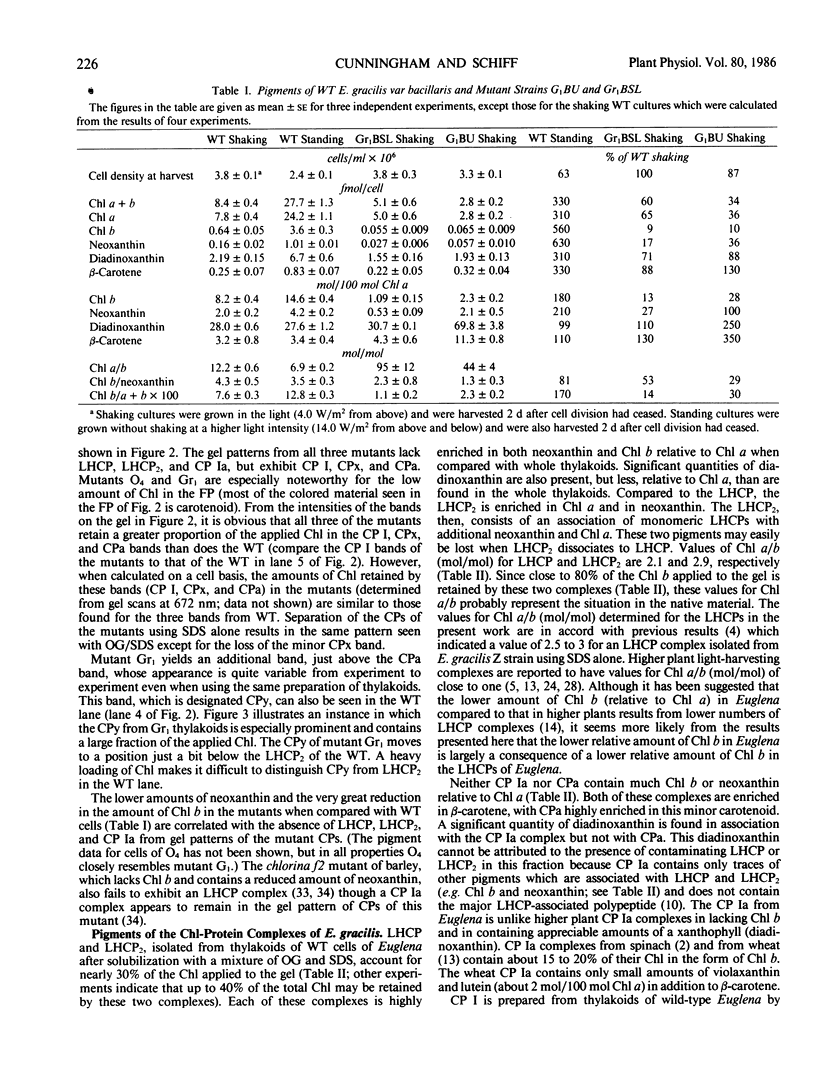
Abstract
The use of n-octyl-β-d-glucopyranoside along with sodium dodecyl sulfate improves the retention of chlorophyll (Chl) by chlorophyll-protein complexes (CPs) prepared from thylakoids of Euglena gracilis Klebs var bacillaris Cori and yields several additional complexes. Thylakoids from wild-type (WT) cells, solubilized in these detergents and subjected to polyacrylamide gel electrophoresis at 0°C, yield the following CPs, in order of relative molecular weight, containing the pigments shown in parentheses with their respective molar ratios where determined: CP Ia (Chl a, diadinoxanthin and β-carotene; 100:12:5); CP I (Chl a and β-carotene; 100:6-12); CPx (Chl and carotenoids); LHCP2 (light-harvesting CP oligomer) (Chl a, Chl b, diadinoxanthin and neoxanthin; 12:4:3:1); CPy (Chl a, diadinoxanthin and β-carotene; 100:14:8); CPa (Chl a and β-carotene; 100:18-25) and LHCP (monomer) (Chl a, Chl b, diadinoxanthin and neoxanthin; 12:6:4:1). The LHCP complexes retain up to 40% of the total Chl and 80% of the Chl b in the thylakoids. CP Ia contains only a trace of Chl b (Chl a/b [mol/mol] = 62). The lower amount of Chl b in Euglena (about 10% of Chl a + b) compared to higher plants (about 30% of Chl a + b) is probably a consequence of the lower Chl b (relative to Chl a) in the LHCPs of Euglena rather than of fewer LHCPs being present. G1BU, Gr1BSL, and O4BSL, mutants of bacillaris low in Chl b (1-2% of Chl a + b), lack the CP Ia, LHCP, and LHCP2 found in wildtype (WT); G1 and O4 also lack CPy. The mutants contain reduced amounts of Chl a (two-thirds of WT in Gr1 and one-third in G1 and O4) and neoxanthin (20-40% of WT) but retain levels of β-carotene and diadinoxanthin close to those in cells of WT. The CPs remaining in the mutants have pigment compositions very similar to their counterparts from WT.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. M. P-700 content and polypeptide profile of chlorophyll-protein complexes of spinach and barley thylakoids. Biochim Biophys Acta. 1980 Jun 10;591(1):113–126. doi: 10.1016/0005-2728(80)90225-x. [DOI] [PubMed] [Google Scholar]
- BRUINSMA J. A comment on the spectrophotometric determination of chlorophyll. Biochim Biophys Acta. 1961 Sep 30;52:576–578. doi: 10.1016/0006-3002(61)90418-8. [DOI] [PubMed] [Google Scholar]
- Bennett J. Regulation of photosynthesis by reversible phosphorylation of the light-harvesting chlorophyll a/b protein. Biochem J. 1983 Apr 15;212(1):1–13. doi: 10.1042/bj2120001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camm E. L., Green B. R. Fractionation of Thylakoid Membranes with the Nonionic Detergent Octyl-beta-d-glucopyranoside: RESOLUTION OF CHLOROPHYLL-PROTEIN COMPLEX II INTO TWO CHLOROPHYLL-PROTEIN COMPLEXES. Plant Physiol. 1980 Sep;66(3):428–432. doi: 10.1104/pp.66.3.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham F. X., Schiff J. A. Chlorophyll-Protein Complexes from Euglena gracilis and Mutants Deficient in Chlorophyll b: II. Polypeptide Composition. Plant Physiol. 1986 Jan;80(1):231–238. doi: 10.1104/pp.80.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskins K., Duysen M. E., Olson L. Pigment analysis of chloroplast pigment-protein complexes in wheat. Plant Physiol. 1983 Apr;71(4):777–779. doi: 10.1104/pp.71.4.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genge S., Pilger D., Hiller R. G. The relationship between chlorophyll b and pigment-protein complex II. Biochim Biophys Acta. 1974 Apr 23;347(1):22–30. doi: 10.1016/0005-2728(74)90196-0. [DOI] [PubMed] [Google Scholar]
- Hager A., Stransky H. Das Carotinoidmuster und die Verbreitung des lichtinduzierten Xanthophyllcyclus in verschiedenen Algenklassen. V. Einzelne Vertreter der Cryptophyceae, Euglenophyceae, Bacillariophyceae, Chrysophyceae und Phaeophyceae. Arch Mikrobiol. 1970;73(1):77–89. [PubMed] [Google Scholar]
- Kirk J. T., Allen R. L. Dependence of chloroplast pigment synthesis on protein synthesis: effect of actidione. Biochem Biophys Res Commun. 1965 Dec 21;21(6):523–530. doi: 10.1016/0006-291x(65)90516-4. [DOI] [PubMed] [Google Scholar]
- Krinsky N. I., Gordon A., Stern A. I. The Appearance of Neoxanthin during the Regreening of Dark-grown Euglena. Plant Physiol. 1964 May;39(3):441–445. doi: 10.1104/pp.39.3.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang T. Y., Argyroudi-Akoyunoglou J. H., Nakatani H. Y., Watson J., Arntzen C. J. The origin of the long-wavelength fluorescence emission band (77 degrees K) from photosystem I. Arch Biochem Biophys. 1984 Dec;235(2):618–627. doi: 10.1016/0003-9861(84)90236-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Ryrie I. J., Anderson J. M., Goodchild D. J. The role of the light-harvesting chlorophyll a/b protein complex in chloroplast membrane stacking. Cation-induced aggregation of reconstituted proteoliposomes. Eur J Biochem. 1980 Jun;107(2):345–354. doi: 10.1111/j.1432-1033.1980.tb06035.x. [DOI] [PubMed] [Google Scholar]
- Thornber J. P., Highkin H. R. Composition of the photosynthetic apparatus of normal barley leaves and a mutant lacking chlorophyll b. Eur J Biochem. 1974 Jan 3;41(1):109–116. doi: 10.1111/j.1432-1033.1974.tb03250.x. [DOI] [PubMed] [Google Scholar]
- Waldron J. C., Anderson J. M. Chlorophyll-protein complexes from thylakoids of a mutant barley lacking chlorophyll b. Eur J Biochem. 1979 Dec 17;102(2):357–362. doi: 10.1111/j.1432-1033.1979.tb04250.x. [DOI] [PubMed] [Google Scholar]




