Abstract
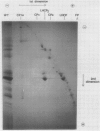
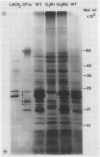
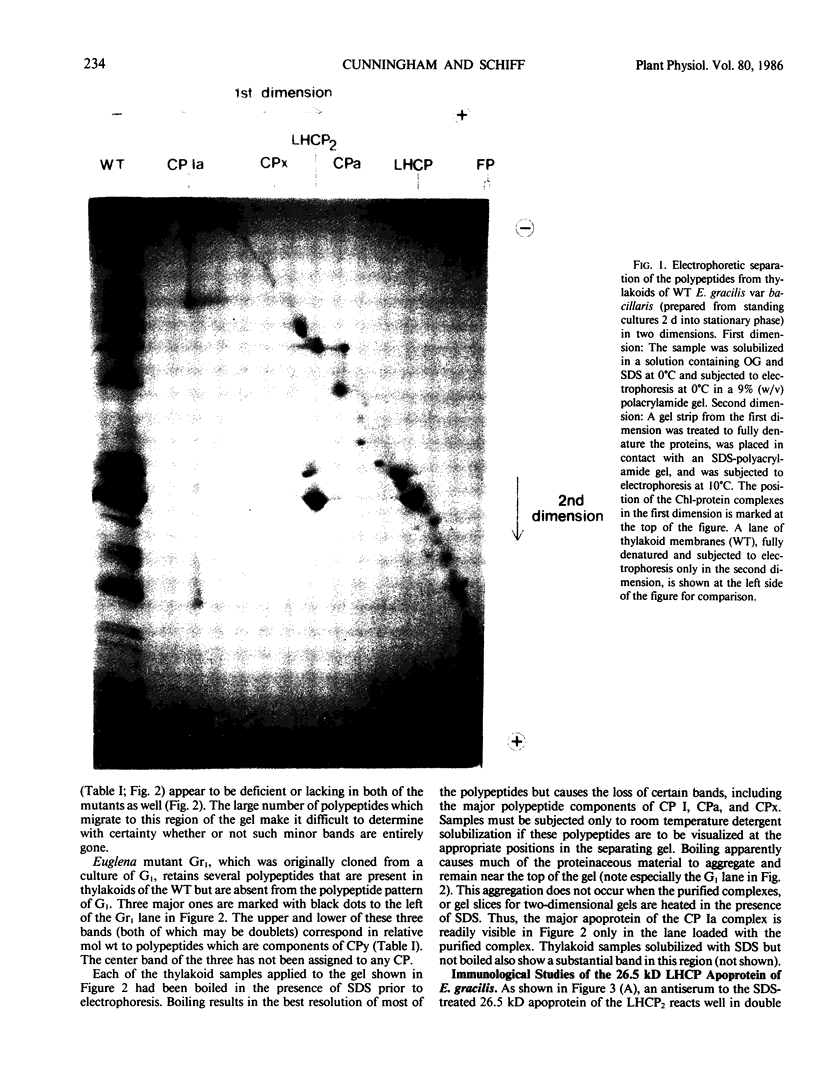
Chlorophyll-protein complexes (CPs) obtained from thylakoids of Euglena gracilis Klebs var bacillaris Cori contain the following polypeptides (listed in parentheses in order of prominence after Coomassie R-250 staining of polyacrylamide gels): CP Ia (66, 18, 22, 22.5, 27.5, 21, 28, 24, 25.5, and 26 kilodaltons [kD]); CP I (66 kD); CPx (41 kD); LHCP2 (an oligomer of LHCP) (26.5, 28, and 26 kD); CPy (27 and 19 kD); CPa (54 kD); and LHCP (26.5, 28, and 26 kD). Mutants of bacillaris low in chlorophyll b (Gr1BSL, G1BU, and O4BSL; Chl a/b [mol/mol] = 50-100) which lack CP Ia, LHCP2, and LHCP also lack or are deficient in polypeptides associated with these complexes in wild-type cells. Mutants G1 and O4, which also lack CPy, lack the CPy-associated polypeptides found in wild-type and Gr1. Using an antiserum which was elicited by and reacts strongly and selectively with the SDS-treated major polypeptide (26.5 kD) of the LHCP complexes of wild-type, this polypeptide is undetectable in the mutants (≪0.25% of the level in wild-type on a cell basis); the antiserum does not react with the SDS-treated 28 kD polypeptide of the Euglena LHCP complexes and cross-reacts only very weakly with components in SDS-treated cells of Chlamydomonas reinhardtii Dangeard and chloroplasts of Spinacia oleracea L. cv Winter Bloomsdale. Rates of photosynthesis of the wild-type and mutant cells of Euglena are approximately equal on a cell basis when measured at light saturation, consistent with the selective loss of major antenna components but not CP I or CPa from the mutants.
Full text
PDF



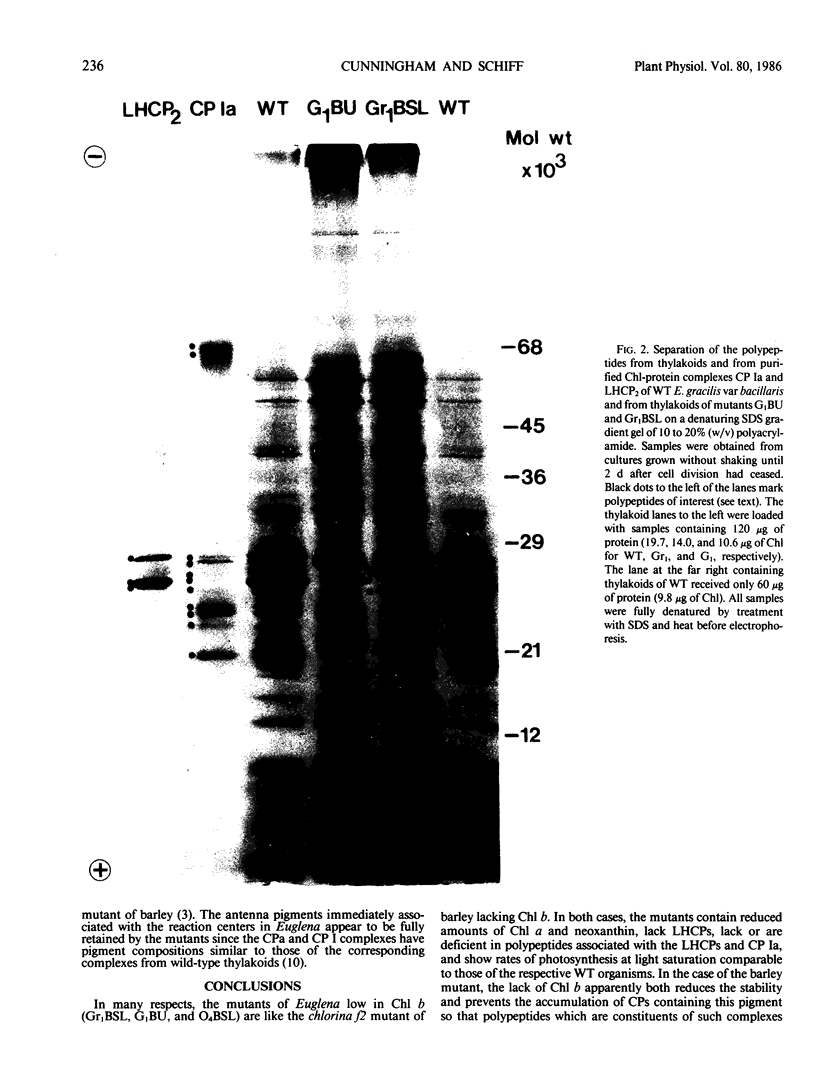
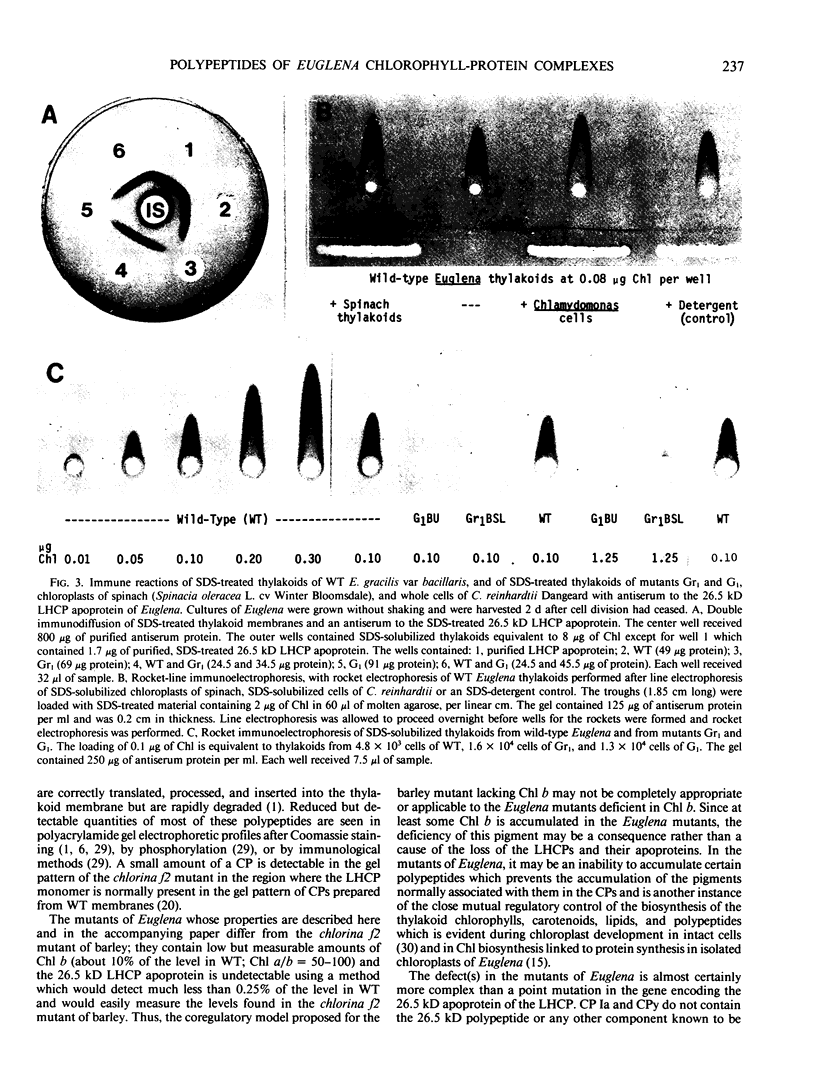

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellemare G., Bartlett S. G., Chua N. H. Biosynthesis of chlorophyll a/b-binding polypeptides in wild type and the chlorina f2 mutant of barley. J Biol Chem. 1982 Jul 10;257(13):7762–7767. [PubMed] [Google Scholar]
- Bingham S., Schiff J. A. Events surrounding the early development of Euglena chloroplasts. 16. Plastid thylakoid polypeptides during greening. Biochim Biophys Acta. 1979 Sep 11;547(3):531–543. doi: 10.1016/0005-2728(79)90032-x. [DOI] [PubMed] [Google Scholar]
- Boardman N. K., Highkin H. R. Studies on a barley mutant lacking chlorophyll b. I. Photochemical activity of isolated chloroplasts. Biochim Biophys Acta. 1966 Oct 10;126(2):189–199. doi: 10.1016/0926-6585(66)90054-9. [DOI] [PubMed] [Google Scholar]
- Brandt P., von Kessel B. Cooperation of cytoplasmic and plastidial translation in formation of the photosynthetic apparatus and its stage-specific efficiency. Plant Physiol. 1983 Jul;72(3):616–619. doi: 10.1104/pp.72.3.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke J. J., Steinback K. E., Arntzen C. J. Analysis of the Light-harvesting Pigment-Protein Complex of Wild Type and a Chlorophyll-b-less Mutant of Barley. Plant Physiol. 1979 Feb;63(2):237–243. doi: 10.1104/pp.63.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camm E. L., Green B. R. Widespread distribution of some minor chlorophyll-protein complexes in some plants and algae. Plant Physiol. 1981 May;67(5):1061–1063. doi: 10.1104/pp.67.5.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua N. H., Blomberg F. Immunochemical studies of thylakoid membrane polypeptides from spinach and Chlamydomonas reinhardtii. A modified procedure for crossed immunoelectrophoresis of dodecyl sulfate.protein complexes. J Biol Chem. 1979 Jan 10;254(1):215–223. [PubMed] [Google Scholar]
- Cunningham F. X., Schiff J. A. Chlorophyll-Protein Complexes from Euglena gracilis and Mutants Deficient in Chlorophyll b: I. Pigment Composition. Plant Physiol. 1986 Jan;80(1):223–230. doi: 10.1104/pp.80.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delepelaire P., Chua N. H. Electrophoretic purification of chlorophyll a/b-protein complexes from Chlamydomonas reinhardtii and spinach and analysis of their polypeptide compositions. J Biol Chem. 1981 Sep 10;256(17):9300–9307. [PubMed] [Google Scholar]
- Freyssinet G., Harris G. C., Nasatir M., Schiff J. A. Events Surrounding the Early Development of Euglena Chloroplasts: 14. Biosynthesis of Cytochrome c-552 in Wild Type and Mutant Cells. Plant Physiol. 1979 May;63(5):908–915. doi: 10.1104/pp.63.5.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Markwell J. P., Webber A. N., Lake B. Mutants of Sweetclover (Melilotus alba) Lacking Chlorophyll b: Studies on Pigment-Protein Complexes and Thylakoid Protein Phosphorylation. Plant Physiol. 1985 Apr;77(4):948–951. doi: 10.1104/pp.77.4.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Tolbert N. E., Bieber L. L. Protein determination in membrane and lipoprotein samples: manual and automated procedures. Methods Enzymol. 1981;72:296–303. doi: 10.1016/s0076-6879(81)72018-4. [DOI] [PubMed] [Google Scholar]
- Plumley F. G., Schmidt G. W. Rocket and crossed immunoelectrophoresis of proteins solubilized with sodium dodecyl sulfate. Anal Biochem. 1983 Oct 1;134(1):86–95. doi: 10.1016/0003-2697(83)90267-1. [DOI] [PubMed] [Google Scholar]
- Ryrie I. J. Immunological evidence for apoproteins of the light-harvesting chlorophyll-protein complex in a mutant of barley lacking chlorophyll b. Eur J Biochem. 1983 Mar 1;131(1):149–155. doi: 10.1111/j.1432-1033.1983.tb07242.x. [DOI] [PubMed] [Google Scholar]