacheilidae | Sisoridae | Mugilidae | Aphanidae | Mastacembelidae | Salmonidae | Gobiidae | Gobionidae | Poeciliidae | Cichlidae | Danionidae | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Site | Capoeta coadi | Capoeta aculeata | Capoeta trutta | Carassius gibelio | Arabibarbus grypus | Cyprinus carpio | Garra rufa | Garra gymnothorax | Barbus lacerta | Barbus karunensis | Luciobarbus barbulus | Carasobarbus luteus | Carasobarbus kosswigi | Cyprion macrostomus | Chondrostoma regium | Squalius berak | Squalius lepidus | Acanthobrama marmid | Alburnus sellal | Alburnus caeruleus | Alburnus doriae | Alburnoides idignesis | Hemiculter leucisculus | Ctenopharyngodon idella | Turcinoemacheilus saadii | Turcinoemacheilus hafezi | Oxynoemacheilus euphraticus | Glyptothorax galaxias | Glyptothorax alidaeii | PlaniPlaniliza abu | Esmaeilius vladykovi | Mastacembelus mastacembelus | Oncorhynchus mykiss | Rhinogobius lindbergi | Pseudorasbora parva | Gambusia holbrooki | Oreochromis aureus | Coptodon zillii | Bariliusmesopotamicus |
| 1 | + | + | + | − | − | − | − | − | − | − | − | − | − | − | + | + | + | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| 2 | + | + | − | − | − | − | − | − | + | − | − | − | − | − | + | − | − | − | − | − | − | − | − | − | + | − | − | + | + | − | − | − | − | − | − | − | − | − | − |
| 3 | + | − | − | − | − | − | + | + | + | − | − | − | − | − | + | − | − | − | − | − | + | − | − | − | + | − | − | − | − | − | + | − | − | − | − | − | − | − | − |
| 4 | + | − | − | − | − | − | − | − | − | − | − | − | − | − | + | − | − | − | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| 5 | + | + | − | + | − | − | − | − | − | − | − | − | − | − | + | − | − | − | − | − | + | − | − | − | − | − | − | − | − | − | + | − | − | − | − | − | − | − | − |
| 6 | + | − | − | + | − | − | + | + | −− | − | + | − | − | + | − | − | − | − | + | − | − | − | − | − | − | − | − | + | + | + | − | − | − | − | − | − | − | − | − |
| 7 | + | − | + | − | + | − | − | − | − | − | − | − | + | + | − | − | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| 8 | + | − | + | + | − | − | + | + | + | − | − | − | − | − | + | − | − | − | + | − | − | + | − | − | − | − | −− | − | − | − | − | − | − | − | − | − | − | − | − |
| 9 | + | − | + | − | − | − | − | − | + | − | − | − | − | − | + | − | − | − | + | − | + | + | − | − | + | + | − | − | − | − | − | − | − | − | − | − | − | − | − |
| 10 | + | − | − | + | − | − | − | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | + | − | − | − | − | − | + | − | − | − | − | + | + | − | − | − |
| 11 | + | − | + | − | − | − | − | − | + | − | − | − | − | − | + | − | − | − | + | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| 12 | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | − | + | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| 13 | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | − | + | − | − | − | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − |
| 14 | + | − | − | − | − | − | + | + | − | − | − | − | − | − | + | − | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| 15 | + | − | − | − | − | − | − | − | − | − | + | − | − | − | + | − | − | − | + | − | − | − | − | − | − | − | − | + | + | − | − | − | − | − | − | − | − | − | − |
| 16 | + | + | −− | − | − | − | + | + | − | + | + | − | − | − | + | + | + | − | + | − | + | − | − | − | − | − | − | − | − | − | − | − | + | − | − | − | − | − | − |
| 17 | + | + | − | + | − | − | + | + | − | + | − | − | − | − | + | + | + | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| 18 | + | + | − | + | − | − | + | + | − | − | − | − | − | − | + | − | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| 19 | + | + | + | + | − | − | + | + | − | + | − | − | − | + | + | + | + | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| 20 | + | + | + | − | − | − | + | + | − | − | + | − | − | − | + | + | + | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| 21 | + | − | + | + | − | − | + | + | − | − | + | − | − | − | + | − | − | − | + | − | − | − | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| 22 | − | − | + | − | − | − | + | + | − | − | − | − | − | − | + | + | + | − | + | − | + | − | − | − | − | − | − | + | + | − | − | − | − | − | − | − | − | − | − |
| 23 | + | + | − | − | − | − | − | − | − | − | + | − | − | − | + | + | + | − | − | − | + | − | − | − | − | − | − | + | + | − | − | − | − | − | − | − | − | − | − |
| 24 | − | + | + | − | − | − | + | + | − | − | − | − | − | − | + | + | + | − | + | − | + | − | − | − | − | − | − | + | − | + | − | − | − | − | − | − | − | − | − |
| 25 | + | − | + | − | − | − | + | + | − | − | − | − | − | + | + | − | − | − | + | − | − | − | − | − | − | − | − | − | − | + | − | − | − | − | − | − | − | − | − |
| 26 | − | − | + | − | + | − | + | + | − | − | − | − | − | + | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | − | − | − | − | − |
| 27 | − | − | − | + | − | − | + | + | − | − | − | − | − | + | − | − | − | − | − | + | − | − | − | − | − | − | − | − | − | + | − | − | − | + | − | − | + | + | − |
| 28 | − | − | − | + | − | − | − | − | − | − | − | + | − | + | + | − | − | − | + | − | + | − | + | − | − | − | − | − | − | + | − | − | − | − | − | − | + | + | + |
| 29 | − | − | + | + | − | − | + | + | − | − | − | − | − | + | + | − | − | + | − | − | − | − | + | − | − | − | − | − | − | +− | − | − | − | − | − | − | + | + | − |
| 30 | − | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | − | − | − | + | − | − | − | − | − | − | − | − | + | − | − | − | − | + | + | + |
| 31 | − | − | − | + | − | − | + | + | − | − | − | − | − | + | + | − | − | + | + | − | + | − | + | − | − | − | − | − | − | + | − | − | − | − | − | − | + | + | + |
| 32 | − | − | − | + | − | − | − | − | − | − | − | + | − | + | + | − | ++ | − | − | − | − | + | − | − | − | − | − | − | + | − | − | − | − | − | − | + | + | +− | |
| 33 | − | − | − | + | − | − | + | + | − | − | − | + | − | − | − | − | − | + | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| 34 | − | − | + | + | − | + | − | − | − | − | − | − | − | + | + | − | − | + | + | − | − | − | − | − | − | − | − | − | − | + | − | + | − | − | − | − | + | + | − |
| 35 | − | − | + | + | − | − | + | + | − | − | − | + | − | + | + | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
Note: Alien fish species are represented in bold columns, and the sites with red numbers are distributed in the downstream part of the Karun River Basin.
Appendix C
Figure A2.

Pictures of Alien Fish Species in the Karun River Basin, Iran. Photo Credit: M. Zare Shahraki.
Appendix D
Table A2.
Substrate size in different sites in the Karun River Basin.
| Site Code | Substrate Characteristics |
|---|---|
| 1 | Cobbles |
| 2 | Cobbles |
| 3 | Boulders (small) |
| 4 | Boulders (small) |
| 5 | Boulders (small) |
| 6 | Cobbles |
| 7 | Cobbles |
| 8 | Cobbles |
| 9 | Cobbles |
| 10 | Gravel (Fine) |
| 11 | Boulders (small) |
| 12 | Cobbles |
| 13 | Boulders (small) |
| 14 | Cobbles |
| 15 | Boulders (small) |
| 16 | Cobbles |
| 17 | Cobbles |
| 18 | Cobbles |
| 19 | Boulders (small) |
| 20 | Boulders (small) |
| 21 | Cobbles |
| 22 | Cobbles |
| 23 | Cobbles |
| 24 | Boulders (small) |
| 25 | Boulders (small) |
| 26 | Cobbles |
| 27 | Sand |
| 28 | Sand |
| 29 | Mud |
| 30 | Mud |
| 31 | Mud |
| 32 | Mud |
| 33 | Mud |
| 34 | Gravel (Coarse) |
| 35 | Sand |
Footnotes
Institutional Review Board Statement: All sampling has been conducted as per the guidelines of the Institutional Animal Ethics Committee, Department of Natural Resources, Isfahan University of Technology, Isfahan, Iran. There is no established ethical approval system in Iran. We adopt a cautious approach in our research. Instead of collecting and transporting all fish samples to the lab, we identify them in the field and release them back into the river. Only a few fish samples are taken for more precise identification in the laboratory. The ethical approval is waived.
Conflicts of Interest: The authors declare no conflict of interest.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
Data Availability Statement:
There are no supplementary data parts and no publicly archived datasets were analyzed or generated during the study. The data and the location of sampling sites are available on request after publishing.
References
- 1.Bănăduc D; Simić VM; Cianfaglione K; Barinova S; Afanasyev S; Öktener A; McCall G; Simi SB Freshwater as a Sustainable Resource and Generator of Secondary Resources in the 21st Century: Stressors, Threats, Risks, Management and Protection Strategies, and Conservation Approaches. Int. J. Environ. Res. Public Health 2022, 19, 16570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wahltinez SJ; Kroll KJ; Behringer DC; Arnold JE; Whitaker B; Newton AL; Edmiston K; Hewson I; Stacy NI Common Sea Star (Asterias rubens) Coelomic Fluid Changes in Response to Short-Term Exposure to Environmental Stressors. Fishes 2023, 8, 51. [Google Scholar]
- 3.Bănăduc D; Barinova S; Cianfaglione K; Curtean-Bănăduc A Editorial: Multiple freshwater stressors-Key drivers for the future of freshwater environments. Front. Environ. Sci 2023, 11, 92. [Google Scholar]
- 4.Navarro-Ortega A; Acuña V; Bellin A; Burek P; Cassiani G; Choukr-Allah R; Dolédec S; Elosegi A; Ferrari F; Ginebreda A; et al. Managing the effects of multiple stressors on aquatic ecosystems under water scarcity. The GLOBAQUA project. Sci. Total Environ 2015, 503–504, 3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Curtean-Bănăduc A; Olosutean H; Bănăduc D Influence of Environmental Variables on the Structure and Diversity of Ephemeropteran Communities: A Case Study of the Timis River, Romania. Acta Zool. Bulg 2016, 68, 215–224. [Google Scholar]
- 6.Wheeler C. The Ecosystem Role of Fishes in Lotic Environments. Ph.D. Thesis, Utah State University, Logan, UT, USA, 2014; p. 3694. [Google Scholar]
- 7.Villéger S; Brosse S; Mouchet MA; Mouillot D; Vanni MJ Functional ecology of fish: Current approaches and future challenges. Aquat. Sci 2017, 79, 783–801. [Google Scholar]
- 8.Holmlund CM; Hammer M Ecosystem services generated by fish populations. Ecol. Econ 1999, 29, 253–268. [Google Scholar]
- 9.Zubcov N; Zubcov E; Schnenk D The dynamics of metals in fish from Nistru and Prut rivers (Moldova). Transylv. Rev. Syst. Ecol. Res 2008, 6, 51–58. [Google Scholar]
- 10.Curtean-Bănăduc A; Marić S; Gabor G; Didenko A; Rey Planellas S; Bănăduc D Hucho hucho (Linnaeus, 1758): Last natural viable population in the Eastern Carpathians—Conservation elements. Turk. J. Zool 2019, 43, 215–223. [Google Scholar]
- 11.Jeeva V; Kumar S; Verma D; Rumana HS River fragmentation and connectivity problems in Gange River of upper Himalayas: The effect on the fish communities (India). Transylv. Rev. Syst. Ecol. Res 2011, 12, 75–90. [Google Scholar]
- 12.Bănăduc D; Sas A; Cianfaglione K; Barinova S; Curtean-Bănăduc A The role of aquatic refuge habitats for fish, and threats in the context of climate change and human impact, during seasonal hydrological drought in the Saxon Villages area (Transylvania, Romania). Atmosphere 2021, 12, 1209. [Google Scholar]
- 13.Sosai AS Illegal fishing in southern Mannar Island coastal area (Sri Lanka). Transylv. Rev. Syst. Ecol. Res 2015, 17, 95–108. [Google Scholar]
- 14.Zare-Shahraki M; Ebrahimi-Dorche E; Bruder A; Flotermersch J; Blocksom K; Bănăduc D Fish species composition, distribution and community structure in relation to environmental variation in a semi-arid mountainous river basin, Iran. Water 2022, 14, 2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khoshnood Z. Effects of environmental pollution on fish: A short review. Transylv. Rev. Syst. Ecol. Res 2017, 19, 49–60. [Google Scholar]
- 16.Bourillon B; Feunteun E; Acou A; Trancart T; Teichert N; Belpaire C; Dufour S; Bustamante P; Aarestrup K; Walker A; et al. Anthropogenic Contaminants Shape the Fitness of the Endangered European Eel: A Machine Learning Approach. Fishes 2022, 7, 274. [Google Scholar]
- 17.Baker SM; Reyier EA; Ahr BJ; Cook GS Assessing the Effects of Physical Barriers and Hypoxia on Red Drum Movement Patterns to Develop More Effective Management Strategies. Fishes 2023, 8, 171. [Google Scholar]
- 18.Kar D. Wetlands and their fish diversity in Assam (India). Transylv. Rev. Syst. Ecol. Res 2019, 21, 47–94. [Google Scholar]
- 19.Bănăduc D; Maric S; Cianfaglione K; Afanasyev S; Somogyi D; Nyeste K; Antal L; Kosco J; Caleta M; Wanzenbock J; et al. Stepping Stone Wetlands, Last Sanctuaries for European Mudminnow: How Can the Human Impact, Climate Change, and Non-Native Species drive a Fish to the Edge of Extinction. Sustainability 2022, 14, 13493. [Google Scholar]
- 20.Siddique MAB; Ahammad AKS; Mahalder B; Alam MM; Hasan NA; Bashar A; Biswas JC; Haque MM Perceptions of the Impact of Climate Change on Performance of Fish Hatcheries in Bangladesh: An Empirical Study. Fishes 2022, 7, 270. [Google Scholar]
- 21.Curtean-Bănăduc A; Mihut C; Burcea A; McCall GS; Matei C; Bănăduc D Screening for Microplastic Uptake in an Urbanized Freshwater Ecosystem; Chondrostoma nasus (Linnaeus, 1758) Case Study. Water 2023, 15, 1578. [Google Scholar]
- 22.Taiwo IO; Olopade OA; Bamidele NA Heavy metal concentration in eight fish species from Epe Lagoon (Nigeria). Transylv. Rev. Syst. Ecol. Res 2019, 21, 69–82. [Google Scholar]
- 23.Bănăduc D; Joy M; Olosutean H; Afanasyev S; Curtean-Bănăduc A Natural and anthropogenic driving forces as key elements in the Lower Danube Basin–South-Eastern Carpathians–North-Western Black Sea coast area lakes: A broken stepping stones for fish in a climatic change scenario? Environ. Sci. Eur 2020, 32, 73. [Google Scholar]
- 24.Mathers KL; Guareschi S; Pattison Z Biological invasions in rivers and associated ecosystems: New insights, challenges, and methodological advancements. River Res. Appl 2022, 38, 1351–1355. [Google Scholar]
- 25.Seebens H; Bacher S; Blackburn TM; Capinha C; Dawson W; Dullinger S; Genovesi P; Hulme PE; van Kleunen M; Kühn I; et al. Projecting the continental accumulation of alien species through to 2050. Glob. Change Biol 2021, 27, 970–982. [DOI] [PubMed] [Google Scholar]
- 26.Ricciardi A; Kipp R Predicting the number of ecologically harmful exotic species in an aquatic system. Divers. Distrib 2008, 14, 374–380. [Google Scholar]
- 27.Fedorenkova A; Vonk JA; Breure AM; Hendriks AJ; Leuven RSEW Tolerance of native and non-native fish species to chemical stress: A case study for the River Rhine. Aquat. Invasions 2013, 8, 231–241. [Google Scholar]
- 28.Piria M; Simonović P; Kalogianni E; Vardakas L; Koutsikos N; Zanella D; Ristovska M; Apostolou A; Adrović A; Mrdak D; et al. Alien freshwater fish species in the Balkans—Vectors and pathways of introduction. Fish Fish. 2018, 19, 138–169. [Google Scholar]
- 29.WWF. Living Planet Report 2022 – Building a Nature-Positive Society; Almond REA, Grooten M, Juffe Bignoli D, Petersen T, Eds.; WWF: Gland, Switzerland, 2000. [Google Scholar]
- 30.Gozlan RE; Andreou D; Asaeda T; Beyer K; Bouhadad R; Burnard D; Caiola N; Cakic P; Djikanovic V; Esmaeili HR; et al. Pan-continental invasion of Pseudorasbora parva: Towards a better understanding of freshwater fish invasions. Fish Fish. 2010, 11, 315–340. [Google Scholar]
- 31.Falk-Petersen J; Bohn T; Sandlund OT On the numerous concepts in invasion biology. Biol. Invasions 2006, 8, 1409–1424. [Google Scholar]
- 32.Alves CBM; Vieira F; Magalhaes ALB; Brito MFG Impacts of Non-Native Fish Species in Minas Gerais, Brazil: Present Situation and Prospects. In Ecological and Genetic Implications of Aquaculture Activities; Springer: Dordrecht, The Netherlands, 2007; pp. 291–314. [Google Scholar]
- 33.Jawad LA (Ed.) Tigris and Euphrates Rivers: Their Environment from Headwaters to Mouth; Aquatic Ec. Springer: Auckland, New Zealand, 2021. [Google Scholar]
- 34.Veer G; Nentwig W Environmental and economic impact assessment of alien and invasive fish species in Europe using the generic impact scoring system. Ecol. Freshw. Fish 2015, 24, 646–656. [Google Scholar]
- 35.Mousavi-Sabet H. Exotic ornamental fishes in Iranian inland water basins: An updated checklist. J. Anim. Divers 2019, 1, 1–10. [Google Scholar]
- 36.Patimar R. Fish species diversity in the lakes of Alma-Gol, Adji-Gol, and Ala-Gol, Golestan province, northern Iran. J. Ichthyol 2008, 48, 911–917. [Google Scholar]
- 37.Jha DN; Joshi KD; Alam MA; Das SCS; Kumar V Dominance of exotic fishes in the River Ganga at Allahabad stretch. J. Kalash Sci 2016, 4, 1–6. [Google Scholar]
- 38.Sala OE; Chapin FS; Armesto JJ; Berlow E; Bloomfield J; Dirzo R; Huber-Sanwald E; Huenneke LF; Jackson RB; Kinzig A; et al. Global biodiversity scenarios for the year 2100. Science 2000, 287, 1770–1774. [DOI] [PubMed] [Google Scholar]
- 39.Leprieur F; Beauchard O; Blanchet S; Oberdorff T; Brosse S Fish invasions in the world’s river systems: When natural processes are blurred by human activities. PLoS Biol. 2008, 6, 404–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rahel FJ; Bierwagen B; Taniguchi Y Managing aquatic species of conservation concern in the face of climate change and invasive species. Conserv. Biol 2008, 22, 551–561. [DOI] [PubMed] [Google Scholar]
- 41.Bani L; Orioli V; Trasforini S; Puzzi CM; Sibilia A; Dondina O; Tirozzi P The spread of exotic fish species in Italian rivers and their effect on native fish fauna since 1990. Biodiversity 2021, 22, 4–12. [Google Scholar]
- 42.Adha K; Esa Y; Arshad A The Influence of Alien Fish Species on Native Fish Community Structue in Malaysian Waters. Kuroshio Sci. 2013, 7, 81–93. [Google Scholar]
- 43.Britton JR; Davies GD; Harrod C Trophic interactions and consequent impacts of the invasive fish Pseudorasbora parva in a native aquatic foodweb: A field investigation in the UK. Biol. Invasions 2010, 12, 1533–1542. [Google Scholar]
- 44.Gozlan R; Hilaire S; Feist SW; Martin P; Kent ML Biodiversity: Disease threat to European fish. Nature 2005, 435, 1046. [DOI] [PubMed] [Google Scholar]
- 45.Leuven R; Hendriks A; Huijbregts M; Lenders H; Matthews J; Van Der Velde G Differences in sensitivity of native and exotic fish species to changes in river temperature. Curr. Zool 2011, 57, 852–862. [Google Scholar]
- 46.Singh AK; Kumar D; Srivastava SC; Ansari A; Jena JK; Sarkar UK Invasion and impacts of alien fish species in the Ganga River, India. Aquat. Ecosyst. Health Manag 2013, 16, 408–414. [Google Scholar]
- 47.Kennard MJ; Arthington AH; Pusey J; Harch BD Are alien fish a reliable indicator of river health? Freshw. Biol 2005, 50, 174–193. [Google Scholar]
- 48.Jouladeh Roudbar A; Ghanavi HR; Doadrio I Ichthyofauna from iranian freshwater: Annotated checklist, diagnosis, taxonomy, distribution and conservation assessment. Zool. Stud 2020, 59, 1–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tabasian H; Abdoli A; Khorasani N; Dehghan Madiseh S The reproductive biology of the invasive redbelly tilapia, Coptodon zillii, case study: Shadegan Wetland, Iran. J. Wildl. Biodivers 2022, 6, 244971122. [Google Scholar]
- 50.Altun T. Tilapia Culture and Its Problems in Turkey. J. Fish Aquat. Sci 2006, 23, 473–478. [Google Scholar]
- 51.Al-Faisal AJ; Mutlak FM; Abdullah SA Exotic freshwater fishes in the southern Iraq. Marsh Bull. 2014, 9, 65–78. [Google Scholar]
- 52.Mohamed A-R; Abood AN Dispersal of the exotic fish in the Shatt Al-Arab. J. Agric. Vet. Sci 2017, 10, 50–57. [Google Scholar]
- 53.Coad B; Abdoli A Exotic fish species in the fresh waters of Iran. Zool. Middle East 1993, 9, 65–80. [Google Scholar]
- 54.Waldron A; Miller DC; Redding D; Mooers A; Kuhn TS; Nibbelink N; Roberts JT; Tobias JA; Gittleman JL Reductions in global biodiversity loss predicted from conservation spending. Nature 2017, 551, 364–367. [DOI] [PubMed] [Google Scholar]
- 55.Coelho PN; Henry R The small foreigner: New laws will promote the introduction of non-native zooplankton in Brazilian aquatic environments. Acta Limnol. Bras 2017, 29, e7. [Google Scholar]
- 56.Esmaeili HR; Teimori A; Owfi F; Abbasi K; Coad BW Alien and invasive freshwater fish species in Iran: Diversity, environmental impacts and management. Iran. J. Ichthyol 2014, 1, 61–72. [Google Scholar]
- 57.Naddafi K; Honari H; Ahmadi M Water quality trend analysis for the Karoon River in Iran. Environ. Monit. Assess 2007, 134, 305–312. [DOI] [PubMed] [Google Scholar]
- 58.Fathi P; Dorche EE; Shahraki MZ; Stribling J; Kashkooli OB; Ofogh AE; Bruder A Revised Iranian Water Quality Index (RIWQI): A tool for the assessment and management of water quality in Iran. Environ. Monit. Assess 2022, 194, 504. [DOI] [PubMed] [Google Scholar]
- 59.Rahimi D; Hasheminasab S; Abdollahi K Assessment of temperature and rainfall changes in the Karoun River basin. Theor. Appl. Climatol 2019, 137, 2829–2839. [Google Scholar]
- 60.Rahimi Y; Saghafian B; Banihashemi MA Hydrological and Hydraulic Uncertainty Analysis in Probabilistic Design of Flood Diversion Systems Using NSGAII and Bivariate Frequency Analysis. Iran. J. Sci. Technol. Trans. Civ. Eng 2020, 45, 2651–2662. [Google Scholar]
- 61.Sadeghianl MS; Hassunizadeh H; McNaughton AN Optimising the River Karun system, Iran. Trans. Ecol. Environ 2003, 60, 109–118. [Google Scholar]
- 62.Kottek M; Grieser J; Beck C; Rudolf B; Rubel F World Map of the Köppen-Geiger climate classification updated. Meteorol. Z 2006, 15, 259–263. [Google Scholar]
- 63.Khosravi M; Siadatmousavi SM; Yari S; Azizpour J Observation of currents in Karun River. Res. Mar. Sci 2017, 2, 50–58. [Google Scholar]
- 64.Fisher RA; Corbet AS; Williams CB The Relation Between the Number of Species and the Number of Individuals in a Random Sample of an Animal Population. J. Anim. Ecol 1943, 12, 42. [Google Scholar]
- 65.Zare Shahraki M; Ebrahimi Dorche E; Keivany Y; Blocksom KA; Bruder A; Flotemersch JE A fish-based multi-metric assessment index in the Karun River Basin, Iran. River Res. Appl 2022, 38, 573–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Keivany Y; Nasri M; Abbasi K; Abdoli A Atlas of Inland Water Fishes of Iran; Iran Department of Environment: Tehran, Iran, 2016. [Google Scholar]
- 67.Froese R; Pauly D Fish Base. World Wide Web Electronic Publication. Version (12/2019). 2019. Available online: www.fishbase.org (accessed on 20 March 2019). [Google Scholar]
- 68.Ter Braak C; Smilauer P CANOCO Reference Manual and CanoDraw for Windows User’s Guide: Software for Canonical Community Ordination, Version 4.5; 2002; (Microcomputer Power; ); Available online: www.canoco.com; https://edepot.wur.nl/405659; (accessed on 12 October 2023). [Google Scholar]
- 69.Leps J; Smilauer P Multivariate Analysis of Ecological Data Using Canoco 5; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- 70.Milardi M; Gavioli A; Soininen J; Castaldelli G Exotic species invasions undermine regional functional diversity of freshwater fish. Sci. Rep 2019, 9, 17921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Milardi M; Aschonitis V; Gavioli A; Lanzoni M; Fano EA; Castaldelli G Run to the hills: Exotic fish invasions and water quality degradation drive native fish to higher altitudes. Sci. Total Environ 2018, 624, 1325–1335. [DOI] [PubMed] [Google Scholar]
- 72.Huang J; Huang L; Wu Z; Mo Y; Zou Q; Wu N; Chen Z Correlation of fish assemblages with habitat and environmental variables in a headwater stream section of Lijiang River, China. Sustainability 2019, 11, 1135. [Google Scholar]
- 73.Purevdorj Z; Munkhbayar M; Paek WK; Ganbold O; Jargalsaikhan A; Purevee E; Amartuvshin T; Genenjamba U; Nyam B; Lee JW Relationships between Bird Assemblages and Habitat Variables in a Boreal Forest of the Khentii Mountain, Northern Mongolia. Forests 2022, 13, 1037. [Google Scholar]
- 74.Blocksom KA; Johnson BR Development of a regional macroinvertebrate index for large river bioassessment. Ecol. Indic 2009, 9, 313–328. [Google Scholar]
- 75.Shahraki MZ; Dorche EE; Fathi P; Flotemersch J; Blocksom K; Stribling J; Keivany Y; Kashkooli OB; Scown M; Bruder A Defining a Disturbance Gradient in a Middle-Eastern River Basin. Limnologica 2021, 91, 125923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2020. [Google Scholar]
- 77.Oksanen F. Vegan: Community Ecology Package; 2017; R package Version 2.4-3; Available online: https://CRAN.R-project.org/package=vegan (accessed on 12 October 2023).
- 78.Wickham H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. Available online: https://ggplot2.tidyverse.org (accessed on 12 October 2023). [Google Scholar]
- 79.DIVA-GIS. Free Spatial Data by Country. 2017. Available online: http://www.diva-gis.org/gdata (accessed on 15 February 2018).
- 80.ESRI. ArcGIS Desktop; Release 10; Environmental Systems Research Institute: Redlands, CA, USA, 2011. [Google Scholar]
- 81.US Geological Survey. U.S. Geological Survey. National Water Information System Data Available on the World Wide Web. 2020. Available online: https://waterdata.usgs.gov/nwis/ (accessed on 30 June 2018).
- 82.Hosseini SA; Mirvaghefi A; Alishahi M; Genetics F; Yasuj M; Rastiannasab A Measurement Some of Immunological Parameters Cyprinus Carpio in the Polluted (Ahwaz) and Non-Polluted (Shoushtar) Area in Karun Riverian Iran. J. Appl. Biol. Sci 2014, 8, 81–85. [Google Scholar]
- 83.Arthington A; McKenzie F Review of Impacts of Displaced/Introduced Fauna Associated with Inland Waters. Australia: State of the Environment Technical Paper Series (Inland waters); Department of the Environment: Canberra, Australia, 1997; 69p. [Google Scholar]
- 84.Chapman DV; WHO; UNESCO; Programme, U.N.E. Water Quality Assessments: A Guide to the Use of Biota, Sediments and Water in Environmental Monitoring; Chapman D, Ed.; Chapman and Hall Ltd.: London, UK, 1996; p. 651. [Google Scholar]
- 85.Rahel FJ; Olden JD Assessing the effects of climate change on aquatic invasive species. Conserv. Biol. J. Soc. Conserv. Biol 2008, 22, 521–533. [DOI] [PubMed] [Google Scholar]
- 86.Záhorská E. Climate warming and invasive fish species: Will they replace native fish species in waters of temperate zones? Biologia 2016, 71, 727–735. [Google Scholar]
- 87.Okun N; Brasil J; Attayde J; Costa I Omnivory does not prevent trophic cascades in pelagic food webs. Freshw. Biol 2007, 53, 129–138. [Google Scholar]
- 88.Lusk S; Luskova V; Hanel L Alien fish species in the Czech Republic and their impact on the native fish fauna. Folia Zool. 2010, 59, 57–72. [Google Scholar]
- 89.Gavioli A; Milardi M; Castaldelli G; Fano EA; Soininen J Diversity patterns of native and exotic fish species suggest homogenization processes, but partly fail to highlight extinction threats. Divers. Distrib 2019, 25, 983–994. [Google Scholar]
- 90.Taylor CA; Knouft JH; Hiland TM Consequences of stream impoundment on fish communities in a small North American drainage. Regul. Rivers Res. Manag 2001, 17, 687–698. [Google Scholar]
- 91.El-Sayed AFM; Kawanna M Optimum water temperature boosts the growth performance of Nile tilapia (Oreochromis niloticus) fry reared in a recycling system. Aquac. Res 2008, 39, 670–672. [Google Scholar]
- 92.Jones PE; Tummers JS; Galib SM; Woodford DJ; Hume JB; Silva LGM; Braga RR; de Leaniz CG; Vitule JRS; Herder JE; et al. The Use of Barriers to Limit the Spread of Aquatic Invasive Animal Species: A Global Review. Front. Ecol. Evol 2021, 9, 1–19. [Google Scholar]
- 93.Rakocy JE FAO—Oreochromis Niloticus. 2009. Available online: https://www.fao.org/fishery/docs/CDrom/aquaculture/I1129m/file/en/en_niletilapia.htm (accessed on 25 December 2021).
- 94.Zeng L; Zhou L; Guo D-L; Fu D-H; Xu P; Zeng S; Tang Q-D; Chen A-L; Chen F-Q; Luo Y; et al. Ecological effects of dams, alien fish, and physiochemical environmental factors on homogeneity/heterogeneity of fish community in four tributaries of the Pearl River in China. Ecol. Evol 2017, 7, 3904–3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Welcomme R. A history of international introductions of inland aquatic species. Mar. Sci. Symp 1992, 194, 3–14. [Google Scholar]
- 96.Valikhani H; Abdoli A; Hassanzade Kiabi B; Sadeghsaba M; Khosravi M A Study on the status of invasive tilapia species (Coptodon zillii Gervais, 1848 and Oreochromis aureus Steindachner, 1864) in aquatic ecosystems of Khuzestan Province, Iran. Environ. Sci 2018, 15, 29–44. [Google Scholar]
- 97.Cruz EM; Ridha M Overwintering tilapia, Oreochromis spilurus (Gunther), fingerlings using warm underground sea water. Aquac. Fish. Manag 1994, 25, 865–871. [Google Scholar]
- 98.Khaefi R; Esmaeili HR; Zareian H; Babaei S The first record of the redbelly tilapia, Tilapia zillii (Gervais, 1848), in freshwaters of Iran. Turk. J. Zool 2014, 38, 96–98. [Google Scholar]
- 99.Peterson MS; Slack WT; Woodley CM The occurrence of non-indigenous Nile tilapia, Oreochromis niloticus (linnaeus) in coastal Mississippi, USA: Ties to aquaculture and thermal effluent. Wetlands 2005, 25, 112–121. [Google Scholar]
- 100.Martin CW; Valentine MM; Valentine JF Competitive interactions between invasive nile tilapia and native fish: The potential for altered trophic exchange and modification of food webs. PLoS ONE 2010, 5, e14395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Xia Y; Zhao W; Xie Y; Xue H; Li J; Li Y; Chen W; Huang Y; Li X Ecological and economic impacts of exotic fish species on fisheries in the Pearl River basin. Manag. Biol. Invasions 2019, 10, 127–138. [Google Scholar]
- 102.Abdullah AHJ; Abdullah SA; Yaseen AT A composition and abundance of alien fish species in inland waters, southern iraq. Iraqi J. Sci 2020, 62, 373–386. [Google Scholar]
- 103.Mohamed AR; Abood AN Compositional change in fish assemblage structure in the Shatt Al-Arab River, Iraq. Asian J. Appl. Sci 2017, 5, 944–958. [Google Scholar]
- 104.Gu DE; Ma GM; Zhu YJ; Xu M; Luo D; Li YY; Wei H; Mu XD; Luo JR; Hu YC The impacts of invasive Nile tilapia (Oreochromis niloticus) on the fisheries in the main rivers of Guangdong Province, China. Biochem. Syst. Ecol 2015, 59, 1–7. [Google Scholar]
- 105.Hashemi S; Eskandary G; Ansary H; Yooneszadeh M Stock Assessment and Production of Fish Species in the Shadegan Wetland, Iran. World J. Fish Mar. Sci 2011, 3, 502–508. [Google Scholar]
- 106.Hashemi SAR; Ansary H Biomass and production of fish species in the Shadegan Wetland, Iran. Glob. Vet 2012, 9, 123–128. [Google Scholar]
- 107.Razlutskij V; Mei X; Maisak N; Sysova E; Lukashanets D; Makaranka A; Jeppesen E; Zhang X Omnivorous Carp (Carassius gibelio) Increase Eutrophication in Part by Preventing Development of Large-Bodied Zooplankton and Submerged Macrophytes. Water 2021, 13, 1497. [Google Scholar]
- 108.Tapkir S; Boukal D; Kalous L; Bartoň D; Souza AT; Kolar V; Soukalová K; Duchet C; Gottwald M; šmejkal M Invasive gibel carp (Carassius gibelio) outperforms threatened native crucian carp (Carassius carassius) in growth rate and effectiveness of resource use: Field and experimental evidence. Aquat. Conserv. Mar. Freshw. Ecosyst 2022, 32, 1901–1912. [Google Scholar]
- 109.Cucherousset J; Aymes J; Poulet N; Santoul F; Cereghino R Do native brown trout and non-native brook trout interact reproductively? Naturwissenschaften 2008, 95, 647–654. [DOI] [PubMed] [Google Scholar]
- 110.El-Matbouli M; Soliman H Transmission of Cyprinid herpesvirus-3 (CyHV-3) from goldfish to naive common carp by cohabitation. Res. Vet. Sci 2011, 90, 536–539. [DOI] [PubMed] [Google Scholar]
- 111.Azevedo-Santos VM; Pelicice FM; Lima-Junior DP; Magalhães ALB; Orsi ML; Vitule JRS; Agostinho AA How to avoid fish introductions in Brazil: Education and information as alternatives. Nat. E Conserv 2015, 13, 123–132. [Google Scholar]
- 112.CABI. Invasive Species Compendium. 2021. Available online: www.cabi.org/isc (accessed on 10 May 2023).
- 113.FAO (Food and Agriculture Organization of the United Nations). The State of World Fisheries and Aquaculture; FAO: Rome, Italy, 2015. [Google Scholar]
- 114.Ahmed KKU; Hambrey JB Studies on the fish catch efficiency of different types of fishing gear in Kaptai Reservoir, Bangladesh. Lakes Reserv. Res. Manag 2005, 10, 221–234. [Google Scholar]
- 115.Laxmappa B; Bakshi RR Types of fishing gears operating and their impact on Krishna river fishery in Mahabubnagar district, India. Int. J. Fish. Aquat. Stud 2014, 2, 30–41. [Google Scholar]
- 116.Oliveira AG; Gomes LC; Latini JD; Agostinho AA Implications of using a variety of fishing strategies and sampling techniques across different biotopes to determine fish species composition and diversity. Nat. Conserv 2014, 12, 112–117. [Google Scholar]
- 117.Dadebo E; Eyayu A; Sorsa S; Tilahun G Food and Feeding Habits of the Common Carp (Cyprinus carpio L. 1758) (Pisces: Cyprinidae) in Lake Koka, Ethiopia. Momona Ethiop. J. Sci 2015, 7, 16–31. [Google Scholar]
- 118.Erarto F; Getahun A Impacts of introductions of alien species with emphasis on fishes. Int. J. Fish. Aquat. Stud 2020, 8, 207–216. [Google Scholar]
- 119.Eagderi S; Nasri M; Cicek E First record of the Amur goby Rhinogobius lindbergi Berg 1933 (Gobiidae) from the Tigris River drainage, Iran. Int. J. Aquat. Biol 2018, 6, 202–207. [Google Scholar]
- 120.Sadeghi R; Esmaeili HR; Zarei F; Esmaeili A; Abbasi K The taxonomic status of an introduced freshwater goby of the genus Rhinogobius to Iran (Teleostei: Gobiidae). Zool. Middle East 2019, 65, 51–58. [Google Scholar]
- 121.Kalogianni E; Koutsikos N; Vardakas L; Giakoumi S; Chatzinikolaou Y; Oikonomou A Impacts of the alien mosquitofish on the abundance and condition of two Mediterranean native fish. Mediterr. Mar. Sci 2019, 20, 727–735. [Google Scholar]
- 122.Coad BW Zoogeography of the fishes of the tigris-euphrates basin. Zool. Middle East 1996, 13, 51–70. [Google Scholar]
- 123.Kumar AB Exotic fishes and freshwater fish diversity. Zoos’ Print J. 2000, 15, 363–367. [Google Scholar]
- 124.Segev O; Mangel M; Blaustein L Deleterious effects by mosquitofish (Gambusia affinis) on the endangered fire salamander (Salamandra infraimmaculata). Anim. Conserv 2009, 12, 29–37. [Google Scholar]
- 125.Ekmekçï F; Kirankaya Ş Distribution of an Invasive Fish Species, Pseudorasbora parva (Temminck & Schlegel, 1846) in Turkey. Turk. J. Zool 2006, 30, 329–334. [Google Scholar]
- 126.Taybi AF; Mabrouki Y; Doadrio I The occurrence, distribution and biology of invasive fish species in fresh and brackish water bodies of ne Morocco. Arxius de Miscellania Zoologica. 2020, 18, 59–73. [Google Scholar]
- 127.Grabowska J; Przybylski M Life-history traits of non-native freshwater fish invaders differentiate them from natives in the Central European bioregion. Rev. Fish Biol. Fish 2015, 25, 165–178. [Google Scholar]
- 128.Blinn DW; Runck C; Clark DA; Rinne JN Notes: Effects of Rainbow Trout Predation on Little Colorado Spinedace. Trans. Am. Fish. Soc 1993, 122, 139–143. [Google Scholar]
- 129.Aalipour M; Keivany Y; Ebrahimi E Feeding habits of Rainbow Trout (Oncorhynchus mykiss) in Beheshtabad River of Chaharmahal & Bakhtiari Province. Appl. Biol 2019, 32, 76–97. [Google Scholar]
- 130.Dudgeon D; Arthington AH; Gessner MO; Kawabata Z-I; Knowler DJ; Lévêque C; Naiman RJ; Prieur-Richard A-H; Soto D; Stiassny MLJ; et al. Freshwater biodiversity: Importance, threats, status and conservation challenges. Biol. Rev. Camb. Philos. Soc 2006, 81, 163–182. [DOI] [PubMed] [Google Scholar]
- 131.Brown LR; Ford T Effects of flow on the fish communities of a regulated California river: Implications for managing native fishes. River Res. Appl 2002, 18, 331–342. [Google Scholar]
- 132.Ostberg S; Schaphoff S; Lucht W; Gerten D Three centuries of dual pressure from land use and climate change on the biosphere. Environ. Res. Lett 2015, 10, 044011. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
There are no supplementary data parts and no publicly archived datasets were analyzed or generated during the study. The data and the location of sampling sites are available on request after publishing.


