Abstract
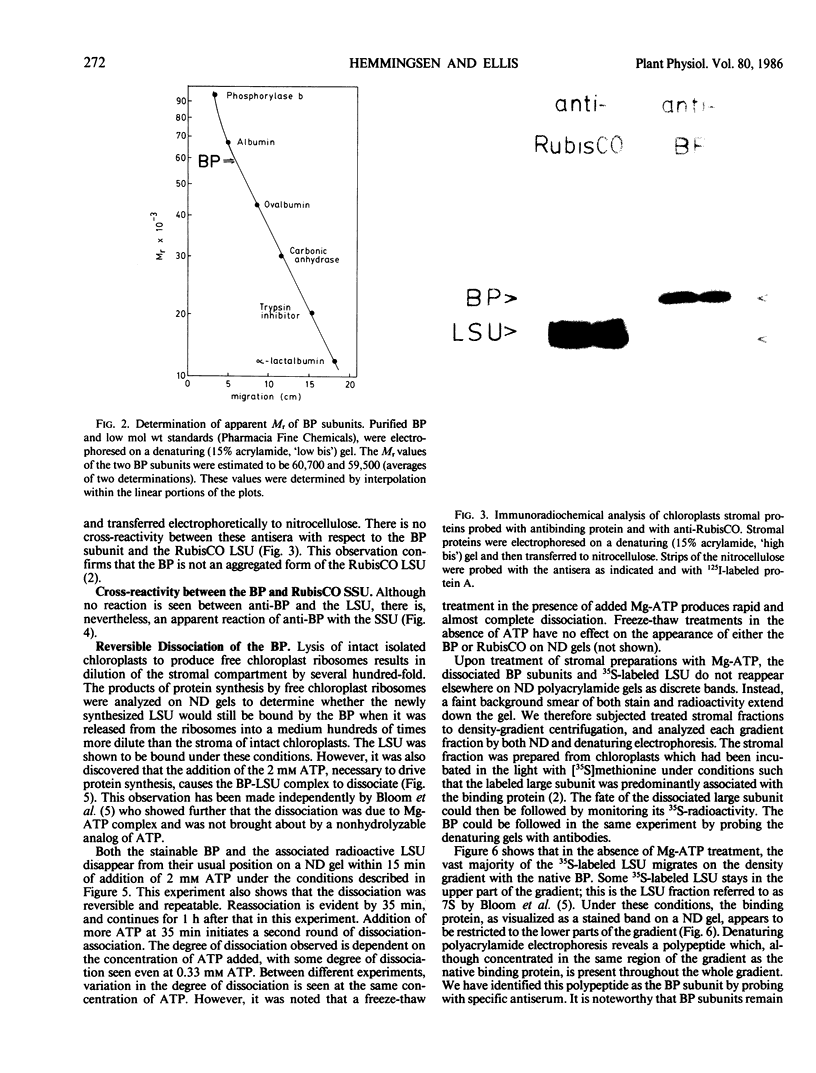
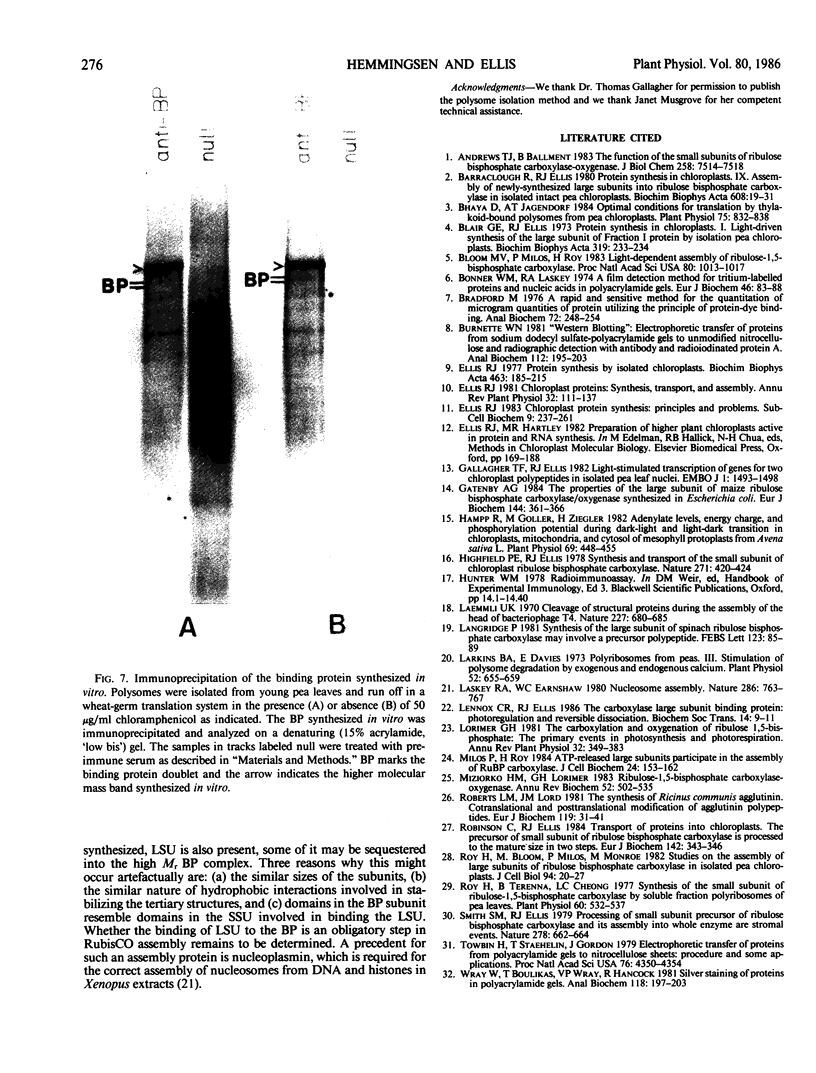
The large subunit binding protein, an abundant plastid protein implicated in the assembly of ribulose-1,5-bisphosphate carboxylase-oxygenase (RubisCO), has been highly purified from leaves of Pisum sativum. The 720 kilodaltons purified binding protein is composed of two types of subunits of 60 and 61 kilodaltons. Highly specific polyclonal antibodies have been raised against the binding protein. The antibodies do not cross-react with the large subunit nor do anti-RubisCO antibodies cross-react with the binding protein. A higher molecular weight form of the binding protein is immunoprecipitated from products of P. sativum polysomes translated in a wheat-germ system, indicating that the binding protein is synthesized by cytoplasmic ribosomes. Immunoblotting reveals the presence of binding protein in extracts of tobacco, wheat and barley leaves and castor bean endosperm.
The previously reported dissociation of the binding protein-large subunit complex upon addition of ATP in vitro has been confirmed and the fates of the dissociated subunits further investigated. The dissociated binding protein subunits are not phosphorylated or adenylated in vitro by added ATP.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews T. J., Ballment B. The function of the small subunits of ribulose bisphosphate carboxylase-oxygenase. J Biol Chem. 1983 Jun 25;258(12):7514–7518. [PubMed] [Google Scholar]
- Barraclough R., Ellis R. J. Protein synthesis in chloroplasts. IX. Assembly of newly-synthesized large subunits into ribulose bisphosphate carboxylase in isolated intact pea chloroplasts. Biochim Biophys Acta. 1980 Jun 27;608(1):19–31. doi: 10.1016/0005-2787(80)90129-x. [DOI] [PubMed] [Google Scholar]
- Bhaya D., Jagendorf A. T. Optimal conditions for translation by thylakoid-bound polysomes from pea chloroplasts. Plant Physiol. 1984 Jul;75(3):832–838. doi: 10.1104/pp.75.3.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair G. E., Ellis R. J. Protein synthesis in chloroplasts. I. Light-driven synthesis of the large subunit of fraction I protein by isolated pea chloroplasts. Biochim Biophys Acta. 1973 Aug 24;319(2):223–234. doi: 10.1016/0005-2787(73)90013-0. [DOI] [PubMed] [Google Scholar]
- Bloom M. V., Milos P., Roy H. Light-dependent assembly of ribulose-1,5-bisphosphate carboxylase. Proc Natl Acad Sci U S A. 1983 Feb;80(4):1013–1017. doi: 10.1073/pnas.80.4.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Ellis R. J. Chloroplast protein synthesis: principles and problems. Subcell Biochem. 1983;9:237–261. doi: 10.1007/978-1-4613-3533-7_2. [DOI] [PubMed] [Google Scholar]
- Gallagher T. F., Ellis R. J. Light-stimulated transcription of genes for two chloroplast polypeptides in isolated pea leaf nuclei. EMBO J. 1982;1(12):1493–1498. doi: 10.1002/j.1460-2075.1982.tb01345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatenby A. A. The properties of the large subunit of maize ribulose bisphosphate carboxylase/oxygenase synthesised in Escherichia coli. Eur J Biochem. 1984 Oct 15;144(2):361–366. doi: 10.1111/j.1432-1033.1984.tb08472.x. [DOI] [PubMed] [Google Scholar]
- Hampp R., Goller M., Ziegler H. Adenylate Levels, Energy Charge, and Phosphorylation Potential during Dark-Light and Light-Dark Transition in Chloroplasts, Mitochondria, and Cytosol of Mesophyll Protoplasts from Avena sativa L. Plant Physiol. 1982 Feb;69(2):448–455. doi: 10.1104/pp.69.2.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larkins B. A., Davies E. Polyribosomes from Peas: III. Stimulation of Polysome Degradation by Exogenous and Endogenous Calcium. Plant Physiol. 1973 Dec;52(6):655–659. doi: 10.1104/pp.52.6.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskey R. A., Earnshaw W. C. Nucleosome assembly. Nature. 1980 Aug 21;286(5775):763–767. doi: 10.1038/286763a0. [DOI] [PubMed] [Google Scholar]
- Lennox C. R., Ellis R. J. The carboxylase-large-subunit-binding protein: photoregulation and reversible dissociation. Biochem Soc Trans. 1986 Feb;14(1):9–11. doi: 10.1042/bst0140009. [DOI] [PubMed] [Google Scholar]
- Milos P., Roy H. ATP-released large subunits participate in the assembly of RuBP carboxylase. J Cell Biochem. 1984;24(2):153–162. doi: 10.1002/jcb.240240206. [DOI] [PubMed] [Google Scholar]
- Miziorko H. M., Lorimer G. H. Ribulose-1,5-bisphosphate carboxylase-oxygenase. Annu Rev Biochem. 1983;52:507–535. doi: 10.1146/annurev.bi.52.070183.002451. [DOI] [PubMed] [Google Scholar]
- Roberts L. M., Lord J. M. The synthesis of Ricinus communis agglutinin, cotranslational and posttranslational modification of agglutinin polypeptides. Eur J Biochem. 1981 Sep;119(1):31–41. doi: 10.1111/j.1432-1033.1981.tb05573.x. [DOI] [PubMed] [Google Scholar]
- Robinson C., Ellis R. J. Transport of proteins into chloroplasts. The precursor of small subunit of ribulose bisphosphate carboxylase is processed to the mature size in two steps. Eur J Biochem. 1984 Jul 16;142(2):343–346. doi: 10.1111/j.1432-1033.1984.tb08292.x. [DOI] [PubMed] [Google Scholar]
- Roy H., Bloom M., Milos P., Monroe M. Studies on the assembly of large subunits of ribulose bisphosphate carboxylase in isolated pea chloroplasts. J Cell Biol. 1982 Jul;94(1):20–27. doi: 10.1083/jcb.94.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy H., Terenna B. Synthesis of the Small Subunit of Ribulose-1,5-bisphosphate Carboxylase by Soluble Fraction Polyribosomes of Pea Leaves. Plant Physiol. 1977 Oct;60(4):532–537. doi: 10.1104/pp.60.4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]








