Abstract
Objective
Donation after circulatory death (DCD) procurement and transplantation after thoracoabdominal normothermic regional perfusion (TA-NRP) remains a novel technique to improve cardiac and hepatic allograft preservation but may be complicated by lung allograft pulmonary edema. We present a single-center series on early implementation of a lung-protective protocol with strategies to mitigate posttransplant pulmonary edema in DCD lung allografts after TA-NRP procurement.
Methods
Data from all lung transplantations performed using a TA-NRP procurement strategy from October 2022 to April 2023 are presented. Donor management consisted of key factors to reduce lung allograft pulmonary edema: aggressive predonation and early posttransplant diuresis, complete venous drainage at TA-NRP initiation, and early pulmonary artery venting upon initiation of systemic perfusion. Donor and recipient characteristics, procurement characteristics such as TA-NRP intervals, and 30-day postoperative outcomes were assessed.
Results
During the study period, 8 lung transplants were performed utilizing TA-NRP procurement from DCD donors. Donor ages ranged from 16 to 39 years and extubation time to declaration of death ranged from 10 to 90 minutes. Time from declaration to TA-NRP initiation was 7 to 17 minutes with TA-NRP perfusion times of 49 to 111 minutes. Median left and right allograft warm ischemia times were 55.5 minutes (interquartile range, 46.5-67.5 minutes) and 41.0 minutes (interquartile range, 39.0-53.0 minutes, respectively, with 2 recipients supported with cardiopulmonary bypass or venoarterial extracorporeal membrane oxygenation during implantation. No postoperative extracorporeal membrane oxygenation was required. There were no pulmonary-related deaths; however, 1 patient died from complications of severe necrotizing pancreatitis with a normal functioning allograft. All patients were extubated within 24 hours. Index intensive care unit length of stay ranged from 3 to 11 days with a hospital length of stay of 13 to 37 days.
Conclusions
Despite concern regarding quality of DCD lung allografts recovered using the TA-NRP technique, we report initial success using this procurement method. Implementation of strategies to mitigate pulmonary edema can result in acceptable outcomes following lung transplantation. Demonstration of short- and long-term safety and efficacy of this technique will become increasingly important as the use of TA-NRP for thoracic and abdominal allografts in DCD donors expands.
Key Words: thoracoabdominal normothermic regional perfusion, donation after circulatory death, lung transplantation
Graphical abstract
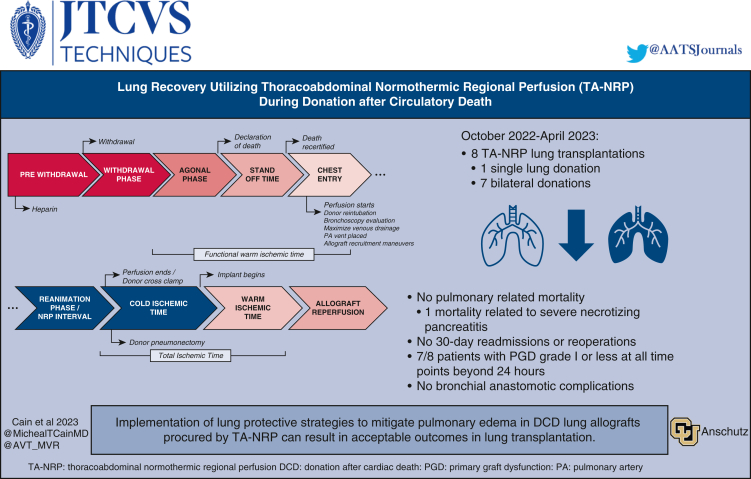
Schematic demonstration of the TA-NRP donation timeline with key time points outlined.
Central Message.
We report initial success with DCD lung allografts recovered using the TA-NRP technique with strategies to mitigate pulmonary edema that result in acceptable lung transplantation outcomes.
Perspective.
As the use of TA-NRP for recovery of thoracic and abdominal organs in DCD donors expands, it is increasingly important to optimize this technique to protect lung allografts from any potential insult and thus improve efficiency in donor yield. Here we demonstrate encouraging early results with a lung protective strategy used during TA-NRP DCD lung recovery.
See Discussion on page 359.
Donation after circulatory death (DCD) has been increasingly utilized for thoracic organ transplantation as a strategy to expand the heart and lung donor pool. DCD lung procurement has shown similar outcomes to donation after brain death (DBD), despite a longer donor ischemia time, with no differences in mortality or primary graft dysfunction (PGD).1, 2, 3 Concerns have arisen with expansion of DCD heart and abdominal transplantation regarding the influence of warm ischemia time on these more metabolically active organs that serve as a barrier to broader use.4 Abdominal grafts obtained through DCD procurement have been associated with a higher risk of ischemic cholangiopathy resulting in more biliary complications, retransplantation, and higher mortality than those procured from DBD donors.1,5
Thoracoabdominal normothermic regional perfusion (TA-NRP) has emerged as an alternative strategy to direct procurement DCD, with or without ex vivo perfusion, to limit donor warm ischemia time while allowing for in situ examination of the donor graft function after reinstitution of thoracoabdominal flow with extracorporeal membrane oxygenation (ECMO).6,7 Early results have been promising for cardiac allografts, and adoption of this technique has expanded across the United States.6, 7, 8, 9, 10 Utilization of TA-NRP for abdominal allograft recovery has also demonstrated distinct benefits, with reductions in biliary complications, rates of graft failure, and PGD in comparison to ex vivo perfusion or direct procurement DCD.11 Despite these advantages, TA-NRP in lung recovery remains understudied and hesitancy remains in utilizing lungs procured via this method due to concerns over pulmonary edema, which may negatively influence lung graft function. With the growing utilization of TA-NRP as a strategy for DCD cardiac recovery and abdominal recovery, it will become increasingly important to evaluate the feasibility of lung recovery utilizing this technique and to optimize protocols for DCD lung procurement utilizing TA-NRP to reduce PGD.
Here we present a single-institution experience demonstrating outcomes after implementation of a lung-protective protocol for TA-NRP DCD lung transplantation to mitigate posttransplant pulmonary edema in DCD lung allografts. We aim to describe early postoperative outcomes with this technique and highlight key technical points we believe are important to optimize graft function.
Methods
A retrospective review of a prospectively collected single institution TA-NRP database was conducted. All patients older than age 18 years who received single or bilateral orthotopic lung transplant using a DCD TA-NRP procurement strategy from October 2022 (TA-NRP program start date) to April 2023 were included for analysis. Donor and patient characteristics were evaluated in addition to procurement and intraoperative surgical characteristics. Perioperative and early postoperative outcomes were evaluated with specific focus on mortality, pulmonary-related mortality, and early postoperative pulmonary function utilizing International Society for Heart and Lung Transplantation 2016 consensus guidelines for definition of PGD.12 This study was evaluated and approved by the local institutional review board (No. 20-0081; approved April 12, 2023).
Statistical Analysis
This was primarily a descriptive study, and collected demographic and hemodynamic values were reported as mean ± SD or median with corresponding interquartile range (IQR) as dictated by the dataset distribution. Variables were checked for the Gaussian distribution using normal plots and using D'Agostino-Pearson, Shapiro-Wilk, and Kolmogorov-Smirnov tests. Given the small sample size, a nonparametric data distribution was observed.
Donor Evaluation
DCD lung donors are evaluated similarly to any other donor with several important caveats. Given limitations in the ability to perform predonation recruitment maneuvers such as therapeutic bronchoscopy, prone positioning, and mechanical ventilatory recruitment, a slightly lower partial pressure of oxygen, arterial, to fraction of inspired oxygen ratio (P:F) cutoff >200 is allowed. Despite this, preoperative imaging in the form of chest radiograph, and chest computed tomography (when clinically indicated) should still demonstrate no consolidations, gross anatomical abnormalities, or evidence of aspiration. Any bronchial alveolar lavage or sputum cultures obtained are evaluated, and procedural reports from any therapeutic bronchoscopy are reviewed.
Predonation Allograft Management and Intraoperative Evaluation
Suitable donors should have robust urine output or receive diuresis with 20 to 40 mg furosemide every 8 hours to aid in counteracting the expected pulmonary edema related to their critical condition. This will further aid in preventing overall pulmonary edema following TA-NRP and cold static storage.
Once TA-NRP perfusion commences, evaluation of the lung allograft mirrors any other procurement. Endobronchial anatomy and presence of endobronchial pathology is assessed after reintubation. Visual inspection includes assessment for damage, contusions, consolidations, nodules, inadequate elastic recoil, or aberrant anatomy. The pleural space is similarly evaluated for pathologic findings, including the pleural fluid quality. Atelectasis is recruited. If concern exists for the ability of an individual lung lobe to oxygenate blood, pulmonary vein sampling after weaning completely from TA-NRP may be performed. This sampling should be deferred until complete weaning from TA-NRP because the circuit flow and oxygenator will confound interpretation of these blood gases. Our practice does not include routine assessment of individual lobar blood gasses but rather assessment of the lung in total.
Withdrawal and Procurement Policy
Our institution's policy on donor withdrawal and allograft procurement policy has been described previously.7 Briefly, decisions regarding withdrawal of life support and organ donation are made by a patient's family in consultation with the patient's medical team. The choice to pursue organ donation is independent of the decision to proceed with withdrawal of care and does not influence the continued medical care received by a patient before organ donation.
Location of withdrawal of care, preparation of a donor for the operating room, timing of heparin administration, and the length of the standoff period are dictated by the local organ procurement organization and recovery hospital policy. A predonation conference with all participating teams ensures efficiency in maximizing organ donation while adhering to the highest technical and ethical standards. It is important to coordinate with any other procuring teams and agree on an organized approach to procurement. Whereas TA-NRP can adequately support all thoracoabdominal grafts as a standalone strategy, any planned use of organ care systems or direct procurement should be discussed among all team because these can alter surgical approach and/or require a significant autologous blood prime, which influences implementation TA-NRP. Our policy is to independently confirm asystole after declaration of death and before incision. Additionally, a pre-reperfusion time out is performed before initiation of TA-NRP where interruption of cerebral circulation is confirmed.
Ethical Considerations
During DCD, a patient is declared dead by circulatory standards. This standard includes irreversible cessation of circulatory and respiratory function. In the United States, the circulatory standard for declaration of death is absolute and therefore the so-called dead donor rule is respected. Using the TA-NRP technique, circulatory death is permanent and physiologic function is not spontaneously restored. After circulatory death, spontaneous resuscitation with a sustained blood pressure and blood flow to support physiologic needs is not possible and has never been reported. However, in all DCD cases a mandatory no-touch period is observed, thereby eliminating the theoretical risk of autoresuscitation. A more thorough description of the ethical considerations are outlined by Entwistle and colleagues.13
Procurement Technique and Graft Evaluation
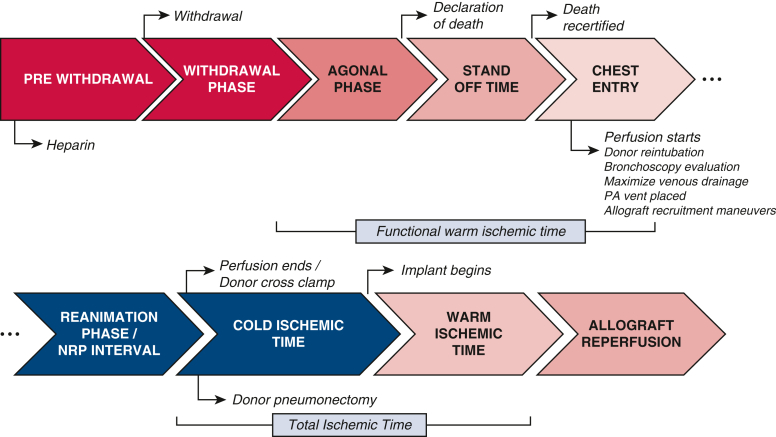
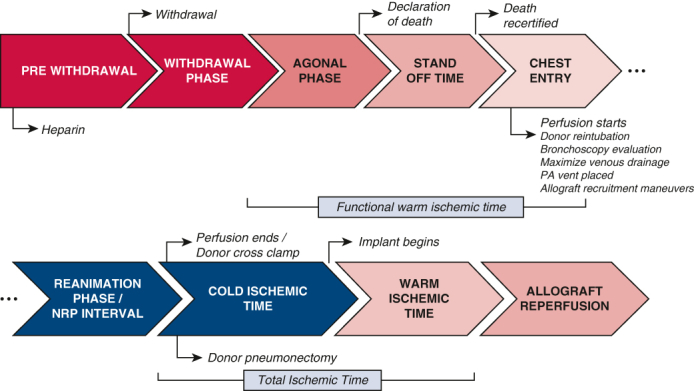
A schematic of the donation timeline is provided in Figure 1. The patient is anticoagulated with 30,000 U heparin at a minimum of 3 minutes before terminal extubation. Following declaration of death by an independent provider and appropriate standoff period as determined by local institutional protocols, the sternum is opened over the midline and retractor is placed. A pericardial incision is made and continued cranially up to the pericardial reflection at the proximal aortic arch where the innominate vein is retracted cranially or divided with a vascular stapler, if not being procured with the heart. The brachiocephalic trunk, left carotid artery, and left subclavian artery are then exposed and clamped. A right atriotomy is then performed and a dual stage venous cannula is placed. At this point, venous drainage is started. An aortic cannula is inserted at the proximal aorta arch and de-aired carefully, and TA-NRP is initiated. The donor is then reintubated early in the course of TA-NRP, and ventilation is started with a tidal volume of 6 to 8 mL/kg body weight, positive end-expiratory pressure of 5 cm H2O, and fraction of inspired oxygen of 0.5. Peripheral arterial monitoring is often unreliable, and so an aortic root cannula with a vent line is inserted and connected to a pressure transducer to guide pressure management. A right-angle metal tip cannula is inserted into the main pulmonary artery and connected the drainage line to aid in pulmonary artery decompression during TA-NRP to augment pulmonary edema. This same cannula is used for the pulmonary preservation solution administration/flush following TA-NRP. Once support is stabilized, allograft evaluation commences as noted above.
Figure 1.
Schematic demonstration the thoracoabdominal normothermic regional perfusion (NRP) donation timeline with key time points outlined. Agonal phase: A variable phase is defined by different hemodynamic parameters in each organ. As a result, at our center for both donation after circulatory death NRP heart and lung procurement, we no longer define agonal hemodynamics and instead favor terminal extubation and waiting until declaration or recovery is terminated by the time limit set by the hospital and/or the organ procurement organization, whichever comes first. PA, Pulmonary artery.
Complications and risks during TA-NRP procurement center largely around the need for expedient sternotomy and cannulation, including risk for inferior vena cava or hepatic venous injury during atrial cannulation, aortic hematoma or dissection during cannulation, arterial cannula malposition, and right ventricular laceration or pulmonary laceration during sternotomy and pericardiotomy. Predonation arrhythmia is also a potential risk related to coronary air embolism during rapid cannulation. These risks are minimized through clear predonation communication between all procuring surgeons clarifying the technical approach, and through employing a dedicated procurement teams experienced with the technique.
We aim for an ideal TA-NRP duration of 45 to 60 minutes to allow for graft recovery and limit the theoretical risk of pulmonary edema accumulation. Competing interests exist in multivisceral procurement, and extended durations of TA-NRP from 90 to 120 minutes may be preferred for abdominal procurement. We have found a compromise of 60 to 80 minutes to be reasonable to prevent the theoretical risk of excessive pulmonary edema. Following TA-NRP, the aorta is crossclamped, the lungs are flushed with 4 L Perfadex (Xvivo) and 500 μg prostaglandin E1 in the standard fashion through the previously placed pulmonary arterial cannula. Ventilation is continued until the lungs are excised. An extra 1 to 2 L Perfadex is given retrograde via the pulmonary veins on back table to minimize risk of retained pulmonary emboli.
TA-NRP Circuit and Management
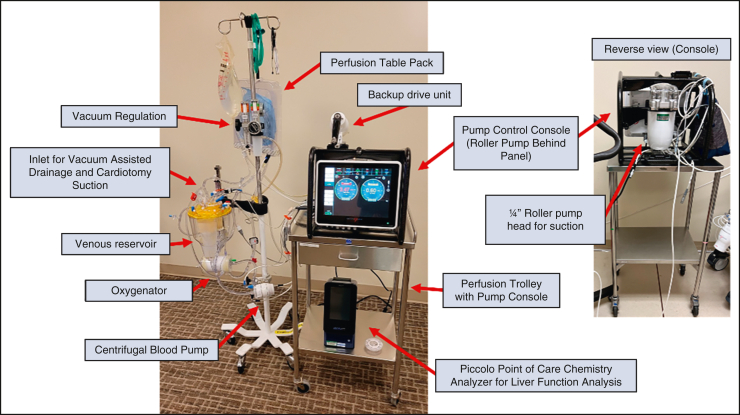
Our TA-NRP platform is based on the Spectrum Medical ECLS hardware platform (Spectrum Medical Inc). This portable extracorporeal life support platform consists of a perfusion interface workstation mounted on a carbon fiber frame with the ability to operate both a centrifugal pump and a roller pump to provide independent blood suction and venting capability independent of arterial blood flow management. This platform also incorporates an integrated data management system that creates an electronic record of the NRP procedure. The extracorporeal circuit contains a standard cardiopulmonary bypass (CPB) hard shell venous reservoir and membrane oxygenator (LivaNova), centrifugal blood pump, and three-eighths-inch venous arterial tubing loop. Venous return is controlled using regulated vacuum applied to the venous reservoir (Boehringer Laboratories) (Figure 2).
Figure 2.
Standard thoracoabdominal normothermic regional perfusion circuit. The use of a roller pump in our circuit facilitates the return of blood to the circuit without compromising suction to the blood reservoir.
The circuit is primed with 800 mL Plasmalyte A (Baxter), 50 mEq 8.4% sodium bicarbonate, 10,000 U heparin, 500 mg methylprednisone, and 200 mg rocuronium. After sternotomy a 36 to 46 venous cannula (Edwards Lifesciences) is placed, and 1000 to 1500 mL blood is collected via the venous line to the CPB reservoir. This blood is recirculated and oxygenated through a recirculation line integrated in the circuit. After isolation of the arch vessels and placement of a 21 Fr aortic cannula, the recirculation line is clamped and CPB is initiated. Blood flow is based on an adjusted cardiac index target of 1.8 to 2.0 L/minute/m2 body surface area and adjusted to normalize oxygen delivery and maintain the mixed venous saturation >70%. Serial blood gas and electrolyte analysis are performed to normalize blood chemistries. Blood pressure is supported with bolus injections of phenylephrine or vasopressin to maintain a mean arterial pressure >50 mm Hg.
Results
During the study period, 8 patients underwent lung transplantation using a TA-NRP donation strategy with 1 single left lung donation and 7 bilateral donations. Donor characteristics are shown in Table 1 and demonstrate a median age of 32.5 years (IQR, 26.0-37.0 years) and male gender in 4 out of 8 (50%) donors. Trauma was the most frequent cause of intensive care unit admission for donors (4 out of 8; 50.0%). Predonation gas exchange demonstrated a peak median P:F of 493 (IQR, 424.0-542.5). All donors had normal anatomy and thin secretions on bronchoscopic evaluation.
Table 1.
Donor baseline demographics (n = 8)
| Donor characteristic | Result |
|---|---|
| Age (y) | 32.5 (26.0-37.0) |
| Male sex | 4 (50) |
| Race/ethnicity | |
| White | 6 (83) |
| Black | 1 (12.5) |
| Hispanic | 1 (12.5) |
| Other | 0 |
| Cause of death | |
| Anoxia | 1 (12.5) |
| Cerebrovascular/stroke or head trauma | 2 (25.0) |
| Trauma | 4 (50.0) |
| Other | 1 (12.5) |
| Abnormal chest radiograph | 4 (50.0) |
| ≥20 pack-year smoking history | 0 |
| Highest P:F | 493 (424.0-542.5) |
| Hepatitis C + | 0 |
| Donor BMI | 22.8 (19.7-23.1) |
| Abnormal donor bronchoscopy? | 0 |
| Lung procured/transplanted | |
| Bilateral | 7 (87.5) |
| Isolated right | 0 |
| Isolated left | 1 (12.5) |
| Total recipients per donor, all organs | 5 (4.5-5) |
Values are presented as median (interquartile range) or n (%). P:F, Partial pressure of oxygen, arterial, to fraction of inspired oxygen ratio; BMI, body mass index.
Recipients for transplantation had a median age of 61 years (IQR, 51.0-67.25 years) and 4 out of 8 (50.0%) of recipients were men. Complete recipient characteristics are shown in Table 2. Indications for transplantation were chronic obstructive lung disease in 3 out of 8 (37.5%) patients and restrictive idiopathic pulmonary fibrosis in 4 out of 8 (50.0%). All patients were admitted from home immediately before transplantation. None required mechanical ventilation or ECMO during the preoperative period.
Table 2.
Recipient baseline demographic data (n = 8)
| Recipient characteristic | Result |
|---|---|
| Age (y) | 61 (51.0-67.25) |
| Male sex | 4 (50) |
| Ethnicity | |
| White | 6 (75) |
| Black | 2 (25) |
| Hispanic | 0 |
| Other | 0 |
| Diagnosis | |
| Obstructive disease | 3 (37.5) |
| Pulmonary vascular disease | 0 |
| Cystic fibrosis | 1 (12.5) |
| Restrictive disease | 4 (50) |
| Other | 0 |
| Lung allocation score | 35.15 (33.012-36.66) |
| Recipient 6-min walk test | |
| Distance (ft) | 1350 (1250-1550) |
| Oxygen requirement (L) | 8 (5-12.5) |
| Recipient ventilation and perfusion distribution | |
| Left ventilation | 48.3 (46.25-53.65) |
| Right ventilation | 51.7 (46.35-53.75) |
| Left perfusion | 47.58 (43.24-53.5) |
| Right perfusion | 52.43 (47.00-56.77) |
| Recipient pulmonary vascular resistance (Woods units) | 2.96 (1.87-3.58) |
| Recipient prior chest surgery | 1 (12.5) |
| Recipient prior chest radiation? | 0 |
| Total days on waitlist | 49.5 (10.75-96.25) |
| BMI | 26.3 (23.4-28.7) |
| Preoperative ECMO? | 0 |
| Preoperative mechanical ventilation? | 0 |
Values are presented as median (interquartile range) or n (%). BMI, Body mass index; ECMO, extracorporeal membrane oxygenation.
Complete donation, warm, and cold ischemia times are shown in Table 3. Median asystole to reperfusion time was 9.5 minutes (IQR, 8.75-12.5 minutes). Total TA-NRP perfusion time was 86.5 minutes (IQR, 79.75-92.5 minutes) reflecting a slight preference for longer reperfusion time favored in multivisceral and hepatic procurement. No surgical damage was noted due to TA-NRP procurement for any candidate organs, including lung allografts. TA-NRP donation resulted in a median of 5 (IQR, 4.5-5) organ recipients per donor. Mechanical circulatory support was required during implantation for 2 out of 8 (25.0%) recipients. One of these recipients was planned mechanical support due to severe pulmonary hypertension, with preoperative pulmonary vascular resistance of 11.1 Woods units. The second required support during contralateral implantation due to graft edema and impaired gas exchange, which improved quickly postoperatively.
Table 3.
Thoracoabdominal normothermic regional perfusion (TA-NRP) and ischemic times (n = 8)
| Procedure-related characteristic | Result |
|---|---|
| Bilateral orthotopic lung transplant? | 7 (87.5) |
| Withdrawal of life support to asystole time (min) | 19 (14-28.25) |
| Asystole to reperfusion (min) | 9.5 (8.75-12.5) |
| Standoff time | 6 (5-7) |
| TA-NRP perfusion time (min) | 86.5 (79.75-92.5) |
| Cold ischemia time (min) | |
| Left∗ | 202 (191-419) |
| Right† | 275 (146-335) |
| Warm ischemia time (min) | |
| Left | 55.5 (46.5-67.5) |
| Right‡ | 41.0 (39.0-53.0) |
| Total ischemia time (min) | |
| Left∗ | 243 (223.5-414.0) |
| Right† | 221 (154.0-375.0) |
| Intraoperative CPB or ECMO support required? | 2 (25.0) |
| CPB/ECMO duration, if used | 191/170 |
Values are presented as median (interquartile range), n (%) or n/N. TA-NRP, Thoracoabdominal normothermic regional perfusion; CPB, cardiopulmonary bypass; ECMO, extracorporeal membrane oxygenation.
n = 6.
n = 5.
n = 7.
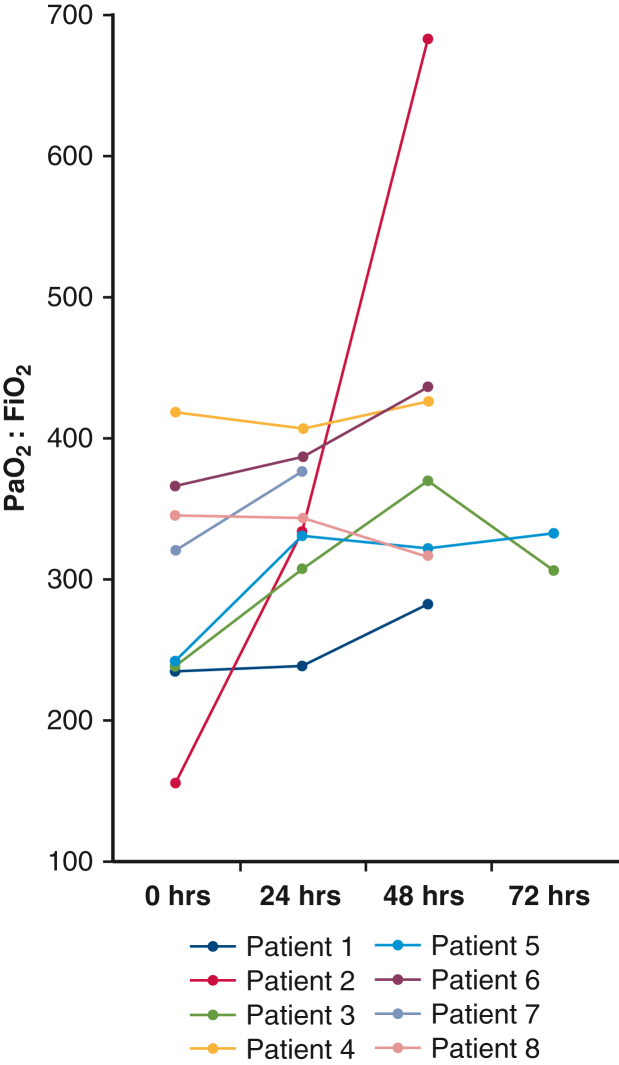
There were no early postoperative pulmonary related mortalities observed. One patient died from complications related to severe necrotizing pancreatitis on postoperative day 28. There were no 30-day readmissions or reoperations. Median postoperative duration of intubation was 15.5 hours (IQR, 9.0-15.5 hours) and 7 out of 8 patients demonstrated PGD grade 1 or less at all time points beyond 24 hours. Postoperative P:Fs are shown in Figure 3. PGD grade II was observed in the lone left single transplant recipient at 0 to 48 hours. Total duration of hospitalization was a median of 17 days (IQR, 14.5-28 days). No bronchial anastomotic complications were noted during the index hospitalization or during postoperative surveillance bronchoscopy for any individuals (Table 4).
Figure 3.
Recipient partial pressure of oxygen, arterial, to fraction of inspired oxygen ratio (Pao2:Fio2) on intensive care unit arrival and at 24 to 72 hours at all clinically indicated time points.
Table 4.
Recipient perioperative and early postoperative outcomes (n = 8)
| Postoperative outcome | Result |
|---|---|
| Duration of mechanical ventilation (h) | 15.5 (9.0-15.5) |
| Ventilatory support >48 h | 0 |
| Intubation at 72 h | 0 |
| ECMO use postoperatively? | 0 |
| Duration of postoperative ECMO | 0 |
| Postoperative P:F | |
| POD 0 | 280.5 (236-355.5) |
| POD 1 | 338 (318.5-381) |
| POD 2∗ | 370 (316.0-436.0) |
| POD 3† | 319 (312.5-325.5) |
| Reintubation? | 2 (25.0) |
| Predischarge acute rejection | 0 |
| Postoperative rejection on biopsy? | 2 (25.0) |
| Bronchial anastomotic complication? | 0 |
| Postoperative dialysis | 0 |
| ICU length of stay | 4.5 (3.5-5.5) |
| Hospital length of stay (d) | 17 (14.5-28) |
| Survival at 30-d posttransplant | 7 (87.5) |
| Survival at 60-d posttransplant‡ | 5 (87) |
| Survival at 90-d posttransplant‡ | 5 (87) |
| Death from pulmonary cause? | 0 |
| Postoperative readmission | |
| 30-d | 0 |
| Overall during follow-up | 4 (50) |
| Reoperation postoperatively? | 0 |
Values are presented as median (interquartile range) or n (%). ECMO, Extracorporeal membrane oxygenation; P:F, partial pressure of oxygen, arterial, to fraction of inspired oxygen ratio; POD, postoperative day; ICU, intensive care unit.
n = 7.
n = 4.
n = 6.
Discussion
Here we present early exploratory results utilizing a lung protective strategy for TA-NRP during DCD procurement of thoracic organs. We sought to outline the technical feasibility and early pulmonary allograft outcomes utilizing this approach. Our cohort experienced no pulmonary related mortalities with 1 early postoperative death related to severe acute pancreatitis (Table 4). Early graft function was excellent in all recipients, with 7 out of 8 patients demonstrating PGD grade 1 or less at 24 hours and all patients were extubated within 24 hours of implantation. We observed no airway complications in the perioperative or early postoperative period. Notable in this cohort of donors was a nearly complete utilization of thoracic and abdominal organs from each donor, with a median of 5 transplant recipients per DCD donor with this single perfusion modality. This may serve as an opportunity for increased efficiency in donor yield because recipient yield using conventional DCD procurement with or without use of ex vivo organ perfusion has been shown to be substantially lower in ischemia sensitive organs when TA-NRP is not used during procurement.14 Because DCD procurement for thoracic and abdominal organs continues to play a larger role in addressing donor shortages, techniques that maximize organ recovery and function without adding additional cost will become increasingly important to provide equity in access to transplantation.
We have noted several beneficial technical points to employing TA-NRP that combat a slight propensity for these grafts to accumulate pulmonary edema. Although the lung allograft is relatively tolerant of the ischemia during the DCD process, this early edema can impair immediate postoperative graft function. We have found a strategy of predonation diuresis, early venous cannulation with subsequent donor decongestion before initiating TA-NRP, early pulmonary arterial venting, early reintubation and ventilation during TA-NRP, and limitation of NRP duration to be beneficial in mitigating these changes. Mechanisms for pulmonary edema in these allografts include both increased aortic afterload and increased bronchial blood flow, as well as lack of cardiac contractility during TA-NRP initiation; all resulting in transient left atrial hypertension. Prolonged reperfusion with TA-NRP and its associated volume shifts may exacerbate pulmonary edema. We hypothesize this contributed to early pulmonary edema in patient 2 of this series, an individual who had a TA-NRP duration of 111 minutes and early pulmonary edema contributing to impaired graft gas exchange. It should be noted that early postoperative diuresis in this recipient resulted in quick resolution of the edema and an otherwise normal postoperative course (Figure 3). Following this observation, we have favored shorter TA-NRP for thoracic organs, although the decision on duration of TA-NRP is collaborative among procuring surgeons, and intended to maximize function of all organs.
Early investigation of TA-NRP during DCD lung procurement without subsequent implementation of ex vivo lung perfusion has, to date, been limited largely to case anecdotes with and without the use of ex vivo lung perfusion in conjunction with TA-NRP.15, 16, 17, 18 These reports have been helpful in outlining feasibility but have not allowed for refining TA-NRP as a standalone procurement technique. Despite this, these reports have been encouraging with no reports of primary graft nonfunction, and overall favorable early postoperative pulmonary graft function. In addition to these reports, an indirect evaluation of outcomes and utilization of TA-NRP DCD lung procurement has recently been completed through query of the United Network for Organ Sharing database. This study demonstrated 17 suspected TA-NRP DCD lung procurements from over a 15-month period starting January 2020 as defined by time from asystole to aortic crossclamp >50 minutes. Early postoperative results demonstrated no significant difference in rates of prolonged intubation (>48 hours); ECMO utilization; predischarge acute rejection; and survival at 30, 60, and 90 days compared with recipients of direct DCD procurement lung allografts during the same study period.19 Taken together, these investigations outline feasibility and promise with this technique; however, the important detailed analysis of early patient outcomes with an emerging technique has yet to be completed. This analysis is paramount to maintain quality in thoracic grafts for recipients.
Utilization of DCD procurement for lung transplantation has gained an increasingly important role in addressing the longstanding shortages faced by patients with end-stage lung disease. Utilization of lungs procured using a direct procurement DCD strategy has not been shown to have inferior outcomes related to organs procured from brain-dead donors. Multiple corroborating studies have demonstrated equipoise in recipient survival, PGD, and acute rejection in both 1- and 5-year outcomes.2,20, 21, 22 Comparison of DCD- and DBD-procured lungs during a multiera study of the United Network for Organ Sharing/Organ Procurement and Transplantation Network database from 2005 to 2020 further supported these findings with equivalent survival in recipients despite a trend for DCD recipients to be older, be admitted to the intensive care unit preoperatively, and have higher likelihood for preoperative support with ECMO.1 Concurrent with the 7-fold increase in DCD lung transplantation observed over the era of these prior studies has been the introduction and popularization of DCD heart transplantation, either through direct procurement with use of organ care systems or TA-NRP. To date, DCD heart transplantation with organ care systems or TA-NRP have shown comparable outcomes to DBD donation with cold static storage.23 This progress has been paired with the adoption of TA-NRP in DCD liver procurement to drastically improve biliary complications in DCD liver transplantation.24,25 The emerging role of TA-NRP in optimizing heart and liver procurement creates urgency to optimize this technique to protect the lung allografts from any potential insult. In the present study, we outline our strategy to facilitate this.
We have identified an increased frequency of NRP being used to facilitate recovery of cardiac and abdominal allografts. Given the reality of a national paradigm shift in recovery of DCD organs and the results of our centers success with lung procurement using TA-NRP, we have altered our donor recovery strategy to permit recovery of DCD lung allografts by either direct procurement or by use of the normothermic regional perfusion technique. We currently have no preference as to recovery method and believe that these lung allografts should not be avoided simply because TA-NRP is being used during multiorgan recovery.
Our study has several important limitations. This is an early experience, single-center study with a novel technique for DCD lung procurement using TA-NRP and, as a result, these early results should be interpreted with caution. Long-term outcomes related to graft performance and complications are not described in this study.
Conclusions
Here we demonstrate encouraging early results with a lung protective strategy for implementing TA-NRP for DCD lung recovery. Please see Figure 4 for a graphical abstract of this study. Given the reality of a national paradigm shift in recovery strategies for DCD donation, and a greater implementation of TA-NRP, we have altered our donor recovery strategy with a goal of optimizing recovery of DCD lung procurement by normothermic regional perfusion. We currently have no preference for direct procurement or TA-NRP procurement for DCD lung donation and believe that these lung allografts should not be avoided simply due to utilization of TA-NRP by others during multivisceral recovery.
Figure 4.
Lung recovery utilizing thoracoabdominal normothermic regional perfusion (TA-NRP) during donation after circulatory death (DCD). PGD, Primary graft dysfunction; PA, pulmonary artery.
Conflict of Interest Statement
The authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
References
- 1.Bobba C.M., Whitson B.A., Henn M.C., Mokadam N.A., Keller B.C., Rosenheck J., et al. Trends in donation after circulatory death in lung transplantation in the United States: impact of era. Transpl Int. 2022;35 doi: 10.3389/ti.2022.10172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krutsinger D., Reed R.M., Blevins A., Puri V., De Oliveira N.C., Zych B., et al. Lung transplantation from donation after cardiocirculatory death: a systematic review and meta-analysis. J Heart Lung Transplant. 2015;34:675–684. doi: 10.1016/j.healun.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 3.Van Raemdonck D., Keshavjee S., Levvey B., Cherikh W.S., Snell G., Erasmus M., et al. Donation after circulatory death in lung transplantation-five-year follow-up from ISHLT registry. J Heart Lung Transplant. 2019;38:1235–1245. doi: 10.1016/j.healun.2019.09.007. [DOI] [PubMed] [Google Scholar]
- 4.Kwon J.H., Ghannam A.D., Shorbaji K., Welch B., Hashmi Z.A., Tedford R.J., et al. Early outcomes of heart transplantation using donation after circulatory death donors in the United States. Circ Heart Fail. 2022;15 doi: 10.1161/CIRCHEARTFAILURE.122.009844. [DOI] [PubMed] [Google Scholar]
- 5.Jay C.L., Lyuksemburg V., Ladner D.P., Wang E., Caicedo J.C., Holl J.L., et al. Ischemic cholangiopathy after controlled donation after cardiac death liver transplantation: a meta-analysis. Ann Surg. 2011;253:259–264. doi: 10.1097/SLA.0b013e318204e658. [DOI] [PubMed] [Google Scholar]
- 6.James L., LaSala V.R., Hill F., Ngai J.Y., Reyentovich A., Hussain S.T., et al. Donation after circulatory death heart transplantation using normothermic regional perfusion: the NYU protocol. J Thorac Cardiovasc Surg Tech. 2023;17:111–120. doi: 10.1016/j.xjtc.2022.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoffman J.R.H., McMaster W.G., Rali A.S., Rahaman Z., Balsara K., Absi T., et al. Early US experience with cardiac donation after circulatory death (DCD) using normothermic regional perfusion. J Heart Lung Transplant. 2021;40:1408–1418. doi: 10.1016/j.healun.2021.06.022. [DOI] [PubMed] [Google Scholar]
- 8.Smith D.E., Kon Z.N., Carillo J.A., Chen S., Gidea C.G., Piper G.L., et al. Early experience with donation after circulatory death heart transplantation using normothermic regional perfusion in the United States. J Thorac Cardiovasc Surg. 2022;164:557–568.e1. doi: 10.1016/j.jtcvs.2021.07.059. [DOI] [PubMed] [Google Scholar]
- 9.Joshi Y., Scheuer S., Chew H., Ru Qiu M., Soto C., Villanueva J., et al. Heart transplantation from DCD donors in Australia: lessons learned from the first 74 cases. Transplantation. 2023;107:361–371. doi: 10.1097/TP.0000000000004294. [DOI] [PubMed] [Google Scholar]
- 10.Louca J., Öchsner M., Shah A., Hoffman J., Vilchez F.G., Garrido I., et al. The international experience of in-situ recovery of the DCD heart: a multicentre retrospective observational study. EClinicalMedicine. 2023;58 doi: 10.1016/j.eclinm.2023.101887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaurav R., Butler A.J., Kosmoliaptsis V., Mumford L., Fear C., Swift L., et al. Liver transplantation outcomes from controlled circulatory death donors: SCS vs in situ NRP vs ex situ NMP. Ann Surg. 2022;275:1156–1164. doi: 10.1097/SLA.0000000000005428. [DOI] [PubMed] [Google Scholar]
- 12.Snell G.I., Yusen R.D., Weill D., Strueber M., Garrity E., Reed A., et al. Report of the ISHLT working group on primary lung graft dysfunction, part I: definition and grading-a 2016 consensus group statement of the international society for heart and lung transplantation. J Heart Lung Transplant. 2017;36:1097–1103. doi: 10.1016/j.healun.2017.07.021. [DOI] [PubMed] [Google Scholar]
- 13.Entwistle J.W., Drake D.H., Fenton K.N., Smith M.A., Sade R.M., Cardiothoracic Ethics Forum Normothermic regional perfusion: ethical issues in thoracic organ donation. J Thorac Cardiovasc Surg. 2022;164:147–154. doi: 10.1016/j.jtcvs.2022.01.018. [DOI] [PubMed] [Google Scholar]
- 14.Bekki Y., Croome K.P., Myers B., Sasaki K., Tomiyama K. Normothermic regional perfusion can improve both utilization and outcomes in DCD liver, kidney, and pancreas transplantation. Transplant Direct. 2023;9 doi: 10.1097/txd.0000000000001450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vandendriessche K., Tchana-Sato V., Ledoux D., Degezelle K., Rex S., Neyrinck A., et al. Transplantation of donor hearts after circulatory death using normothermic regional perfusion and cold storage preservation. Eur J Cardio Thorac Surg. 2021;60:813–819. doi: 10.1093/ejcts/ezab139. [DOI] [PubMed] [Google Scholar]
- 16.Urban M., Castleberry A.W., Markin N.W., Chacon M.M., Strah H.M., Um J.Y., et al. Successful lung transplantation with graft recovered after thoracoabdominal normothermic perfusion from donor after circulatory death. Am J Transplant. 2022;22:294–298. doi: 10.1111/ajt.16806. [DOI] [PubMed] [Google Scholar]
- 17.Urban M., Bishawi M., Castleberry A.W., Markin N.W., Chacon M.M., Um J.Y., et al. Novel use of mobile ex-vivo lung perfusion in donation after circulatory death lung transplantation. Prog Transplant. 2022;32:190–191. doi: 10.1177/15269248221087437. [DOI] [PubMed] [Google Scholar]
- 18.Boelhouwer C., Vandendriessche K., Van Raemdonck D., Jochmans I., Monbaliu D., Pirenne J., et al. Lung donation and transplantation following thoraco-abdominal normothermic regional perfusion: a case series. J Heart Lung Transplant. 2022;41:S252–S253. doi: 10.1016/j.healun.2022.01.1775. [DOI] [PubMed] [Google Scholar]
- 19.Zhou A.L., Ruck J.M., Casillan A.J., Larson E.L., Shou B.L., Karius A.K., et al. Early United States experience with lung donation after circulatory death using thoracoabdominal normothermic regional perfusion. J Heart Lung Transplant. 2023;42:693–696. doi: 10.1016/j.healun.2023.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levvey B., Keshavjee S., Cypel M., Robinson A., Erasmus M., Glanville A., et al. Influence of lung donor agonal and warm ischemic times on early mortality: analyses from the ISHLT DCD Lung Transplant Registry. J Heart Lung Transplant. 2019;38:26–34. doi: 10.1016/j.healun.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 21.Cypel M., Levvey B., Van Raemdonck D., Erasmus M., Dark J., Love R., et al. International Society for Heart and Lung Transplantation donation after circulatory death registry report. J Heart Lung Transplant. 2015;34:1278–1282. doi: 10.1016/j.healun.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 22.Reeb J., Keshavjee S., Cypel M. Expanding the lung donor pool: advancements and emerging pathways. Curr Opin Organ Transplant. 2015;20:498–505. doi: 10.1097/mot.0000000000000233. [DOI] [PubMed] [Google Scholar]
- 23.Schroder J.N., Patel C.B., DeVore A.D., Bryner B.S., Casalinova S., Shah A., et al. Transplantation outcomes with donor hearts after circulatory death. N Engl J Med. 2023;388:2121–2131. doi: 10.1056/NEJMoa2212438. [DOI] [PubMed] [Google Scholar]
- 24.Watson C.J.E., Hunt F., Messer S., Currie I., Large S., Sutherland A., et al. In situ normothermic perfusion of livers in controlled circulatory death donation may prevent ischemic cholangiopathy and improve graft survival. Am J Transplant. 2019;19:1745–1758. doi: 10.1111/ajt.15241. [DOI] [PubMed] [Google Scholar]
- 25.Sellers M.T., Nassar A., Alebrahim M., Sasaki K., Lee D.D., Bohorquez H., et al. Early United States experience with liver donation after circulatory determination of death using thoraco-abdominal normothermic regional perfusion: a multi-institutional observational study. Clin Transplant. 2022;36 doi: 10.1111/ctr.14659. [DOI] [PubMed] [Google Scholar]