Abstract
Objectives
Mitral annular calcification remains a formidable lesion in cardiac surgery with significant perioperative morbidity and mortality, particularly when en bloc annular decalcification is implemented. Respect strategies and hybrid approaches have provided safe alternatives. We report the short-term results of our institution's experience with mitral valve replacement in patients with extensive annular calcification.
Methods
This is a retrospective review of 72 consecutive patients with extensive annular calcification who underwent open surgical mitral valve replacement from January 1, 2013, to September 31, 2022. Degree of annular calcification was graded as partial, horseshoe, or circumferential. We excluded patients with calcification involving less than one-third of the annulus and patients with rheumatic heart disease.
Results
Mean patient age was 71.6 ± 10.9 years, and 50 (69.4%) were female. There were 51 patients (70.8%) with New York Heart Association class 3 or greater and 47 patients (65.3%) with pulmonary hypertension. There were 41 patients (56.9%) with partial, 12 patients (16.7%) with horseshoe, and 19 patients (26.4%) with circumferential calcification. Fifty-six patients (77.8%) underwent conventional valve replacement. Sixteen patients underwent a hybrid procedure using balloon-expandable devices. Concomitant procedures were performed in 61 patients (84.7%). In-hospital mortality and 1-year survival were 3.57% and 82.8% in the standard valve replacement cohort and 25.0% and 54.7% in the hybrid cohort, respectively.
Conclusions
Conventional mitral valve replacement using respect strategies is safe and associated with good outcomes in patients with extensive annular calcification. Hybrid approaches using novel devices should remain as a bailout in select patients because of higher perioperative risks and poor short-term outcomes.
Key Words: annular calcification, hybrid valve replacement, mitral valve
Graphical abstract

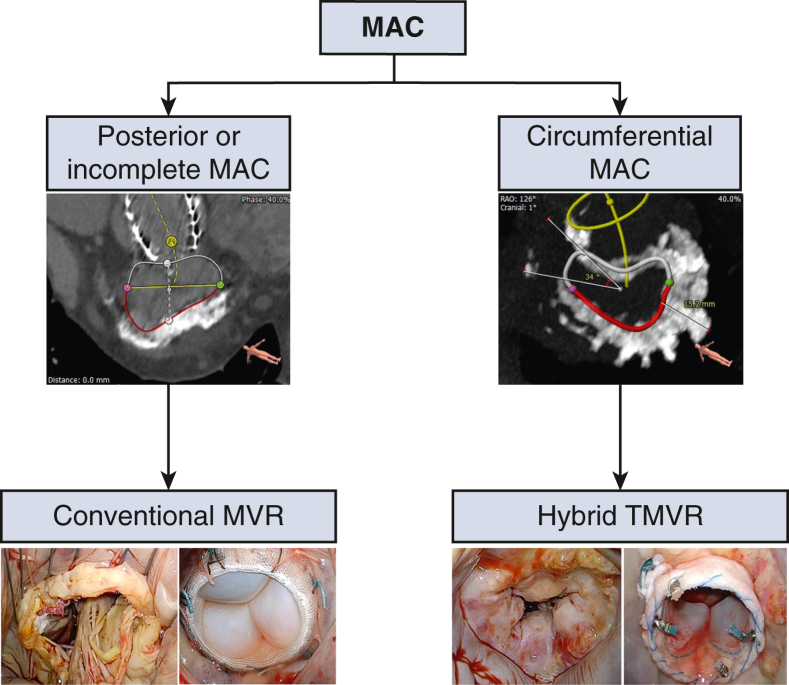
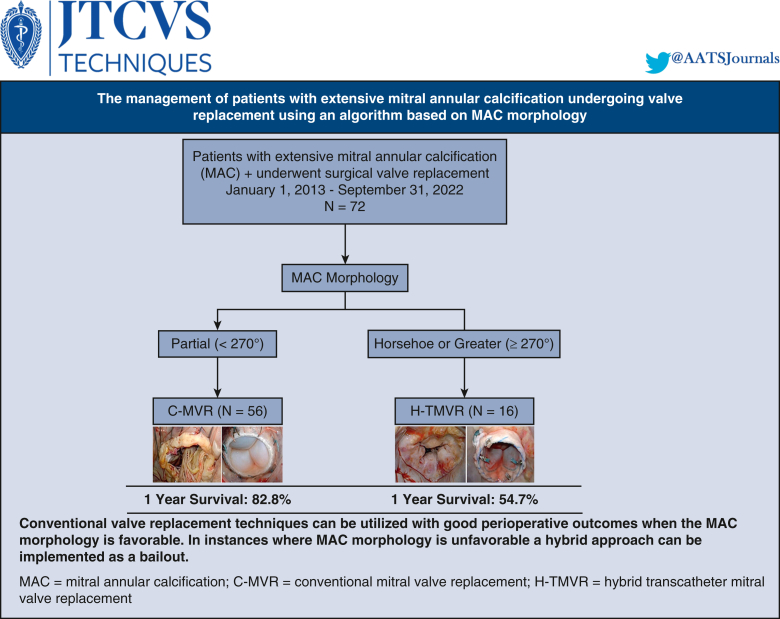
Algorithm for surgical management of patients with extensive MAC.
Central Message.
Contemporary techniques for surgical mitral valve replacement in extensive MAC are safe and feasible. Hybrid approaches should remain a bailout in select patients because of their higher risk.
Perspective.
C-MVR using respect strategies without en bloc annular decalcification is safe and feasible in most patients with extensive annular calcification. Hybrid approaches using balloon-expandable devices under direct vision should be reserved as a bailout to standard surgery in highly selected patients because of the increased perioperative risks.
See Discussion on page 13.
Despite the recent advances in the field of cardiovascular surgery and interventions, mitral annular calcification (MAC) remains a challenging anatomic and physiologic entity in cardiovascular surgery with high perioperative and periprocedural morbidity and mortality, as well as poor long-term outcomes.
Although the risk factors associated with MAC are becoming better defined, its cause remains unclear. In today's population, MAC has a higher prevalence among elderly patients and women, and in the presence of comorbidities such as hypertension, diabetes, end-stage renal disease, aortic stenosis, hypertrophic obstructive cardiomyopathy, and advanced degenerative mitral disease.1 Mediastinal radiation is also an increasingly recognized cause of a more extensive form of MAC as part of radiation heart disease, commonly seen in survivors of mantle field radiation for Hodgkin's lymphoma.
There has been a recent increase in patients presenting with symptomatic severe MAC due to an aging population in addition to the increase in the referral for surgery in high-risk patients with MAC who failed screening for transcatheter therapy. For many decades, MAC has carried a formidable reputation in cardiac surgery with several historical reports of high risk for perioperative complications particularly when en bloc annular decalcification/annular reconstruction techniques were implemented, including risk of atrioventricular (AV) groove disruption, prosthetic paravalvular leak (PVL), stroke, and myocardial ischemia.2,3 However, with the evolution of cardiac imaging, transcatheter therapies, and balloon-expandable devices, novel techniques were explored in patients with extensive MAC not amenable for standard valve replacement techniques. Hybrid procedures using transcatheter valves (hybrid transcatheter mitral valve replacement [H-TMVR]) via direct access implantation of balloon-expandable devices in MAC during open surgery have recently gained interest in select patients with MAC.
In this article, we discuss our decision algorithm, operative strategies, and short-term outcomes for patients with severe MAC using conventional and hybrid surgical approaches.
Material and Methods
Study Population
This is a retrospective review of 72 consecutive patients with symptomatic severe mitral valve disease due to extensive MAC who underwent open surgical mitral valve replacement from January 1, 2013, to September 31, 2022. We defined extensive MAC as MAC involving more than one-third of the annulus on computed tomography (CT) scanning. We excluded patients with MAC involving less than one-third of the annulus and patients with MAC in the context of rheumatic heart disease.
Preoperative Screening, Planning, and Optimization
All patients were admitted to the hospital for preoperative evaluation and planning. A detailed cardiac imaging evaluation of MAC including transthoracic echocardiography, transesophageal echocardiography, and multidetector CT, as well as right and left heart catheterization, was done for all patients. A comprehensive multidisciplinary team evaluation, including consultation by nephrology, pulmonology, neurology, endocrinology, electrophysiology, and heart failure when appropriate, was performed. Medical optimization was initiated and maintained by the Heart Team for decompensated patients, with a focus on optimizing heart rhythm and rate, volume status, pulmonary artery pressure and cardiac output, nutritional status, and physical therapy.
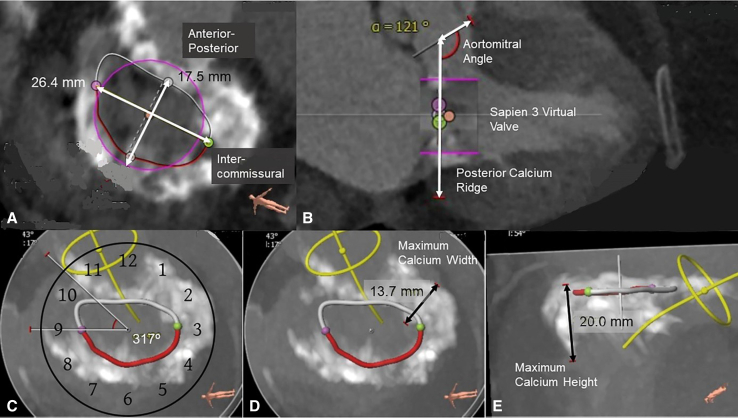
We categorized the extension of MAC based on a specific CT-guided anatomic classification as partial (involving at least one-third of the annular circumference and <270 degrees), horseshoe (commissure-to-commissure, 270-300 degrees) and circumferential (>300 degrees).4 In addition, annular dimensions and eccentricity, maximal thickness and height of the calcium in MAC bar, extension of MAC into the left atrium and ventricle, aortomitral angle, valve sizing, and neo–left ventricular outflow tract (LVOT) anatomy were determined (Figure 1).
Figure 1.
Anatomic analysis of MAC and feasibility of transcatheter valves using multidetector CT. Clockwise from top left: A, Intercommissural and anterior-posterior dimensions are determined. B, Aortomitral angle and a virtual transcatheter valve (Sapien 3, Edwards Lifesciences, LLC) are placed to assess the fitting inside the MAC and LVOT obstruction risk. C, Severity of MAC, using a clockface orientation, is determined, with 270 to 300 degrees deemed horseshoe and more than 300 degrees circumferential. D and E, Maximum calcium thickness and height with the MAC are calculated to determine the extent of anchoring with the transcatheter valve.
Three-dimensional transesophageal echocardiography provided a detailed anatomic assessment of MAC and associated mitral disease, annular dimensions, leaflet anatomy, and risk of LVOT obstruction because of the anterior leaflet or basal septal hypertrophy.
Surgical Technique
In our mitral reference center, all extensive MAC operations were evaluated and performed by a single group of surgeons (D.H.A., A.E-E., P.B., G.H.L.T.). All cases were performed through a median sternotomy. Standard cardiopulmonary bypass techniques with central aortic and bicaval cannulation with aortic crossclamping were used. Myocardial protection was achieved via cold blood cardioplegia given in an antegrade and retrograde fashion. The mitral valve was accessed via Sondergaard's groove or occasionally through a transseptal approach in patients with a small left atrium, complex reoperations, and patients with an in situ aortic prosthesis. In patients with a porcelain aorta, peripheral cannulation was used with hypothermic fibrillatory arrest to avoid aortic manipulation. Intraoperative surgical analysis was done in all patients to confirm the extension of MAC. Valve replacement then proceeded as described in the algorithm outlined in Figure 2.
Figure 2.
Mount Sinai algorithm for management of MAC, where C-MVR is done for partial (<270) MAC and H-TMVR is done for horseshoe and circumferential MAC (>270). MAC, Mitral annular calcification; MVR, mitral valve replacement; TMVR, transcatheter mitral valve replacement.
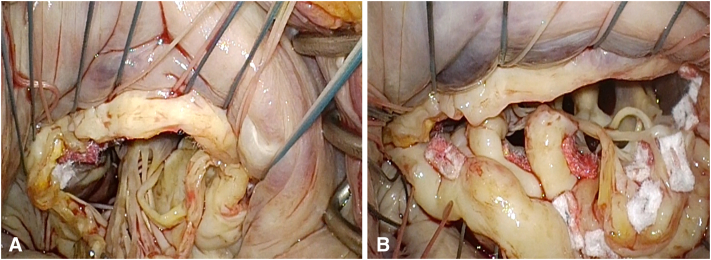
Conventional mitral valve replacement (C-MVR) is a technique preferred for patients with primarily partial MAC. In these patients, the anterior leaflet with its subvalvular apparatus was resected and the posterior leaflet was preserved. The mitral valve was replaced with standard prostheses using a noneverting suture technique with pledgeted sutures placed on the ventricular side. We implemented 3 main suture techniques for C-MVR: periannular sutures using large needles around the calcium bar, intraleaflet implantation where sutures are passed through the posterior leaflet, and a modified anterior leaflet flip technique where the anterior leaflet is detached and flipped toward the posterior leaflet so that both leaflets create a neoannulus buttress to reinforce the suture line and provide protection against ventricular disruption (Videos 1 and 2) (Figure 3). Partial decalcification was performed selectively to even the landing surface and allow for suture placement as well as to debride friable parts of the MAC bar. We preferably used a combination of sharp and rongeur dissection when decalcification was implemented. En bloc decalcification/annular reconstruction was not used in any patient.
Figure 3.
Modified anterior leaflet flip technique for C-MVR. Anterior leaflet disinserted (A) and anterior leaflet flipped toward posterior leaflet creating neo-annulus (B).
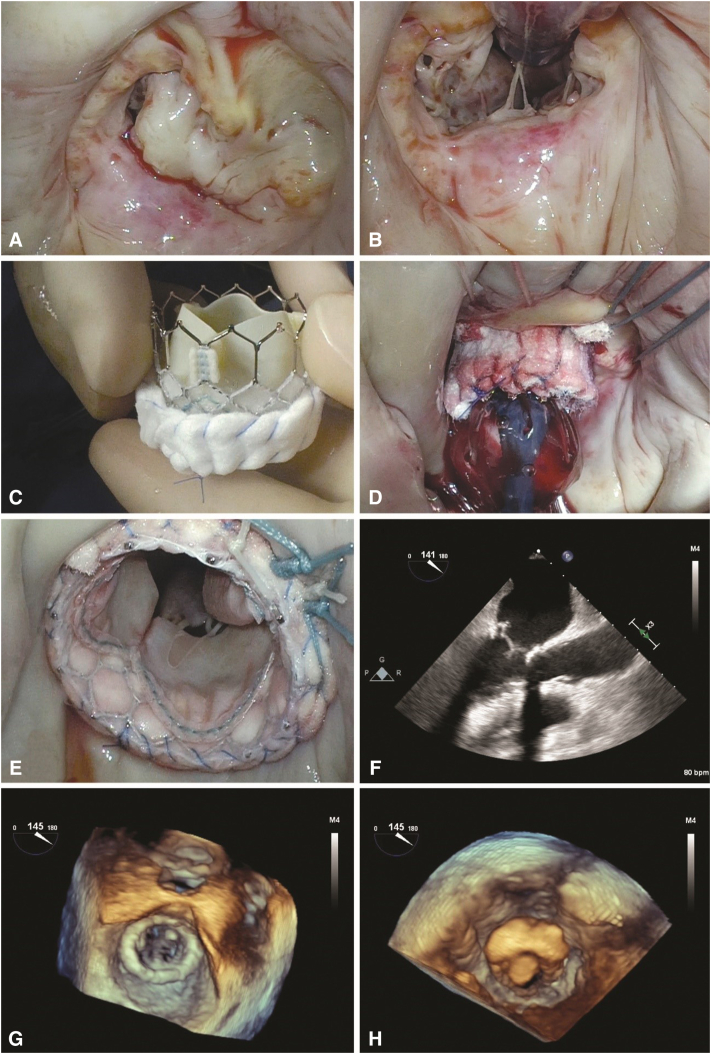
H-TMVR is a transatrial approach that was used in this patient subset. The anterior mitral leaflet was partially resected with its corresponding subvalvular apparatus to reduce the risk of LVOT obstruction. Commissural gaps were closed to circularize the annulus and reduce the risk of paravalvular regurgitation. The Sapien 3 valve (Edwards Lifesciences, LLC) was modified by placing 1 or more rows of Teflon felt at the atrial side to improve sealing against the MAC and reduce the risk of PVL. Sutures placed at noncalcified sections of the annulus were tied to both the Teflon cuff and the valve stent after valve deployment to provide additional reinforcement of the transcatheter prosthesis to reduce the risk of device migration and embolization. This approach was done under direct visualization without the need for guidewires or fluoroscopy and with assistance from an interventional surgeon (G.H.L.T.). Balloon sizing was used for confirmation. The valve was then crimped on the balloon delivery system and deployed into the mitral orifice with proper orientation of the commissures to match the intertrigonal distance while keeping the Teflon cuff more atrial and the open cells toward the LVOT. Sutures were then passed through the Teflon felt ring and stent frame and tied (Video 3 and Figure 4).
Figure 4.
H-TMVR stepwise technique: Valve analysis showing horseshoe MAC (A), anterior leaflet resection (B), Sapien 3 valve wrapped in felt strips (C), valve balloon deployment (D), fully deployed valve (E), and 2-dimensional and 3-dimensional transesophageal echocardiography reconstruction demonstrating well-seated valve (F-H).
Early in the series, we used the Melody valve (Medtronic Inc), which was the only available device, consisting of balloon-expandable stented bovine jugular vein graft primarily designed for the pulmonary position. The insertion technique was similar to that for the Sapien 3 valve except that we did not use Teflon felt wrapping or sutures and the valve was deployed more toward the atrium after folding both valve ends because of its higher profile.5
Postoperative Care
Similar to standard bioprosthetic mitral valve replacement, all patients in the H-TMVR group received lifelong aspirin and a minimum of 3 months of Coumadin with a target international normalized ratio of 2.0 to 3.0. Patients with a contraindication to anticoagulation or who were high risk for bleeding received only aspirin. Transthoracic echocardiography was performed in all patients before discharge.
Data Collection
Clinical variables were identified through retrospective review of the electronic medical record. Information regarding 1-year survival was obtained by reviewing retrospective electronic health records. The study protocol was approved by our local Institutional Review Board (STUDY-22-00800, 08/29/2022) and was compliant with the Health Insurance Portability and Accountability Act regulations and the ethical guidelines of the 1975 Declaration of Helsinki. The approval included a waiver of informed consent. Morbidities were defined according to the 2018 Society of Thoracic Surgeons Adult Cardiac Surgery Risk Models. Censoring was done based on last patient encounter according to electronic health records.
Statistical Analysis
Normally distributed continuous variables were represented as mean ± SD. Nonparametric and categorical variables were represented as median and interquartile range or as the number of patients as a percentage of the sample, respectively. Normality of variables was assessed using the Shapiro–Wilk test and with visual estimation against a normally distributed bell curve and a quantile-quantile plot. Midterm follow-up was done using standard Kaplan–Meier survival curves and modified Kaplan–Meier survival curves to account for the instability in the right-tail of small-risk data set. The statistical analyses were performed with the use of SAS 9.4 statistical software (SAS Institute, Inc).
Results
Patient Characteristics
Mean patient age was 71.6 ± 10.9 years, and 50 (69.4%) were female. There were 51 patients (70.8%) in New York Heart Association class greater than 3, 47 patients (65.3%) with pulmonary hypertension, and 12 patients (16.7%) with mediastinal radiation. The median Society of Thoracic Surgeons score risk for mortality was 4.1% (interquartile range, 2.4%-6.4%). Seven patients (9.7%) had structural valve procedures including transcatheter aortic valve replacement and percutaneous edge-to-edge repair, all in the C-MVR group. Nineteen patients (26.4%) had reoperations. Mitral dysfunction was mixed in 21 patients (29.6%), regurgitant in 31 patients (43.6%), and stenotic in 19 patients (26.8%). There were 41 patients (56.9%) with partial, 12 patients (16.7%) with horseshoe, and 19 patients (26.4%) with circumferential MAC. Patient demographics, comorbidities, clinical characteristics, and preoperative echocardiographic parameters are summarized in Table 1.
Table 1.
Baseline characteristics
| Characteristic | All (N = 72) | C-MVR (N = 56) | H-TMVR (N = 16) | P |
|---|---|---|---|---|
| Age, mean (SD) | 71.6 (10.9) | 70.9 (10.7) | 73.9 (11.3) | .340 |
| Female, n (%) | 50 (69.44%) | 37 (66.07%) | 13 (81.25%) | .245 |
| HTN, n (%) | 55 (76.39%) | 41 (73.21%) | 14 (87.50%) | .235 |
| Pulmonary HTN, n (%) | 47 (65.28%) | 37 (66.07%) | 10 (62.50%) | .791 |
| DM, n (%) | 19 (26.39%) | 14 (25.00%) | 5 (31.25%) | .617 |
| HLD, n (%) | 52 (72.22%) | 40 (71.43%) | 12 (75.00%) | .779 |
| CAD, n (%) | 43 (59.72%) | 31 (55.36%) | 12 (75.00%) | .158 |
| CHF, n (%) | 27 (37.50%) | 22 (39.29%) | 5 (31.25%) | .558 |
| CKD, n (%) | 16 (22.22%) | 13 (23.21%) | 3 (18.75%) | .705 |
| Chronic lung disease, n (%) | 8 (11.11%) | 6 (10.71%) | 2 (12.50%) | .841 |
| Rheumatic disease, n (%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | - |
| Endocarditis, n (%) | 2 (2.78%) | 2 (3.57%) | 0 (0.00%) | .443 |
| Mediastinal radiation, n (%) | 12 (16.67%) | 9 (16.07%) | 3 (18.75%) | .799 |
| Atrial fibrillation, n (%) | 35 (48.61%) | 27 (48.21%) | 8 (50.00%) | .899 |
| MI, n (%) | 9 (12.50%) | 6 (10.71%) | 3 (18.75%) | .391 |
| Previous intervention, n (%) | 26 (36.11%) | 21 (37.50%) | 5 (31.25%) | .646 |
| Median sternotomy | 19 (73.08%) | 14 (66.67%) | 5 (100.00%) | .131 |
| TAVR | 4 (15.38%) | 4 (19.05%) | 0 (0.00%) | .289 |
| MitraClip | 3 (11.54%) | 3 (14.29%) | 0 (0.00%) | .369 |
| NYHA 3+, n (%) | 51 (70.83%) | 39 (69.64%) | 12 (75.00%) | .678 |
| MAC Class, n (%) | .001 | |||
| Circumferential | 19 (26.39%) | 10 (17.86%) | 9 (56.25%) | |
| Horseshoe | 12 (16.67%) | 8 (14.29%) | 4 (25.00%) | |
| Partial | 41 (56.94%) | 38 (67.86%) | 3 (18.75%) | |
| MV disease, n (%) | .002 | |||
| Mixed | 21 (29.58%) | 14 (25.45%) | 7 (43.75%) | |
| Pure regurgitation | 31 (43.66%) | 30 (54.55%) | 1 (6.25%) | |
| Pure stenosis | 19 (26.76%) | 11 (20.00%) | 8 (50.00%) | |
| EF, mean (Standard deviation) | 62.2% (7.5%) | 61.2% (6.9%) | 65.7% (8.9%) | .035 |
| STS score, median (Interquartile range) | 4.1% (2.4%-6.4%) | 4.1% (2.0%-6.0%) | 4.7% (3.3%-8.2%) | .332 |
C-MVR, Conventional mitral valve replacement; H-TMVR, hybrid transcatheter mitral valve replacement; HTN, hypertension; DM, diabetes mellitus; HLD, hyperlipidemia; CAD, coronary artery disease; CHF, congestive heart failure; CKD, chronic kidney disease; MI, myocardial infarction; TAVR, transcatheter aortic valve replacement; NYHA, New York Heart Association; MAC, mitral annular calcification; MV, mitral valve; EF, ejection fraction; STS, Society of Thoracic Surgeons.
Procedural Characteristics
Fifty-six patients (77.8%) underwent C-MVR using standard bioprosthetic valves: 32 (57.1%) using periannular suture placement, 12 (21.4%) with intra-leaflet implantation, 15 (26.8%) using an anterior leaflet flip technique, and 10 (17.9%) using partial annular decalcification (Table 2). We did not encounter any cases of AV groove disruption, midventricular tears, or coronary ischemia in this patient group.
Table 2.
Operative details
| Technique | All (N = 72) | C-MVR (N = 56) | H-TMVR (N = 16) | P |
|---|---|---|---|---|
| MVR technique, n (%) | ||||
| AL flip | 15 (26.79%) | 15 (26.79%) | - | |
| Periannular sutures | 32 (57.14%) | 32 (57.14%) | - | |
| Intraleaflet implant | 12 (21.43%) | 12 (21.43%) | - | |
| Decalcification | 10 (17.86%) | 10 (17.86%) | - | |
| MV prosthesis, n (%) | ||||
| Valve type | ||||
| Bovine | - | 12 (21.43%) | - | |
| Porcine | - | 44 (78.57%) | - | |
| Sapien 3 (Edwards Lifesciences, LLC) | - | - | 10 (62.5%) | |
| Melody (Medtronic) | - | - | 6 (37.5%) | |
| Valve size | ||||
| C-MVR 25 | - | 32 (57.14%) | - | |
| C-MVR 27 | - | 11 (19.64%) | - | |
| C-MVR 29 | - | 9 (16.07%) | - | |
| C-MVR 31 | - | 4 (7.14%) | - | |
| H-TMVR 23 | - | - | 3 (18.75%) | |
| H-TMVR 26 | - | - | 5 (31.25%) | |
| H-TMVR 29 | - | - | 2 (12.5%) | |
| Melody | ||||
| Concomitant procedures, n (%) | 61 (84.72%) | 48 (85.71%) | 13 (81.25%) | .662 |
| TV surgery | 52 (72.22%) | 42 (75.00%) | 10 (62.50%) | .324 |
| Maze | 21 (29.17%) | 19 (33.93%) | 2 (12.50%) | .096 |
| CABG | 11 (15.28%) | 9 (16.07%) | 2 (12.50%) | .726 |
| AVR | 8 (11.11%) | 7 (12.50%) | 1 (6.25%) | .483 |
| TAVR explant | 1 (1.39%) | 1 (1.78%) | 0 (0.00%) | .590 |
| Intraoperative Amplatz plug | 1 (1.39%) | 0 (0.00%) | 1 (6.25%) | .059 |
| CPB time, median (Interquartile range) | 147 (115-178) | 146 (114.5-173) | 151 (132-184) | .697 |
| XC time, median (Interquartile range) | 118.5 (87-138) | 118.5 (87-143) | 118.5 (84.5-127.0) | .551 |
C-MVR, Conventional mitral valve replacement; H-TMVR, hybrid transcatheter mitral valve replacement; MVR, mitral valve replacement; AL, anterior leaflet; MV, mitral valve; TV, tricuspid valve; CABG, coronary artery bypass grafting; AVR, aortic valve replacement; TAVR, transcatheter aortic valve replacement; CPB, cardiopulmonary bypass; XC, crossclamp.
The remaining 16 patients were deemed not to be candidates for C-MVR after valve exploration and surgical analysis, and underwent H-TMVR using a balloon-expandable valve as per our protocol. The device was successfully deployed in all patients. Ten patients had a Sapien 3 valve placed, and 6 patients had a Melody valve inserted early in the series. Two patients required insertion of more than 1 valve. One of them had a Sapien 3 valve that embolized into the left atrium, so a new Sapien 3 valve had to be crimped and deployed after adding more annular sutures that were then passed into the Teflon skirt. The other patient had a Melody valve that embolized into the left atrium. The same Melody valve was retrieved; however, severe transvalvular regurgitation occurred due to balloon over-dilation for stabilization, necessitating a second Melody valve-in-valve.
Concomitant procedures were done in 61 patients (84.7%). The most common procedure was tricuspid intervention in 52 patients (72.2%) and Cryo-Maze ablation in 21 patients (29.1%). Median cardiopulmonary bypass time was 147 (115-178) minutes, and crossclamp time was 118.5 (87-138) minutes.
Perioperative Outcomes
All valve-related complications were seen in the H-TMVR group. They included severe LVOT obstruction in 3 patients (4.2%), valve embolization in 2 patients (2.8%), AV groove disruption in 1 patient (1.4%) requiring conversion to conventional valve replacement, and ventricular perforation (primarily repaired) in 1 patient (1.3%).
On predischarge transthoracic echocardiography, there were 2 patients (2.8%) with PVL of moderate or greater degree, 1 in each group. Both were successfully closed percutaneously due to symptomatic mitral regurgitation. There were 2 (3.6%) in-hospital mortalities in the C-MVR cohort, 1 due to aspiration pneumonia and 1 due to multiorgan failure. In the H-TMVR cohort, 4 patients (25.0%) died in-hospital mostly due to intraoperative complications (2 had severe LVOT obstruction, and 1 had AV groove disruption).
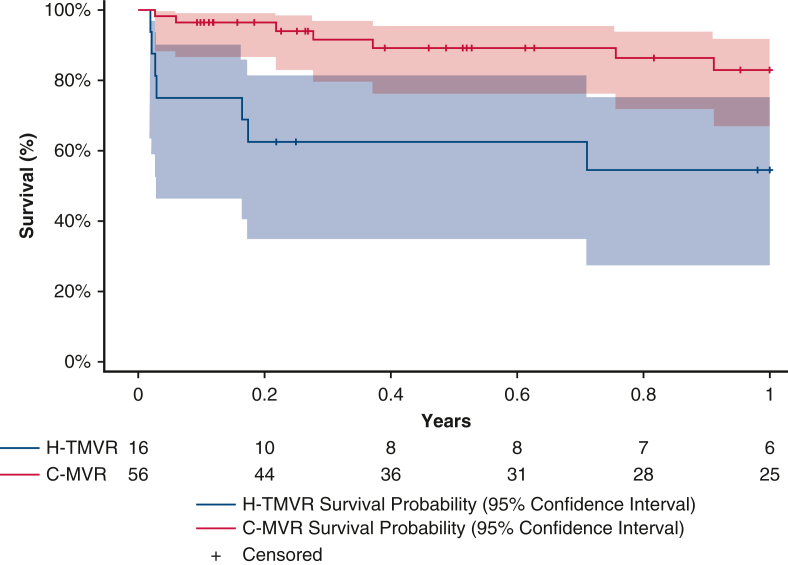
One-year survival was 82.8% in the C-MVR cohort and 54.7% in the H-TMVR cohort. Tables 2 and 3 summarize the operative findings and outcomes for both patient subgroups.
Table 3.
Perioperative and short-term outcomes
| Outcome | All (N = 72) | MVR (N = 56) | H-TMVR (N = 16) | P |
|---|---|---|---|---|
| Valve embolization, n (%) | 2 (2.78%) | 0 (0%) | 2 (12.50%) | .007 |
| AV groove disruption, n (%) | 1 (1.39%) | 0 (0%) | 1 (6.25%) | .059 |
| LCX injury, n (%) | 0 (0%) | 0 (0%) | 0 (0%) | - |
| LVOTO, n (%) | 3 (4.17%) | 0 (0%) | 3 (18.75%) | .001 |
| Second valve placement, n (%) | 2 (2.78%) | 0 (0%) | 2 (13%) | .007 |
| Procedure conversion, n (%) | 1 (1.39%) | 0 (0%) | 1 (6.25%) | .059 |
| IABP, n (%) | 2 (2.78%) | 0 (0%) | 2 (12.50%) | .007 |
| PVL moderate+, n (%) | 2 (2.78%) | 1 (1.79%) | 1 (6.25%) | .338 |
| Valve reintervention, n (%) | 0 (0.00%) | 0 (0%) | 0 (0.00%) | - |
| Stroke, n (%) | 3 (4.17%) | 3 (5.36%) | 0 (0.00%) | .344 |
| New RRT, n (%) | 5 (6.94%) | 3 (5.36%) | 2 (12.50%) | .322 |
| Reoperation for bleed, n (%) | 2 (2.78%) | 1 (1.79%) | 1 (6.25%) | .338 |
| DSWI, n (%) | 2 (2.78%) | 0 (0%) | 2 (13%) | .007 |
| In-hospital mortality, n (%) | 6 (8.33%) | 2 (3.57%) | 4 (25.00%) | .006 |
| 1-y survival, % (95% CI) | 76% (87.63%-64.63%) | 82.82% (94.96%-70.68%) | 54.69% (80.43%-28.95%) | .004 |
| Hospital LOS, median (Interquartile range) | 10.5 (9-15.5) | 10.5 (9-15) | 10.5 (7.5-24.5) | .469 |
| ICU LOS, median (Interquartile range) | 2.0 (4-7) | 2.0 (4-6) | 3.0 (7-10.25) | .992 |
MVR, Mitral valve replacement; H-TMVR, hybrid transcatheter mitral valve replacement; AV, atrioventricular; LCX, left circumflex; LVOTO, left ventricular outflow tract obstruction; IABP, intra-aortic balloon pump; PVL, paravalvular leak; RRT, renal replacement therapy; DSWI, deep sternal wound infection; CI, confidence interval; LOS, length of stay; ICU, intensive care unit.
Discussion
For decades, mitral valve replacement in the setting of severe annular calcification has been associated with high perioperative morbidity and mortality.6 This has been paralleled with an increasing MAC patient population referred for surgery due to the increased screening for transcatheter valve therapies. Thus, there has been an increasing demand for safe and novel surgical strategies to deal with extensive MAC in mitral valve surgery particularly given that the current MAC population are older and have a more extensive form of the disease with multiple comorbidities, whereas significant anatomic limitations remains a major barrier against widespread application of transcatheter technologies in patients with extensive MAC.
Key Findings
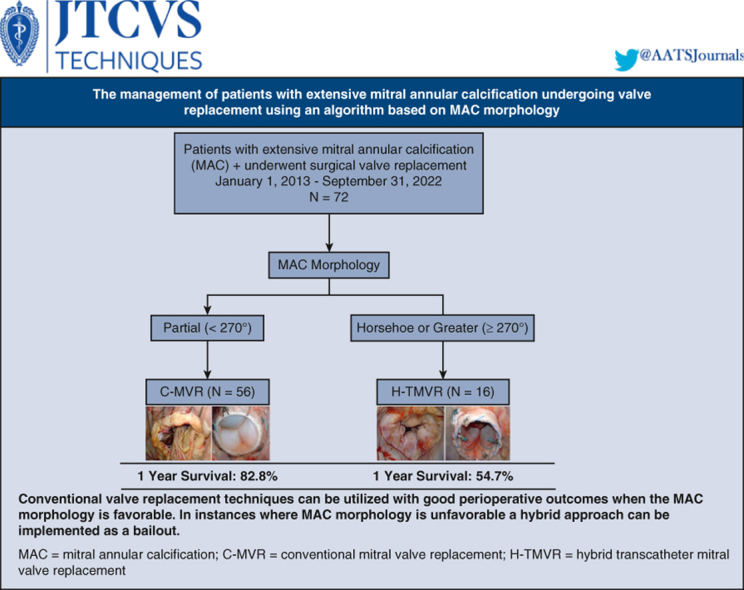
For MAC interventions, a dedicated multidisciplinary team approach is a prerequisite to success. All patients in the current study followed a strict protocol in evaluation based on advanced cardiac imaging and multidisciplinary team assessment to evaluate surgical candidacy, surgical approach, perioperative risk, and medical optimization before surgery. The MAC classification and management algorithm should be used by the cardiac surgeon and the structural heart interventionist to guide management (Figure 5).
Figure 5.
Surgical management of extensive mitral annular calcification. Utilizing CT imaging to delineate annular calcification anatomy can help identify patients who can undergo conventional mitral valve replacement. For those patients with more complex anatomy, transatrial hybrid transcatheter intervention can be a bailout strategy in anatomically feasible cases. C-MVR, Conventional mitral valve replacement; H-TMVR, hybrid transcatheter mitral valve replacement.
Conventional Mitral Valve Replacement Is Safe in Mitral Annular Calcification
In our valve reference center, our default strategy for severe MAC remains valve replacement with standard surgical prostheses using “Respect” surgical techniques for most patients with noncircumferential or partial MAC. Three main techniques for valve implantation were adopted: a modified anterior leaflet flip technique, intraleaflet implantation, and periannular suture placement to avoid en bloc annular decalcification and reconstruction. En bloc annular decalcification/reconstruction of the AV groove was avoided in all patients, given that the current MAC population is high risk due to advanced age, more extensive forms of MAC, and prevalence of extracardiac comorbidities. In fact, several patients in this study were already rejected from other transcatheter valve therapies or had already received transcatheter therapy with transcatheter aortic valve replacement and transcatheter mitral edge-to-edge repair. Given the lack of consensus regarding the safest strategy to replace the mitral valve in patients with extensive MAC, we adopted a more conservative approach using “Respect strategies” with chordal preservation while avoiding en bloc annular decalcification, which likely contributed to our 0% incidence of AV groove disruption in the conventional valve replacement group. However, this strategy did result in using a smaller mitral prosthesis implantation that will require longer-term follow-up to determine if this would have any potential clinical consequences.
We did not encounter any cases of AV groove disruption, ventricular rupture, LVOT obstruction, or perioperative ischemia in the C-MVR cohort, which had an in-hospital mortality of 3.6%. One patient was discharged with moderate paravalvular regurgitation that was amenable to percutaneous closure. Comparing outcomes on C-MVR literature is difficult, because most studies come from case series, with no details on the extent of MAC or the patients' risk profile. A study by Kaneko and colleagues7 provided extensive data from 9551 patients undergoing C-MVR, with estimated higher inpatient mortality of 5.8% among patients with MAC compared with patients without MAC (4.4%). However, this analysis was based on the Society of Thoracic Surgeons Adult Cardiac Surgery Database, which lacks information on the severity of MAC. Our study is unique in that we have a detailed description of MAC severity based on a specific classification in all patients and we excluded rheumatic disease related MAC, which is considered a less severe form of calcification and usually occurs in a younger patient population.
Hybrid Transatrial Approaches Are Feasible in Select Mitral Annular Calcification Patients
H-TMVR via transatrial implantation of a balloon-expandable transcatheter valve after resection of the anterior leaflet can be performed safely in horseshoe or circumferential MAC. We have adopted several techniques that help minimize the risk of operative complications to ensure a more durable valve replacement. The anterior leaflet of the mitral valve can be resected to reduce the risk of LVOT obstruction. Commissural gaps also can be closed to better circularize the annulus and reduce PVL. The Sapien 3 valve can be modified by placing 1 or more rows of Teflon felt at the atrial side to improve sealing against the MAC and reduce the risk of PVL (Figure 4). Sutures can be placed at noncalcified sections of the annulus and tied to the Teflon cuff after valve deployment to provide additional reinforcement of the transcatheter prosthesis to reduce the risk of device migration or embolization. This H-TMVR approach can also offer a unique advantage of allowing the surgeon to perform concomitant procedures.
Before standardization of the H-TMVR technique, we had a challenging learning curve related to patient selection and how to minimize the risks of LVOT obstruction and other intraoperative complications. This learning curve has been made more challenging by the relatively low volume of patients suitable for H-TMVR, given that our default approach is conventional valve replacement.
The Sapien valve provided several advantages because it is a low-profile valve with an open distal stent to minimize the risk of LVOT obstruction, with pericardial leaflets that can sustain the ventricular pressure, as well as a skirt to limit the risk of PVL. However, it remains off-label for use in the mitral position. Future prostheses dedicated for the mitral position might help minimize some of those hurdles encountered in the H-TMVR group.
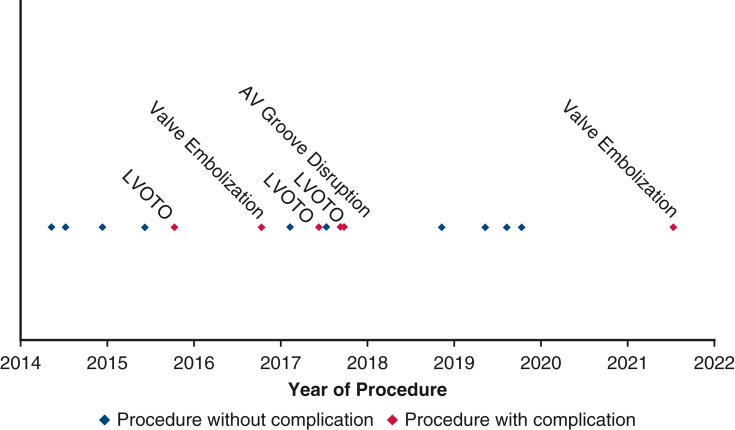
Almost all valve-related complications occurred in the H-TMVR group of patients, particularly early in our experience before standardization of technique and understanding the LVOT obstruction risk. At this early stage, we had incidences of significant LVOT obstruction (3 patients), valve embolization (2 patients), left ventricular tear that was repaired primarily in 1 patient, and AV groove disruption requiring conversion to C-MVR. Our overall in hospital mortality was 25%, and all of those deaths happened in patients with intraoperative complications (Figure E1). Despite the high mortality, these results remain comparable to other studies showing high 30-day mortality in H-TMVR of 25.0% and 34.5%.8,9
Figure E1.
Learning curve for H-TMVR implantation. AV, Atrioventricular; LVOTO, left ventricular outflow tract obstruction.
One-Year Outcomes Are Poor in Patients With Extensive Mitral Annular Calcification Requiring Hybrid Transcatheter Mitral Valve Replacement
One-year survival was 82.8% in the C-MVR (7 mortalities before 1 year) versus 54.7% in the H-TMVR group (7 mortalities before 1 year) (Figure E2). Patients with MAC associated with mitral valve disease had higher 1-year mortality after H-TMVR. Those findings were confirmed by other clinical studies following patients with MAC treated with H-TMVR. Guerrero and colleagues8 and Yoon and colleagues9 had 1-year survivals of 53.7% and 62.8%, respectively. Thus, in patients with extensive MAC, appropriate patient selection is essential to avoid futile procedures. This also could be explained in part by better prosthesis performance in patients receiving C-MVR, with less residual mitral regurgitation and less risk for LVOT obstruction, as well as the higher overall cardiovascular mortality in patients with more MAC burden.10
Figure E2.
Standard Kaplan–Meier curve comparing C-MVR and H-TMVR 1-year survival. H-TMVR, Hybrid transcatheter mitral valve replacement; C-MVR, conventional mitral valve replacement.
Implications for Practice
In a mitral reference center, surgery for extensive MAC is safe and feasible using a dedicated multidisciplinary team, CT-based MAC management algorithms, versatile surgical techniques, and novel hybrid approaches. Figure 5 shows a Graphical Abstract of this study. Futile interventions and surgery should be avoided in patients with more severe forms of MAC because of predictable poor 1-year survival.
Study Limitations
This study has several limitations that may influence the interpretation and generalizability of the results. Our study is a retrospective review and therefore is subject to all the inherent limitations related to this model of analysis. Follow-up was based on our own institutional chart review and therefore lacks complete death data that may impact true postdischarge mortalities for patients who left our health system. Our sample size, particularly in the H-TMVR group, limited the analysis given that our default therapy for extensive MAC is C-MVR in most patients. There was a lack of longer-term follow-up for echocardiography and valve data limiting the ability to determine durability of these surgical techniques. Finally, implementation of the protocol and interpretations of those outcomes has been achieved within a single comprehensive valve center of excellence (level I center of excellence) with a very experienced team, and our results may not be generalizable to non-reference centers.
Conclusions
C-MVR using respect techniques without en bloc annular decalcification remains safe and feasible in most patients with extensive MAC. Hybrid procedures using off-label balloon-expandable devices should be reserved as a bailout in highly select patients because of the increased perioperative risk, unforgiving learning curve, and poor 1-year survival.
Webcast
You can watch a Webcast of this AATS meeting presentation by going to: https://www.aats.org/resources/mitral-valve-replacement-for-extensive-mitral-annular-calcification-surgical-strategies-and-outcomes.

Conflict of Interest Statement
D.H.A. is the National Co-Principal Investigator of the TRILUMINATE U.S. Pivotal Trial (Abbott), the ReChord FDA Pivotal Trial (NeoChord), the APOLLO FDA Pivotal Trial (Medtronic), and the CoreValve U.S. Pivotal Trial (Medtronic). The Icahn School of Medicine at Mount Sinai receives royalty payments from Edwards Lifesciences and Medtronic for intellectual property related to Dr Adams' involvement in the development of 2 mitral valve repair rings and 1 tricuspid valve repair ring. G.H.L.T. is a consultant and physician advisory board member for Medtronic and Abbott Structural Heart, a physician advisory board member for Boston Scientific and JenaValve, and a consultant for NeoChord, and has received speaker's honoraria from Siemens Healthineers and East End Medical. All other authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Footnotes
Institutional Review Board Approval: STUDY-22-00800, August 29, 2022.
Informed Consent Statement: The study protocol was approved by our local Institutional Review Board (STUDY-22-00800, August 29, 2022) and was compliant with the Health Insurance Portability and Accountability Act regulations and the ethical guidelines of the 1975 Declaration of Helsinki. The approval included a waiver of informed consent.
Appendix E1
Supplementary Data
Periannular suture technique: Noneverted sutures are passed behind the calcium bar, and the valve is implanted in a supra-annular position. Video available at: https://www.jtcvs.org/article/S2666-2507(23)00389-9/fulltext.
Anterior leaflet flip technique: The anterior leaflet is disinserted and flipped over toward the posterior leaflet preserving the whole subvalvular apparatus creating a “neo-annulus” in front of the MAC bar. Video available at: https://www.jtcvs.org/article/S2666-2507(23)00389-9/fulltext.
Direct access transatrial implantation of a Sapien 3 valve into MAC. Video available at: https://www.jtcvs.org/article/S2666-2507(23)00389-9/fulltext.
References
- 1.Fann J.I., Ingels N.B., Jr., Miller D.C. In: Cardiac Surgery in the Adult. 5th ed. Cohn L.H., Adams D.H., editors. McGraw-Hill Education; 2018. Pathophysiology of mitral valve disease. [Google Scholar]
- 2.Spencer F., Galloway A., Colvin S. A clinical evaluation of the hypothesis that rupture of the left ventricle following mitral valve replacement can be prevented by preservation of the chordae of the mural leaflet. Ann Surg. 1985;202:673–680. doi: 10.1097/00000658-198512000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roberts W.C. Complications of cardiac valve replacement: characteristic abnormalities of prostheses pertaining to any or specific site. Am Heart J. 1982;103:113–122. doi: 10.1016/0002-8703(82)90537-3. [DOI] [PubMed] [Google Scholar]
- 4.Alexis S.L., Alzahrani T.S., Akkoc D., Salna M., Khalique O.K., El-Eshmawi A., et al. Anatomic classification of mitral annular calcification for surgical and transcatheter mitral valve replacement. J Card Surg. 2021;36:2410–2418. doi: 10.1111/jocs.15535. [DOI] [PubMed] [Google Scholar]
- 5.El-Eshmawi A., Love B., Bhatt H.V., Pawale A., Boateng P., Adams D.H. Direct access implantation of a melody valve in native mitral valve: a hybrid approach in the presence of extensive mitral annular calcification. Ann Thorac Surg. 2015;99:1085. doi: 10.1016/j.athoracsur.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 6.David T.E., Feinde C.M., Armstrong S., Sun Z. Reconstruction of the mitral annulus. J Thorac Cardiovasc Surg. 1995;110:1323–1332. doi: 10.1016/S0022-5223(95)70055-2. [DOI] [PubMed] [Google Scholar]
- 7.Kaneko T., Hirji S., Percy E., Aranki S., McGurk S., Body S., et al. Characterizing risks associated with mitral annular calcification in mitral valve replacement. Ann Thorac Surg. 2019;108:1761–1767. doi: 10.1016/j.athoracsur.2019.04.080. [DOI] [PubMed] [Google Scholar]
- 8.Guerrero M., Urena M., Himbert D., Wang D.D., Eleid M., Kodali S., et al. 1-year outcomes of transcatheter mitral valve replacement in patients with severe mitral annular calcification. J Am Coll Cardiol. 2018;71:1841–1853. doi: 10.1016/j.jacc.2018.02.054. [DOI] [PubMed] [Google Scholar]
- 9.Yoon S.H., Whisenant B.K., Bleiziffer S., Delgado V., Dhoble A., Schofer N., et al. Outcomes of transcatheter mitral valve replacement for degenerated bioprostheses, failed annuloplasty rings, and mitral annular calcification. Eur Heart J. 2019;40:441–451. doi: 10.1093/eurheartj/ehy590. [DOI] [PubMed] [Google Scholar]
- 10.Fox C.S., Larson M.G., Vasan R.S., Guo C.Y., Parise H., Levy D., et al. Cross-sectional association of kidney function with valvular and annular calcification: The Framingham Heart Study. J Am Soc Nephrol. 2006;17:521–527. doi: 10.1681/ASN.2005060627. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Periannular suture technique: Noneverted sutures are passed behind the calcium bar, and the valve is implanted in a supra-annular position. Video available at: https://www.jtcvs.org/article/S2666-2507(23)00389-9/fulltext.
Anterior leaflet flip technique: The anterior leaflet is disinserted and flipped over toward the posterior leaflet preserving the whole subvalvular apparatus creating a “neo-annulus” in front of the MAC bar. Video available at: https://www.jtcvs.org/article/S2666-2507(23)00389-9/fulltext.
Direct access transatrial implantation of a Sapien 3 valve into MAC. Video available at: https://www.jtcvs.org/article/S2666-2507(23)00389-9/fulltext.