Abstract
There is debate as to whether a time-dependent transformation of the episodic-like memory network is observed for nonepisodic tasks, including procedural motor memory. To determine how motor memory networks reorganize with time and practice, mice performed a motor task in a straight alley maze for 1 d (recent), 20 d of continuous training (continuous), or testing 20 d after the original training (remote), and then regional c-Fos expression was assessed. Elevated hippocampal c-Fos accompanied remote, but not continuous, motor task retrieval after 20 d, suggesting that the hippocampus remains engaged for nonhabitual remote motor memory retrieval.
Consolidation of episodic memories, or memories for unique events, requires the hippocampus (Dudai et al. 2015; Sekeres et al. 2018b). Motor memories are considered a form of nondeclarative memory that do not require conscious recollection or the hippocampus, although their retrieval potentially continues to engage an intact hippocampus (Albouy et al. 2008). The development of a new skill like riding a bike is viewed as a motor task, yet the initial acquisition of that skill is linked to the unique experience of the first bike riding event. Dissociating the episodic memory experience from the motor memory component of the event can be difficult. Despite not explicitly recalling the episodic components of that unique first riding experience each time one gets on a bike, the implicit motor memory supports the performance of the task. Like the episodic memory for the event, the motor memory for the task changes over time as it becomes more familiar through repeated performance. This transformed version of the motor memory is accompanied by a reorganization of neural activity (Dayan and Cohen 2011; Albouy et al. 2015; King et al. 2019). It is also difficult to dissociate the effects of the distributed practice of a motor task from the effects of time on memory consolidation. Thus, time and practice are often confounded in studies of human motor memory consolidation to date.
In rodents, common physiological changes have been observed following cellular consolidation of episodic-like memories and motor memories, including dendritic spine growth in the dorsal hippocampus (Restivo et al. 2009) and primary motor cortex (Xu et al. 2009; Hwang et al. 2022; Yang et al. 2022), respectively. These observations suggest that episodic and motor memories undergo common cellular consolidation processes. Large-scale systems consolidation and hippocampal–cortico reorganization of a context fear memory network occur over the course of 2–4 wk, after only a single conditioning trial in the context (Frankland et al. 2004; Winocur et al. 2007; Goshen et al. 2011; Vetere et al. 2017, 2019; Sekeres et al. 2018a, 2020). Conversely, motor skills often require repeated conditioning sessions to successfully acquire the motor memory (Song 2009). With repetition, motor skills become increasingly familiar and habitual and typically recruit the striatum, a neural region known to be engaged during habitual learning and behavior (Shadmehr and Holcomb 1997; Krakauer and Shadmehr 2006; Albouy et al. 2015; Badreddine et al. 2022). The prolonged process of systems consolidation and hippocampal–cortico reorganization of episodic memory networks has been well characterized in rodents (Winocur et al. 2007, 2009; Wheeler et al. 2013; Cullen et al. 2015; Sekeres et al. 2018a, 2020; Vetere et al. 2019; Roy et al. 2022), but whether a similar pattern of recollection network reorganization underlies prolonged procedural motor memory consolidation remains unclear.
To identify the neural network dynamics as a novel motor task becomes familiar over time, we assessed time-dependent and experience-dependent changes in brain activation using immediate–early gene expression of c-Fos across the brain as a mouse learns a motor task over the course of 3 wk. We aimed to determine whether motor memory undergoes a transformation from hippocampal dependency to greater dependency on other brain areas, including the anterior cingulate cortex (aCC) and striatum, as the memory for the experience becomes less unique and more familiar over time. If motor memory network reorganization follows a pattern similar to that of episodic-like declarative memory network reorganization, we predicted that the hippocampus would continue to be engaged during both recent learning and remote memory retrieval of the motor task in the original training context. We also anticipated that the aCC would be more highly active during the remote, relative to the recent, memory retrieval sessions (Frankland et al. 2004). Additionally, by varying the age of the memory (1 or 20 d) and the frequency of training (two training sessions or 20 training sessions), we can dissociate the contributions of time and practice on motor memory systems consolidation and network reorganization. If continuous practice renders the motor memory relatively schematized (Richards et al. 2014; King et al. 2019), we predicted a decline in hippocampal activity during the performance of the continuous motor task, whereas we predicted continued hippocampal engagement during remote memory retrieval in the absence of continued exposure to the task and context.
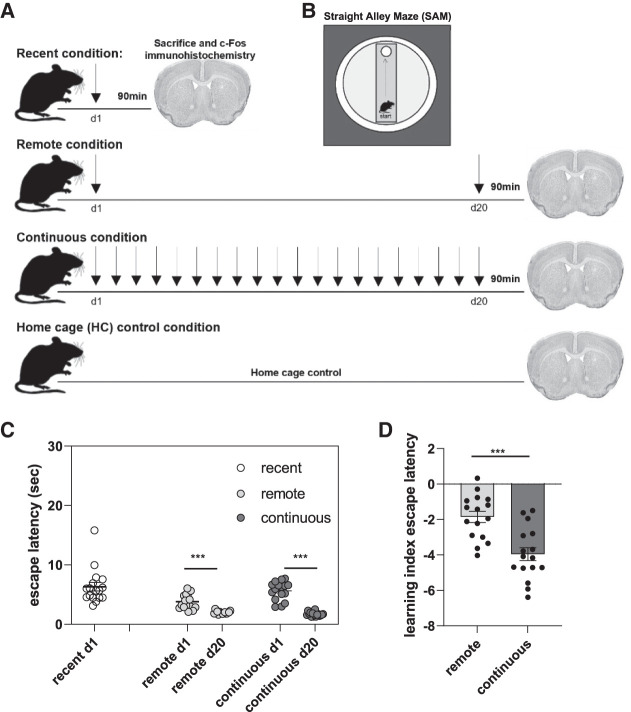
To identify motor learning and memory network activity, we imaged c-Fos expression in the dorsal hippocampus (CA1 and DG), aCC, striatum, and motor cortices (M1 and M2) following training on a motor task in a straight alley maze (SAM). Mice were randomly assigned to one of four conditions: recent training (1-d train, n = 17), remote training (1-d train/18-d rest/1-d train, n = 16), continuous training (1- to 20-d train, n = 16), or home cage (HC) control (n = 8). See Figure 1A for the study design schematic. All procedures were approved by Baylor University's Institutional Care and Use Committee and conducted in accordance with the National Institutes of Health's Guide for the Care and Use of Laboratory Animals.
Figure 1.
Study design and escape latencies. (A) Schematic of the study design. Each arrow indicates a training day (five trials/day). (B) Schematic of the straight alley maze (SAM) apparatus. (C) Escape latency (in seconds) on day 1 (d1) and day 20 (d20) of SAM training. (D) Learning index (LI) measure of escape latency between day 1 and day 20 of SAM training. The LI was calculated by subtracting day 1 latency values from day 20 values to generate a measure of motor learning over time. Individual data points are presented for each subject within the training conditions. Bars represent the group mean, and error bars represent the standard error of the mean (SEM). (***) P < 0.001.
The motor task required mice to learn to swim down the SAM to reach a submerged escape platform. The SAM was housed in a circular pool filled with opaque water, surrounded by a black curtain (Fig. 1B). All training/test days consisted of five nonconsecutive trials, where mice were placed at the end of the SAM opposite the platform and given up to 60 sec to mount the platform. If the mouse failed to mount the platform, it was guided by the experimenter. At the end of each trial, the mouse was given 30 sec to rest atop the platform. Test trials were conducted identically to the training trials. Trials were recorded by an overhead video camera and analyzed using Noldus Ethovision software to assess escape latency, distance, and speed. For the remote and continuous groups, a learning index (LI) was calculated, where day 1 values were subtracted from the day 20 values to generate a measure of behavioral change over time (Badreddine et al. 2022). The HC control group was included to control for baseline expression of c-Fos, a transcriptional regulator and marker of neuronal activity (Cruz et al. 2015). These mice were maintained in their home cages throughout the experiment and did not undergo behavioral training.
Immediately following the final trial, mice were transferred to a quiet holding room. After 90 min, mice were anesthetized and intracardially perfused with PBS and 4% paraformaldehyde. Brains were postfixed for 48 h and then sectioned coronally (30 µm). A subset of brains (eight/group) was randomly selected for c-Fos staining and cell quantification. Ten sections per brain ranging between 2.22 and −3.52 mm from bregma were sampled. Sections were washed in PBS, incubated for 2 h at room temperature in 10% normal goat serum and blocking solution (PBS and 0.3% Triton X-100), and then incubated with rabbit anti-c-Fos polyclonal primary antibody (1:1000; PBS and 0.3% Triton X-100; Abcam ab190289) for 48 h at 4°C. Sections were washed in PBS, incubated with goat antirabbit Alexa 488 secondary antibody (1:200; Invitrogen A-11034) for 2 h at room temperature, washed, mounted with PermaFluor mounting medium (Thermo Scientific) on glass slides, and cover-slipped. Stained sections were imaged and analyzed using a Nikon Eclipse-NI-E fluorescent microscope at 10× magnification and digitally stitched together using NIS-Elements software (Nikon version 4.1.3) to reconstruct each region of interest (ROI; hippocampus, aCC, striatum, and motor cortex). The number of c-Fos+ nuclei within each ROI was counted using ImageJ. The number of c-Fos+ cells was divided by the outlined ROI area to generate a normalized c-Fos+ cells/100,000-µm area value. The mean value for each ROI per brain was calculated, and group means were then calculated for each condition. Univariate ANOVAs were conducted for c-Fos levels and for SAM behavior. Statistical analyses were conducted using SPSS 26.
Univariate ANOVAs assessed group differences in motor performance at each delay. All mice acquired the motor task within the first day of training (Fig. 1C), with all mice mounting the escape platform in <16 sec. Unexpectedly, mice randomly assigned to the remote training condition performed better than the other conditions on training day 1 (F(2,46) = 6.252, P = 0.004, η2 = 0.214), with slightly faster escape latency than the recent (1-d training, P = 0.001) or continuous (20-d consecutive training, P = 0.018) groups. A one-sample t-test comparing escape latency between day 1 and day 20 for the remote condition confirmed that mice in the remote condition did significantly improve their performance on day 20 relative to their day 1 performance (t(15) = 12.373, P < 0.001). A similar comparison of day 1 versus day 20 latency also revealed significant improvements in performance after 20 d of consecutive training in the continuous group (t(15) = 14.554, P < 0.001). Despite both groups showing improved motor memory for the SAM on day 20, a direct comparison of escape latency on day 20 between the remote and continuous groups confirmed better motor performance after 20 d of continuous training in the SAM (t(30) = −2.794, P = 0.009) (Fig. 1C), suggesting that continuous training enhanced motor task performance (see the Supplemental Material for analyses of distance [Supplemental Fig. S1A], speed [Supplemental Fig. S1B], and complete training latency [Supplemental Fig. S1C] for the continuous condition). To control for the difference in baseline performance between the remote and continuous groups, independent sample t-tests (two-tailed) of the LI confirmed the enhanced savings in escape latency for the continuous group relative to the remote group on test day 20 (t(30) = −4.707, P < 0.001), indicating significant improvement in motor task performance following continuous training relative to the equally aged but nonpracticed remote motor task (Fig. 1D).
To test the predictions related to the time-dependent reorganization of network activity typically associated with episodic-like memory, we analyzed c-Fos in the CA1, DG, and aCC. Significant main effects of training condition were observed for hippocampal c-Fos in the CA1 (F(3,25) = 10.121, P < 0.001, η2 = 0.548) and DG (F(3,25) = 13.901, P < 0.001, η2 = 0.635), with all experimental conditions having significant increases in activity-induced c-Fos expression relative to HC control levels (all Ps < 0.02) (Fig. 2B,C). No differences in c-Fos expression were observed between the recent and the remote conditions in the CA1 (P = 0.950) (Fig. 2B) or DG (P = 0.247) (Fig. 2C), suggesting that the hippocampus continues to be engaged during retrieval of the remote but relatively unfamiliar motor memory. This is consistent with observations in the hippocampus when testing episodic-like context fear memory, where comparable levels of c-Fos were observed when tested 24 h or 28 d after conditioning in the original training context (Sekeres et al. 2018a, 2020). Together, these findings suggest that, in healthy brains, the hippocampus continues to be engaged during retrieval of a remote motor task that is still relatively unfamiliar. A different trend was observed when comparing c-Fos in the recent condition with c-Fos levels in the continuous condition in the CA1 (P = 0.060) and DG (P = 0.054), with these regions being less active following the final test session on day 20 (Fig. 2B,C), suggesting that hippocampal engagement declines as the motor task becomes more familiar over time.
Figure 2.
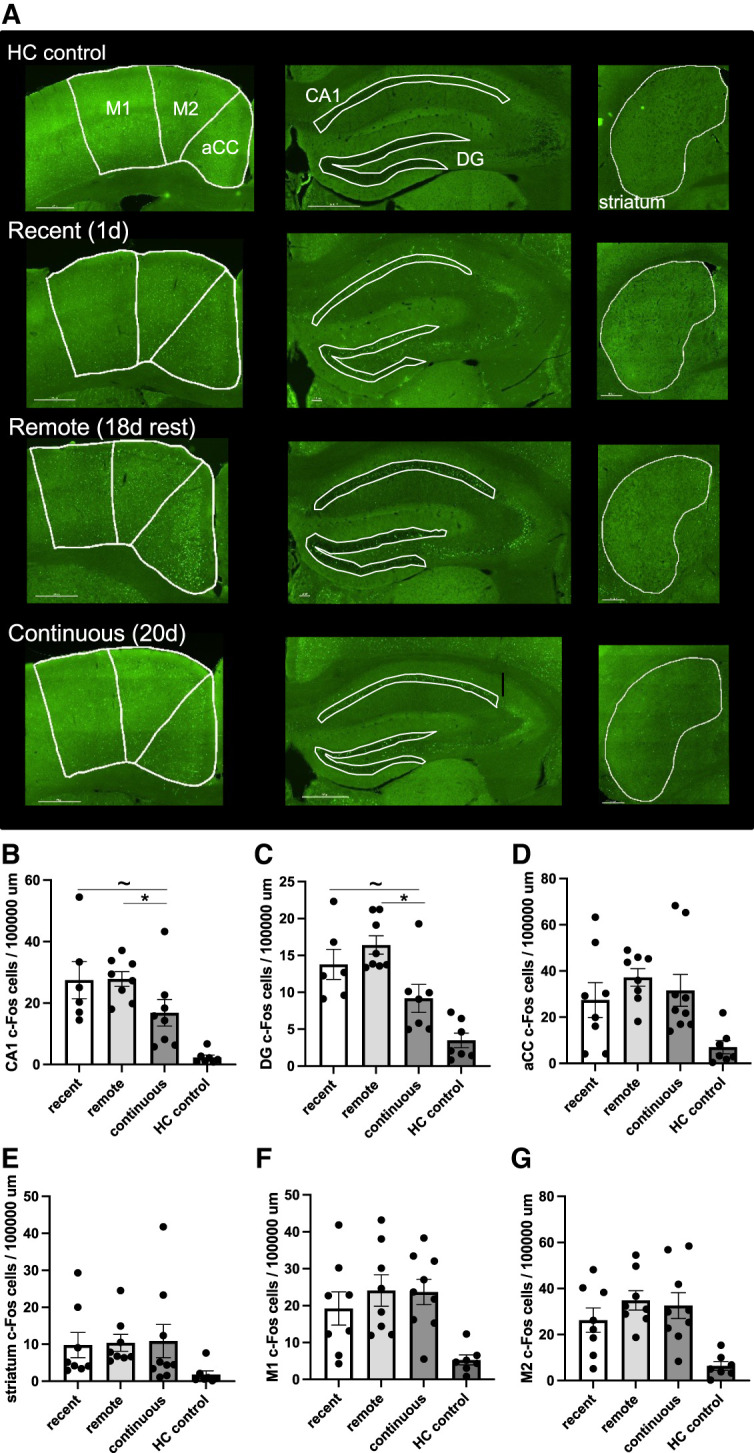
Continued hippocampal engagement over time is sensitive to the relative novelty of the motor task. (A, left) Representative c-Fos expression in the aCC and motor cortices (M1 and M2) for each training condition (recent, remote, and continuous training and home cage [HC] control groups). (Middle) Representative c-Fos expression in the hippocampus (CA1 and DG) for each experimental condition and HC control group. (Right) Representative c-Fos expression in the striatum for each experimental condition and HC control group. Scale bar, 500 µm. (B,C) Mean c-Fos expression levels in the CA1 (B) and DG (C) were significantly higher following remote motor testing relative to the continuous training levels on day 20, suggesting that continued hippocampal engagement over time depends on the relative familiarity of the motor task. (D) Mean c-Fos expression in the aCC was insensitive to age or familiarity of the motor memory. All groups show an activity-induced increase in c-Fos expression relative to HC control levels in the hippocampus and aCC. (E–G) No significant differences in c-Fos expression levels in the striatum (E), M1 (F), or M2 (G) were observed between training conditions. Error bars represent the SEM. (aCC) Anterior cingulate cortex, (DG) dentate gyrus, (d1) training day 1, (d20) training day 20, (HC) home cage control, (M1) motor cortex 1, (M2) motor cortex 2, (SEM) standard error of the mean. (*) P < 0.05, (∼) P < 0.06.
We next directly compared hippocampal c-Fos expression induced by motor task performance on day 20 with groups differing only in the degree of exposure to the task during the 20-d training–test interval. c-Fos expression levels in the remote condition were significantly higher than levels observed in the continuous condition on day 20 (CA1, P = 0.038; DG, P = 0.002) (Fig. 2B,C). These data suggest that continued hippocampal engagement during remote motor memory retrieval is dependent on the relative familiarity of the task, with the less familiar task continuing to strongly engage the hippocampus.
A main effect of training condition was observed in the aCC (F(3,28) = 4.602, P = 0.010, η2 = 0.330), but this was driven by activity-induced c-Fos expression in all training conditions relative to HC controls (all Ps > 0.025) (see Supplemental Table S1 for full statistical results). Neither time nor practice mediated c-Fos expression within the aCC. No differences in aCC activity were observed between recent and remote training (P = 0.248) or recent and continuous training (P = 0.610) (Fig. 2D) conditions. Comparable aCC expression levels were also induced by both remote and continuous training conditions on day 20 (P = 0.491), suggesting that retrieval of a familiar motor memory did not differentially engage the aCC relative to an equally aged yet relatively unfamiliar motor task.
Together, these findings suggest that the pattern of reorganization underlying motor memory consolidation follows a pattern similar to, yet distinct from, what is commonly observed in the episodic-like memory recollection network (Sekeres et al. 2018a, 2020). The results observed in the hippocampus indicate that the memory task retrieved in the original conditioning environment continues to engage the hippocampus, as retrieval of the task is possibly tied to the experience of first learning the motor task within this context. As the task is repeated over time, it is possible that the reduction in hippocampal activity observed following the continuous memory test reflects a schematization of the task during which attention to the environment declines, as the episodic elements of the memory are less salient over time (Tse et al. 2007; King et al. 2019). It remains unclear whether altering the context at the time of remote motor memory retrieval would differently activate the hippocampal–aCC network.
To assess activity in regions more commonly associated with procedural motor learning and memory and motor activity, we next analyzed the expression of c-Fos in the striatum and motor cortices (M1/M2) (Fig. 2A). We did not observe a practice-induced increase in striatal c-Fos, with no main effect of training condition (F(3,28) = 1.535, P = 0.227, η2 = 0.141) (Fig. 2E). While recent evidence suggests that regional differences in medial and lateral dorsal striatal activity differentially mediate motor skill acquisition and learning at early and later stages of training (Badreddine et al. 2022; Wolff et al. 2022), exploratory analyses did not identify regional differences in medial and lateral striatal c-Fos expression between training conditions (all Ps > 0.05) (Supplemental Table S1; Supplemental Fig. S2A,B). It is possible that more sensitive in vivo recordings are needed to detect early- versus late-learning changes in dorso–medial and dorso–lateral striatal activity as a motor task becomes more familiar (Yin et al. 2009; Thorn et al. 2010). An activity-induced main effect of condition was observed in the motor cortices in both M1 (F(3,28) = 5.225, P = 0.005, η2 = 0.359) and M2 (F(3,28) = 6.954, P = 0.001, η2 = 0.427), with all training conditions having significantly higher c-Fos expression relative to HC control mice (all Ps < 0.020) (Fig. 2F,G) but no other between-group differences (all Ps > 0.200) (Supplemental Table S1).
In dissociating the effects of distributed practice from the effects of time on motor memory consolidation, we identified that continued hippocampal c-Fos expression supported remote motor memory retrieval after 20 d, suggesting that hippocampal engagement is dependent on the relative familiarity of the task, with the less familiar task continuing to strongly engage the hippocampus over time. c-Fos levels in the aCC were not significantly different during remote motor testing regardless of the level of familiarity, suggesting that motor memory network reorganization does not follow the same pattern of network reorganization commonly observed in traditional forms of episodic-like memory. To date, investigations of systems-level memory network reorganization at the cellular level have largely been confined to tests of declarative-like memory, such as contextual fear conditioning in rodents (Sekeres et al. 2018b). Similar reorganizational processes of the memory network have also been observed following remote memory training using a prolonged training protocol using spatial versions of the escape-motivated water task (Maviel et al. 2004; Teixeira et al. 2006; Richards et al. 2014), suggesting that a time-dependent increase in aCC activity typically observed during remote memory retrieval also occurs to support remote declarative-like memory acquired over repeated training trials over the course of several weeks. Unlike these studies, the present study did not require the animal to form a conditioned response between a context, spatial cues, or other conditioned stimuli (Takehara et al. 2003) for successful performance. It is possible that increasing task complexity to require the integration of spatial or contextual cues would differentially engage the aCC with time and practice. Additionally, the shift toward increased aCC activity and reduced hippocampal activity during remote declarative memory retrieval is typically accompanied by a loss of contextual specificity of the memory and is reflective of a schematization and contextual generalization of the memory over time (Winocur et al. 2007; Wiltgen and Silva 2007). Future studies will determine how alterations in the testing context differentially engage the hippocampus and aCC as the motor memory potentially schematizes with time and practice and how inhibition of hippocampal input during motor memory encoding or retrieval alters activity and functional connectivity patterns of other nodes of the recollection network. Taken together, the findings partially support the idea that a similar pattern of systems consolidation and memory network reorganizational processes in the hippocampus is observed across declarative (episodic) and nondeclarative (motor) memory, with familiarity, not time, mediating the continued engagement of the hippocampus during task retrieval.
Competing interest statement
The authors declare no competing interests.
Supplementary Material
Acknowledgments
We gratefully acknowledge the technical assistance of vivarium animal care staff Lee Lowe and Natashia Howard. This study was supported by a fellowship from the Courtney Knight Gaines Foundation (to D.G.S.), a Baylor University Research Committee grant (to M.J.S.), a National Sciences and Engineering Research Council Discovery grant (RGPIN-2021-02569 to M.J.S.), and the Canada Research Chairs Program (950-233046 to M.J.S.).
Author contributions: D.G.S. conceptualized the study; performed the methodology, investigation, and formal analysis; acquired the funding; and reviewed and edited the manuscript. P.N., K.G., and M.B.W. performed the investigation and reviewed and edited the manuscript. M.J.S. conceptualized and supervised the study, performed the methodology and formal analysis, acquired the funding, wrote the original draft of the manuscript, and reviewed and edited the manuscript.
Footnotes
[Supplemental material is available for this article.]
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.053792.123.
Freely available online through the Learning & Memory Open Access option.
References
- Albouy G, Sterpenich V, Balteau E, Vandewalle G, Desseilles M, Dang-Vu T, Darsaud A, Ruby P, Luppi PH, Degueldre C, et al. 2008. Both the hippocampus and striatum are involved in consolidation of motor sequence memory. Neuron 58: 261–272. 10.1016/j.neuron.2008.02.008 [DOI] [PubMed] [Google Scholar]
- Albouy G, Fogel S, King BR, Laventure S, Benali H, Karni A, Carrier J, Robertson EM, Doyon J. 2015. Maintaining vs. enhancing motor sequence memories: respective roles of striatal and hippocampal systems. Neuroimage 108: 423–434. 10.1016/j.neuroimage.2014.12.049 [DOI] [PubMed] [Google Scholar]
- Badreddine N, Zalcman G, Appaix F, Becq G, Tremblay N, Saudou F, Achard S, Fino E. 2022. Spatiotemporal reorganization of corticostriatal networks encodes motor skill learning. Cell Rep 39: 110623. 10.1016/j.celrep.2022.110623 [DOI] [PubMed] [Google Scholar]
- Cruz FC, Rubio FJ, Hope BT. 2015. Using c-fos to study neuronal ensembles in corticostriatal circuitry of addiction. Brain Res 1628: 157–173. 10.1016/j.brainres.2014.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen PK, Gilman TL, Winiecki P, Riccio DC, Jasnow AM. 2015. Activity of the anterior cingulate cortex and ventral hippocampus underlie increases in contextual fear generalization. Neurobiol Learn Mem 124: 19–27. 10.1016/j.nlm.2015.07.001 [DOI] [PubMed] [Google Scholar]
- Dayan E, Cohen LG. 2011. Neuroplasticity subserving motor skill learning. Neuron 72: 443–454. 10.1016/j.neuron.2011.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudai Y, Karni A, Born J. 2015. The consolidation and transformation of memory. Neuron 88: 20–32. 10.1016/j.neuron.2015.09.004 [DOI] [PubMed] [Google Scholar]
- Frankland PW, Bontempi B, Talton LE, Kaczmarek L, Silva AJ. 2004. The involvement of the anterior cingulate cortex in remote contextual fear memory. Science 304: 881–883. 10.1126/science.1094804 [DOI] [PubMed] [Google Scholar]
- Goshen I, Brodsky M, Prakash R, Wallace J, Gradinaru V, Ramakrishnan C, Deisseroth K. 2011. Dynamics of retrieval strategies for remote memories. Cell 147: 678–689. 10.1016/j.cell.2011.09.033 [DOI] [PubMed] [Google Scholar]
- Hwang FJ, Roth RH, Wu YW, Sun Y, Kwon DK, Liu Y, Ding JB. 2022. Motor learning selectively strengthens cortical and striatal synapses of motor engram neurons. Neuron 110: 2790–2801. 10.1016/j.neuron.2022.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King BR, Dolfen N, Gann MA, Renard Z, Swinnen SP, Albouy G. 2019. Schema and motor-memory consolidation. Psychol Sci 30: 963–978. 10.1177/0956797619847164 [DOI] [PubMed] [Google Scholar]
- Krakauer JW, Shadmehr R. 2006. Consolidation of motor memory. Trends Neurosci 29: 58–64. 10.1016/j.tins.2005.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maviel T, Durkin TP, Menzaghi F, Bontempi B. 2004. Sites of neocortical reorganization critical for remote spatial memory. Science 305: 96–99. 10.1126/science.1098180 [DOI] [PubMed] [Google Scholar]
- Restivo L, Vetere G, Bontempi B, Ammassari-Teule M. 2009. The formation of recent and remote memory is associated with time-dependent formation of dendritic spines in the hippocampus and anterior cingulate cortex. J Neurosci 29: 8206–8214. 10.1523/JNEUROSCI.0966-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards BA, Xia F, Santoro A, Husse J, Woodin MA, Josselyn SA, Frankland PW. 2014. Patterns across multiple memories are identified over time. Nat Neurosci 17: 981–986. 10.1038/nn.3736 [DOI] [PubMed] [Google Scholar]
- Roy DS, Park YG, Kim ME, Zhang Y, Ogawa SK, DiNapoli N, Gu X, Cho JH, Choi H, Kamentsky L, et al. 2022. Brain-wide mapping reveals that engrams for a single memory are distributed across multiple brain regions. Nat Commun 13: 1799. 10.1038/s41467-022-29384-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekeres MJ, Winocur G, Moscovitch M, Anderson JAE, Pishdadian S, Martin Wojtowicz J, St-Laurent M, McAndrews MP, Grady CL. 2018a. Changes in patterns of neural activity underlie a time-dependent transformation of memory across species. Hippocampus 20: 745–764. 10.1002/hipo.23009 [DOI] [PubMed] [Google Scholar]
- Sekeres MJ, Winocur G, Moscovitch M. 2018b. The hippocampus and related neocortical structures in memory transformation. Neurosci Lett 680: 39–53. 10.1016/j.neulet.2018.05.006 [DOI] [PubMed] [Google Scholar]
- Sekeres MJ, Moscovitch M, Grady CA, Sullens DG, Winocur G. 2020. Reminders reinstate context-specificity to generalized remote memories in rats: relation to activity in the hippocampus and aCC. Learn Mem 27: 1–5. 10.1101/lm.050161.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadmehr R, Holcomb HH. 1997. Neural correlates of motor memory consolidation. Science 277: 821–825. 10.1126/science.277.5327.821 [DOI] [PubMed] [Google Scholar]
- Song S. 2009. Consciousness and the consolidation of motor learning. Behav Brain Res 196: 180–186. 10.1016/j.bbr.2008.09.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takehara K, Kawahara S, Kirino Y. 2003. Time-dependent reorganization of the brain components underlying memory retention in trace eyeblink conditioning. J Neurosci 23: 9897–9905. 10.1523/JNEUROSCI.23-30-09897.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira CM, Pomedli SR, Maei HR, Kee N, Frankland PW. 2006. Involvement of the anterior cingulate cortex in the expression of remote spatial memory. J Neurosci 26: 7555–7564. 10.1523/JNEUROSCI.1068-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorn CA, Atallah H, Howe M, Graybiel AM. 2010. Differential dynamics of activity changes in dorsolateral and dorsomedial striatal loops during learning. Neuron 66: 781–795. 10.1016/j.neuron.2010.04.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse D, Langston RF, Kakeyama M, Bethus I, Spooner PA, Wood ER, Witter MP, Morris RG. 2007. Schemas and memory consolidation. Science 316: 76–82. 10.1126/science.1135935 [DOI] [PubMed] [Google Scholar]
- Vetere G, Kenney JW, Tran LM, Xia F, Steadman PE, Parkinson J, Josselyn SA, Frankland PW. 2017. Chemogenetic interrogation of a brain-wide fear memory network in mice. Neuron 94: 363–374. 10.1016/j.neuron.2017.03.037 [DOI] [PubMed] [Google Scholar]
- Vetere G, Borreca A, Pignataro A, Conforto G, Giustizieri M, Marinelli S, Ammassari-Teule M. 2019. Coincident pre- and post-synaptic cortical remodelling disengages episodic memory from its original context. Mol Neurobiol 56: 8513–8523. 10.1007/s12035-019-01652-3 [DOI] [PubMed] [Google Scholar]
- Wheeler AL, Teixeira CM, Wang AH, Xiong X, Kovacevic N, Lerch JP, McIntosh AR, Parkinson J, Frankland PW. 2013. Identification of a functional connectome for long-term fear memory in mice. PLoS Comp Biol 9: e1002853. 10.1371/journal.pcbi.1002853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltgen BJ, Silva AJ. 2007. Memory for context becomes less specific with time. Learn Mem 14: 313–317. 10.1101/lm.430907 [DOI] [PubMed] [Google Scholar]
- Winocur G, Moscovitch M, Sekeres M. 2007. Memory consolidation or transformation: context manipulation and hippocampal representations of memory. Nat Neurosci 10: 555–557. 10.1038/nn1880 [DOI] [PubMed] [Google Scholar]
- Winocur G, Frankland PW, Sekeres MJ, Fogel S, Moscovitch M. 2009. Changes in context-specificity during memory reconsolidation: selective effects of hippocampal lesions. Learn Mem 16: 722–729. 10.1101/lm.1447209 [DOI] [PubMed] [Google Scholar]
- Wolff SB, Ko R, Ölveczky BP. 2022. Distinct roles for motor cortical and thalamic inputs to striatum during motor skill learning and execution. Sci Adv 8: eabk0231. 10.1126/sciadv.abk0231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Yu X, Perlik AJ, Tobin WF, Zweig JA, Tennant K, Jones T, Zuo Y. 2009. Rapid formation and selective stabilization of synapses for enduring motor memories. Nature 462: 915–919. 10.1038/nature08389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Serrano P, Yin X, Sun X, Lin Y, Chen SX. 2022. Functionally distinct NPAS4-expressing somatostatin interneuron ensembles critical for motor skill learning. Neuron 110: 3339–3355. 10.1016/j.neuron.2022.08.018 [DOI] [PubMed] [Google Scholar]
- Yin HH, Mulcare SP, Hilário MR, Clouse E, Holloway T, Davis MI, Hansson AC, Lovinger DM, Costa RM. 2009. Dynamic reorganization of striatal circuits during the acquisition and consolidation of a skill. Nat Neurosci 12: 333–341. 10.1038/nn.2261 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.