Ventricular septal perforation with dissection following takotsubo cardiomyopathy.
Central Message.
The timing of surgery for interventricular septal dissection complicated by perforation should be carefully considered because of the fragile interventricular septum.
Interventricular septal dissection (IVSD) is a rare condition of the interventricular septum (IVS) that mainly occurs following an aneurysm of the sinuses of Valsalva, bacterial endocarditis, trauma, cardiac surgery, endomyocardial biopsy, congenital myocardial developmental anomalies, or myocardial infarction.1 IVSD following takotsubo cardiomyopathy (TC) is rare.2 The IVS is sometimes perforated, creating a shunt.
We report a case of IVSD complicated by ventricular septal perforation (VSP) following TC (institutional review board 1158 and August 14, 2023). Written informed consent could not be obtained from the patient because of the operative death.
Case Report
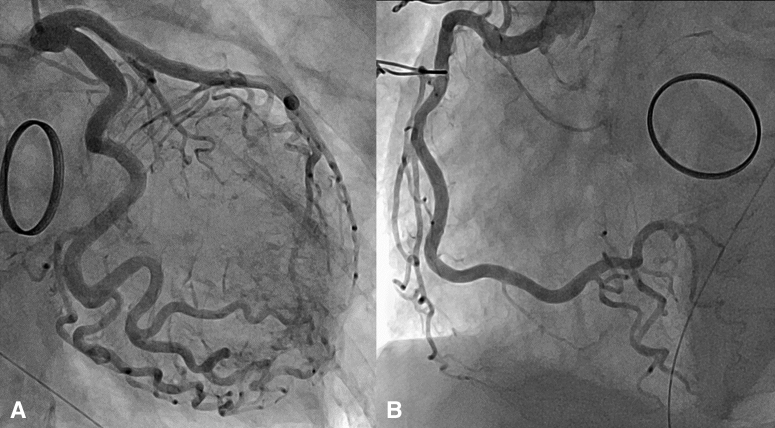
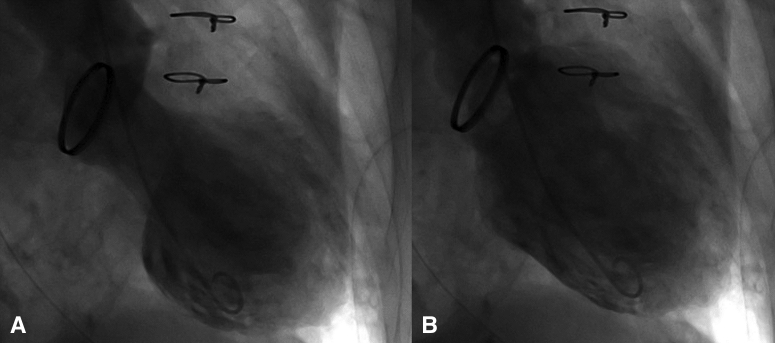
An 82-year-old woman with a history of mechanical mitral valve replacement for infective endocarditis 7 years earlier was transferred to our hospital with complaints of chest discomfort and nausea. Electrocardiography revealed ST-segment elevations in V1-6. However, coronary angiography revealed no lesions, and acute coronary syndrome was ruled out (Figure E1). Left ventriculography revealed apical akinesis (Figure E2), even though her echocardiography 9 months earlier showed normal function of left ventricle (LV). The patient was diagnosed with apical ballooning-type TC and admitted to the intensive care unit.
Figure E1.
Coronary angiography of (A) the left coronary artery and (B) the right coronary artery revealing no lesions.
Figure E2.
Left ventriculography of (A) systole and (B) diastole showing apical akinesis.
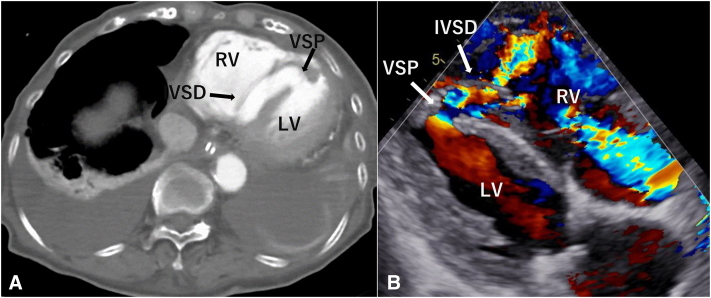
The following day, lactic acidosis progressed and contrast-enhanced computed tomography (CT) revealed an extensive IVSD (Figure 1, A). Transthoracic echocardiography revealed IVSD with a shunt from the LV to the right ventricle (RV) through the VSP (Figure 1, B). Percutaneous cardiopulmonary support with an Impella CP (Abiomed, Inc) was initiated because the patient was in shock.
Figure 1.
A, Contrast-enhanced CT demonstrating extensive IVSD complicated with VSP. B, Transthoracic echocardiography showing the dissection of the IVS in color Doppler indicating plural flows through the VSP, near the apex. VSP, Ventricular septal perforation; RV, right ventricle; IVSD, interventricular septal dissection; LV, left ventricle.
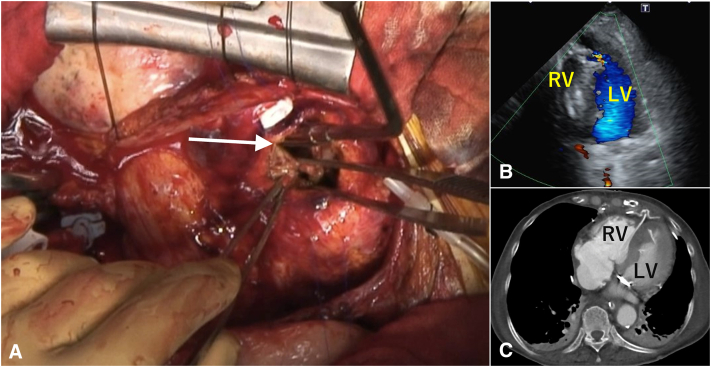
Resternotomy was performed 12 days after diagnosis. We removed the Impella CP and inserted an intra-aortic balloon pump (IABP) 1 hour preoperatively to prevent postoperative bleeding. Under cardiopulmonary bypass with moderate hypothermia, the RV was longitudinally incised 1 cm away from the left anterior descending coronary artery. The ventricular septal wall was dissected with a single tear (1 × 1 cm) into the LV (Figure 2, A). The dissected septum was sufficiently stiff to sew double patches. We performed the “sandwich technique” via an RV incision to repair the VSP.3 We inserted a tailored 1.85-mm Bard polytetrafluoroethylene felt (Becton Dickinson) into the LV. BioGlue Surgical Adhesive (CryoLife) was inserted into the defect. Bard polytetrafluoroethylene felt (1.65 mm) and an Edwards bovine pericardial patch (Edwards Lifesciences) were pasted together. The sheet was cut to use on the RV side. Cardiopulmonary bypass was smoothly weaned using IABP. Transesophageal echocardiography performed during the surgery revealed no residual shunts.
Figure 2.
A, Intraoperative image of a single tear (1 cm × 1 cm) of the ventricular septal wall just under the incision (arrow). The left ventricular septum around the ostium of the dissection tract is not fragile and reveals no ischemic change. B, Postoperative echocardiography showing no shunt after VSP repair. C, Contrast-enhanced CT showing successful VSP repair. RV, Right ventricle; LV, left ventricle.
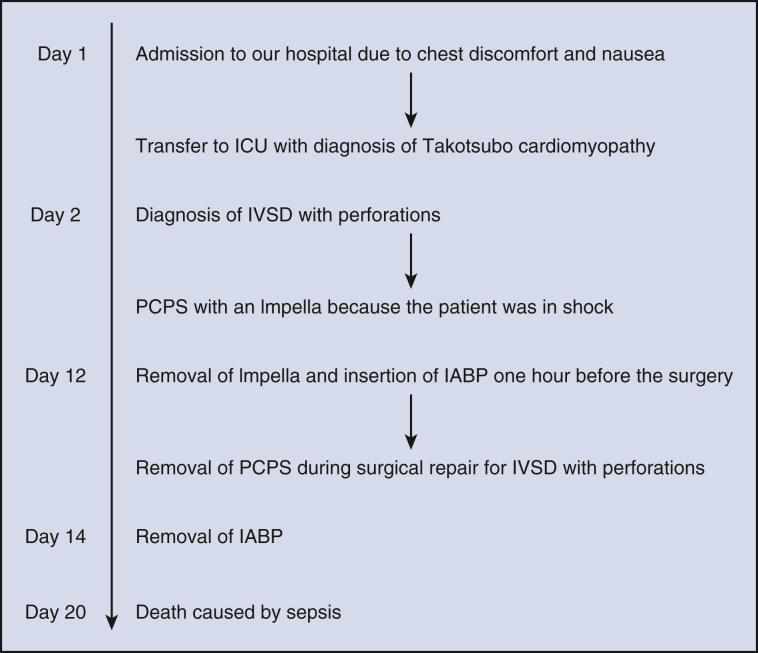
Postoperative hemodynamics were stable; the IABP was removed on postoperative day 2, and CT revealed pneumonia; broad-spectrum antibiotics were immediately initiated. However, 3 days later, CT showed deterioration of pneumonia, a new pulmonary abscess, and fistula due to abscess rupture. Postoperative echocardiography revealed improved contractility of the LV apex and no shunt (Figure 2, B). Contrast-enhanced CT revealed successful VSP repair (Figure 2, C). Despite antibiotic treatment, the patient developed septic shock and died on postoperative day 8 (Figure E3).
Figure E3.
Sequence of events from the admission to postoperative death. ICU, Intensive care unit; IVSD, interventricular septal dissection; PCPS, percutaneous cardiopulmonary support; IABP, intra-aortic balloon pump.
Discussion
The optimal surgical timing for VSP complicated by extended IVSD following TC is unknown because of limited reports. A VSP with dissected IVS is more fragile than an isolated VSP. Miyake and colleagues4 reported fragile and necrotic tissues around the VSP area in an emergency surgical case, whereas relatively firm tissues were observed around the VSP area in an elective surgery case. Furthermore, in most patients with TC, cardiac function improves with conservative therapy. They performed elective surgery 13 days after VSP onset following TC, and the result was uneventful, with no residual shunt.4 In their case, onset of VSP with IVSD occurred 1 day after a diagnosis of apical ballooning-type TC, and the rupture site was anterior. However, their patient had not needed catecholamines or mechanical circulatory support until the surgery.
In our case, we were apprehensive that a secure repair would be extremely difficult because the dissected area extended to almost the entire IVS. In addition, LV function was severely impaired because of TC. Although the patient developed cardiogenic shock at VSP onset, she remained stable after we introduced percutaneous cardiopulmonary support and Impella CP. LV function improved 4 days after VSP onset. However, surgery was delayed until the dissected IVS was sufficiently durable to enable secure repair.
Furui and colleagues5 reported that delayed surgeries for VSP were associated with low reoperation rates for residual shunt, recurrent VSP, LV pseudoaneurysm, and hospital mortality. Conversely, there is a high risk of preoperative intravenous catheter infections, atelectasis, and pneumonia resulting from prolonged immobility.5
Our surgical repair was completed because of sufficient durable tissues around the VSP. However, perioperative infections could not be controlled. If we had performed surgical treatment earlier, we might have prevented this infection. The timing of surgery should be decided based on the advantages of ensuring a secure repair, depending on the etiology, and the disadvantages associated with surgery delay.
Conflict of Interest Statement
The authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Appendix E1
References
- 1.Gu X., He Y., Luan S., Zhao Y., Sun L., Zhang H., et al. Dissection of the interventricular septum: echocardiographic features. Medicine. 2017;96:e6191. doi: 10.1097/MD.0000000000006191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mariscalco G., Cattaneo P., Rossi A., Baravelli M., Piffaretti G., Scannapieco A., et al. Tako-tsubo cardiomyopathy complicated by ventricular septal perforation and septal dissection. Heart Vessels. 2010;25:73–75. doi: 10.1007/s00380-009-1167-9. [DOI] [PubMed] [Google Scholar]
- 3.Isoda S., Osako M., Kimura T., Mashiko Y., Yamanaka N., Nakamura S., et al. Midterm results of the “sandwich technique” via a right ventricle incision to repair post-infarction ventricular septal defect. Ann Thorac Cardiovasc Surg. 2012;18:318–321. doi: 10.5761/atcs.oa.11.01703. [DOI] [PubMed] [Google Scholar]
- 4.Miyake K., Funatsu T., Kondoh H., Taniguchi K. Rare complication of takotsubo cardiomyopathy: ventricular septal perforation with septal dissection. J Card Surg. 2016;31:150–153. doi: 10.1111/jocs.12676. [DOI] [PubMed] [Google Scholar]
- 5.Furui M., Sakurai Y., Kakii B., Asanuma M., Nishioka H., Yoshida T. Benefits and risks of delayed surgery for ventricular septal rupture after acute myocardial infarction. Int Heart J. 2022;63:433–440. doi: 10.1536/ihj.21-581. [DOI] [PubMed] [Google Scholar]