Abstract
Objectives
Autosomal recessive spastic ataxia of Charlevoix-Saguenay (ARSACS) is an early-onset ataxia characterized by cerebellar dysfunction, spasticity, and sensory-motor polyneuropathy due to variations in the SACS gene (13q11). To date, no studies have instrumentally assessed vestibular function in this condition.
Methods
We report a 36-year-old woman with diagnosis of ARSACS syndrome due to homozygous mutation (c.12232 C>T, p.Arg4078Ter) in the SACS gene. Neurologic examination showed spastic-ataxic gait, dysarthric speech, 4-limb ataxia, and spastic hypertonia with lower limb hyperreflexia.
Results
A vestibular instrumental evaluation including bedside oculomotor testing found gaze-evoked and rebound nystagmus on horizontal and vertical gaze, saccadic movements within normality ranges, saccadic pursuit, and slightly impaired visually enhanced vestibulo-ocular reflex (VVOR). A near-normal VOR suppression (VORS) was recorded. Neither head shakings, skull vibrations, nor supine positionings could evoke nystagmus. Finally, the video-head impulse test detected a symmetrical VOR impairment for all the semicircular canals (SCs), mostly involving the horizontal SCs, with corrective saccades in all planes.
Discussion
Vestibular hypofunction may be found in ARSACS syndrome and may represent a possible pitfall in the differential diagnosis of recessive cerebellar and afferent ataxias. In this setting, ARSACS syndrome should be considered in the differential diagnosis of CANVAS.
PRACTICAL IMPLICATIONS
Vestibular hypofunction may be found in ARSACS syndrome and may represent a possible pitfall in the differential diagnosis of recessive cerebellar and afferent ataxias.
Introduction
Autosomal recessive spastic ataxia of Charlevoix-Saguenay (ARSACS) is characterized by early cerebellar dysfunction, spasticity, and sensory-motor polyneuropathy. The disease is caused by variations in the SACS gene (13q11) that lead to absent or severely reduced levels of sacsin.1 The cellular role of sacsin is still a matter of study, but its reduction seems to correlate with disease severity in ARSACS syndrome.1 While many works have described the clinical picture of the disease, no studies have instrumentally assessed vestibular function in this condition so far.
Case Report
A 36-year-old woman with diagnosis of ARSACS syndrome came to our attention. A homozygous mutation (c.12232 C>T, p.Arg4078Ter) in the SACS gene was previously found. Symptoms began at the age of 2 years, with slow progressive decline over the years. During evaluation, the patient complained of gait difficulties with instability and several falls, dysphagia for liquids, fatigue, and distal limb paresthesia; however, no limitation on major activities of daily life were reported. Neurologic examination found spastic-ataxic gait, dysarthric speech, limb ataxia, spastic hypertonia, and lower limb hyperreflexia.
Brain MRI displayed atrophy of the superior vermis and anterior lobes of the cerebellum (Figure 1A). Electroneurography showed a predominant sensory mixed axonal polyneuropathy. Hearing assessment was normal. A vestibular instrumental evaluation including bedside oculomotor testing detected gaze-evoked and rebound nystagmus on horizontal and vertical direction, saccadic movements within normality ranges (Figure 2), saccadic pursuit, and slightly impaired visually enhanced vestibulo-ocular reflex (VVOR). A near-normal VOR suppression (VORS) was recorded. Neither head shakings, skull vibrations, nor supine positionings could evoke nystagmus (Video 1). Finally, the video-head impulse test detected a symmetrical VOR impairment for all the semicircular canals (SCs), mostly involving the horizontal SCs, with corrective saccades in all planes (Figure 3). Perceptual speech assessment found ataxic dysarthria characterized by scanning speech, irregular articulatory breakdowns, distortion of consonant and vowel, variable speech rate, and pneumophonic incoordination with phonation on residual air. Acoustic analysis of speech performed with the open-sources Praat software and the free beta version of Dysarthria Analyzer software2,3 revealed slow speech, diadochokinetic (DDK) irregularity and slow rate, reduction of the maximum phonation time, and higher variability of the power spectral density. Balance, assessed through posturographic study4 performed in different conditions (i.e., eyes open/closed on a firm/soft surface), was abnormal in each of them (Figure 1B). Sway amplitude and area grew with task complexity. Cognitive tasks worsened balance and all the phases of the instrumented TUG test, but not the walking phases.5
Figure 1. Brain MRI and Posturographic Study.
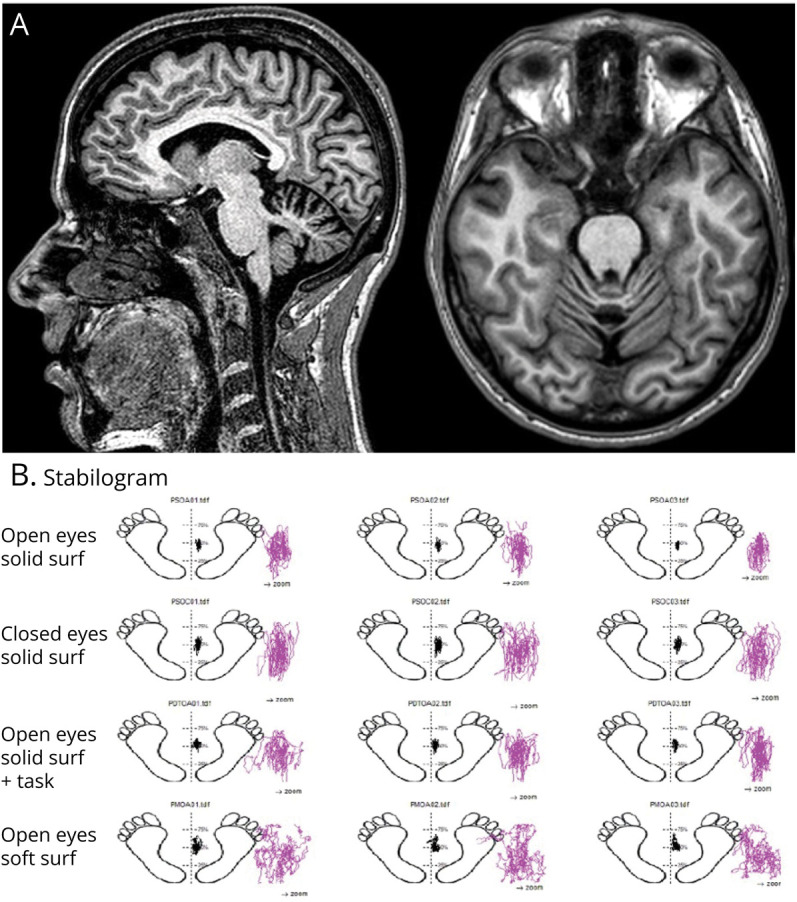
(A) Sagittal and axial T1-weighted showing atrophy of the superior vermis and anterior lobes of the cerebellum; (B) posturographic study.
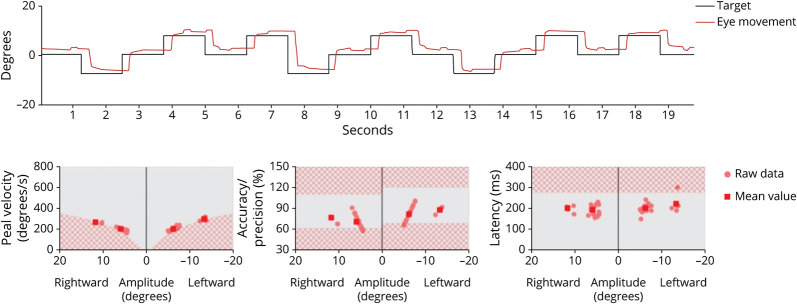
Figure 2. Analysis of Saccadic Movements.
Analysis of saccadic movements using an ICS video-oculographic system (GN Otometrics, Denmark). Right eye position tracking and corresponding analyses of saccadic movements with areas in red squared background corresponding to age-related abnormality ranges. While the analysis shows slightly low mean values of peak velocity, mean values for accuracy/precision and latency are within normality ranges.
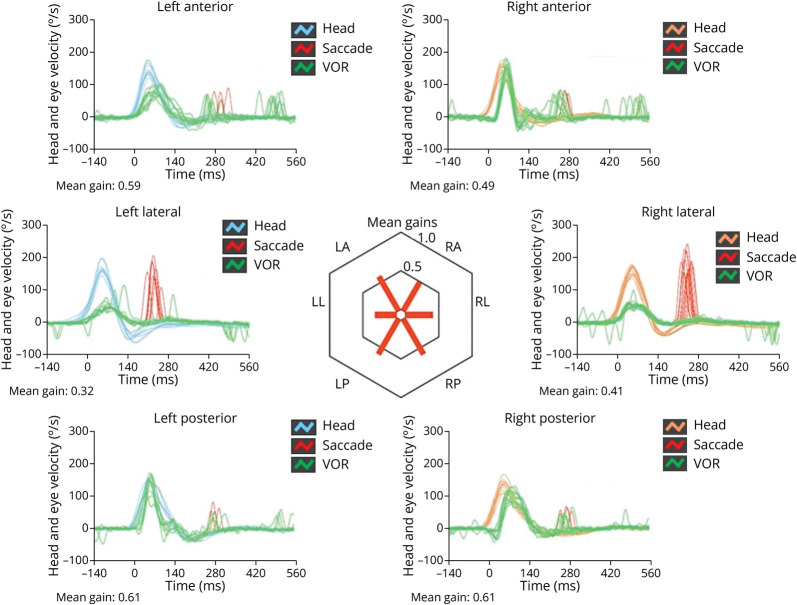
Figure 3. Video-Head Impulse Test Findings.
vHIT measurements with an ICS video-oculographic system (GN Otometrics, Denmark) showing a bilateral and symmetrical VOR impairment involving all the semicircular canals, particularly both horizontal canals, with corrective saccades in all planes. Blue lines represent head impulses exciting left canals, orange lines correspond to impulses for right canals, green lines represent eye movements induced by the activation of VOR following each impulse, and red lines correspond to corrective saccades. The mean value of VOR gain (eye velocity/head velocity) is reported for each canal. The hexagonal plot in the center of the figure summarizes mean VOR gains for each canal. Impaired gains are shown in red. vHIT = video-Head Impulse Test; VOR = vestibulo-ocular reflex.
Download Supplementary Video 1 (110.8MB, mov) via http://dx.doi.org/10.1212/200239_Video_1
Discussion
Aside from the classic triad of cerebellar ataxia, lower limb spasticity, and peripheral neuropathy, some individuals with ARSACS syndrome may present with other features, such as epilepsy, deafness, and intellectual disability.6 Furthermore, the absence of at least one of the defining clinical features has been reported in affected patients.6 To date, only 1 study, performed in 1979, has assessed oculomotor and vestibular function in ARSACS syndrome, finding the presence of several alterations (i.e., horizontal gaze and optokinetic nystagmus, impairment of smooth pursuit, abnormal fixation suppression of caloric nystagmus, and saccadic dysmetria), which confirmed that vermis and vestibulo-cerebellum were mainly affected in this condition.7 The patient reported in this study exhibited similar findings consistent with dysfunction of the same cerebellar structures. Nevertheless, VORS testing was unexpectedly near-normal, likely because the underlying concurrent bilateral vestibular loss did not require any VOR cancellation mechanisms. Accordingly, the lack of evoked nystagmus by head shakings, skull vibrations, and positionings reflected a symmetrical vestibular loss, which is in line with a progressive bilateral impairment of labyrinthine afferents/sensors. The detection of bilateral vestibular hypofunction in the presence of ataxia and polyneuropathy has recently gained a significant role in the differential diagnosis of adult-onset recessive cerebellar and afferent ataxias following the discovery of CANVAS.8 Because the latter syndrome combines peripheral and central dysfunctions, an abnormal VVOR represents its peculiar oculomotor sign because it reflects a compound deficit of different compensatory oculomotor reflex (i.e., VOR, optokinetic reflex, and smooth pursuit).9 Similarly, an unexpected dissociation between smooth pursuit deficits and a “falsely” normal VORS disclose the overlap of central oculomotor dysfunction and bilateral VOR impairment, representing another key feature of CANVAS.8 The patient reported in this study exhibited all the aforementioned vestibular and oculomotor abnormalities.
In this setting, our case expands the possible additional features in ARSACS syndrome, which may be identified by the contemporary presence of progressive cerebellar ataxia, peripheral neuropathy, and bilateral vestibular hypofunction, thus mimicking CANVAS. One important difference between the 2 syndromes that should be kept in mind is represented by the age at onset. Indeed, clinical manifestations of ARSACS syndrome usually start in childhood, as in our case, while CANVAS symptoms usually manifest in adulthood. However, in some cases, ARSACS syndrome may have a later onset in adulthood making its diagnosis more complex.10
Likewise, Friedreich ataxia should be considered in the differential diagnosis. As reviewed by Jordan et al.,11 the combination of neuropathy and cerebellar ataxia should suggest an autosomic recessive ataxia. Among these, Friedreich ataxia should be considered at first; moreover, vestibular hypofunction may be found in patients with Friedreich ataxia.12 Nonetheless, Friedreich ataxia includes other neurologic and non-neurologic clinical features that are lacking in our case.
Based on these premises, when CANVAS is suspected in the presence of the classic triad and a negative genetic testing for variations in its causative genes, it may be reasonable to consider performing additional analysis in the SACS gene in some selected cases with concomitant lower limb spasticity. However, we are describing only a single case that does not allow us to know whether these findings can be generalized and included in the diagnostic workup of recessive cerebellar and afferent ataxias. In this setting, further studies assessing vestibular reflexes in patients with ARSACS syndrome will be needed to evaluate the rate of vestibular dysfunctions in this population.
Appendix. Authors

| Name | Location | Contribution |
| Giacomo Argenziano, MD | Neurology Unit, Department of Biomedical, Metabolic and Neural Science, University of Modena and Reggio Emilia; Neurology Unit, Neuromotor and Rehabilitation Department, Azienda USL-IRCCS di Reggio Emilia, Italy | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; study concept or design; and analysis or interpretation of data |
| Francesco Cavallieri, MD, PhD | Neurology Unit, Neuromotor and Rehabilitation Department, Azienda USL-IRCCS di Reggio Emilia, Italy | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; study concept or design; and analysis or interpretation of data |
| Andrea Castellucci, MD | Otolaryngology Unit, Azienda USL-IRCCS di Reggio Emilia, Italy | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; study concept or design; and analysis or interpretation of data |
| Valentina Fioravanti, MD, PhD | Neurology Unit, Neuromotor and Rehabilitation Department, Azienda USL-IRCCS di Reggio Emilia, Italy | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; study concept or design; and analysis or interpretation of data |
| Giulia Di Rauso, MD | Neurology Unit, Department of Biomedical, Metabolic and Neural Science, University of Modena and Reggio Emilia; Neurology Unit, Neuromotor and Rehabilitation Department, Azienda USL-IRCCS di Reggio Emilia, Italy | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; and analysis or interpretation of data |
| Annalisa Gessani, BSpPath | Neurology, Neuroscience Head Neck Department, Azienda Ospedaliero-Universitaria di Modena, Italy | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; and analysis or interpretation of data |
| Isabella Campanini, PhD | LAM-Motion Analysis Laboratory, Neuromotor and Rehabilitation Department, Azienda USL-IRCCS di Reggio Emilia, Italy | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; and analysis or interpretation of data |
| Andrea Merlo, Eng, PhD | LAM-Motion Analysis Laboratory, Neuromotor and Rehabilitation Department, Azienda USL-IRCCS di Reggio Emilia, Italy | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; and analysis or interpretation of data |
| Manuela Napoli, MD, PhD | Neuroradiology Unit, Azienda USL-IRCCS di Reggio Emilia, Italy | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; and analysis or interpretation of data |
| Sara Grisanti, BME | Clinical and Experimental Medicine PhD Program, University of Modena and Reggio Emilia, Italy | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; and analysis or interpretation of data |
| Jessica Rossi, MD | Neurology Unit, Neuromotor and Rehabilitation Department, Azienda USL-IRCCS di Reggio Emilia; Clinical and Experimental Medicine PhD Program, University of Modena and Reggio Emilia, Italy | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; and analysis or interpretation of data |
| Giulia Toschi, PhD | Neurology Unit, Neuromotor and Rehabilitation Department, Azienda USL-IRCCS di Reggio Emilia, Italy | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; and analysis or interpretation of data |
| Chiara Zini, NP | Neurology Unit, Neuromotor and Rehabilitation Department, Azienda USL-IRCCS di Reggio Emilia, Italy | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; and analysis or interpretation of data |
| Angelo Ghidini, MD | Otolaryngology Unit, Azienda USL-IRCCS di Reggio Emilia, Italy | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; and analysis or interpretation of data |
| Franco Valzania, MD | Neurology Unit, Neuromotor and Rehabilitation Department, Azienda USL-IRCCS di Reggio Emilia, Italy | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; study concept or design; and analysis or interpretation of data |
Study Funding
This study was partially supported by the Italian Ministry of Health—Ricerca Corrente Annual Program 2023.
Disclosure
The authors report no relevant disclosures. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
References
- 1.Longo F, De Ritis D, Miluzio A, et al. Assessment of sacsin turnover in patients with ARSACS: implications for molecular diagnosis and pathogenesis. Neurology. 2021;97(23):e2315-e2327. doi: 10.1212/WNL.0000000000012962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hlavnička J, Růžičková H, Tykalová T, Novotny` M, Rusz J. Dysarthria analyzer. Beta Version. 2022. Accessed December 20, 2022. ([computer program]). dysan.cz/
- 3.Argenziano G, Cavallieri F, Monfrini E, et al. Deconstructing speech alterations in episodic ataxia type 2: perceptual-acoustic analysis in a case due to CACNA1A gene mutation. Parkinsonism Relat Disord. 2023;108:105311. doi: 10.1016/j.parkreldis.2023.105311 [DOI] [PubMed] [Google Scholar]
- 4.Merlo A, Zemp D, Zanda E, et al. Postural stability and history of falls in cognitively able older adults: the Canton Ticino study. Gait Posture. 2012;36(4):662-666. doi: 10.1016/j.gaitpost.2012.06.016 [DOI] [PubMed] [Google Scholar]
- 5.Cavallieri F, Campanini I, Gessani A, et al. Long-term effects of bilateral subthalamic nucleus deep brain stimulation on gait disorders in Parkinson's disease: a clinical-instrumental study. J Neurol. 2023;270(9):4342-4353. doi: 10.1007/s00415-023-11780-5 [DOI] [PubMed] [Google Scholar]
- 6.Vermeer S, van de Warrenburg BP, Kamsteeg EJ, et al. ARSACS. 2003 December 9 [Updated 2020 January 2]. 2020. In: Adam MP, Mirzaa GM, Pagon RA, et al., eds. GeneReviews® [Internet]. University of Washington, Seattle; 1993–2023. ncbi.nlm.nih.gov/books/NBK1255 [PubMed] [Google Scholar]
- 7.Dionne J, Wright G, Barber H, Bouchard R, Bouchard JP. Oculomotor and vestibular findings in autosomal recessive spastic ataxia of Charlevoix-Saguenay. Can J Neurol Sci 1979;6(2):177-184. doi: 10.1017/s0317167100119602 [DOI] [PubMed] [Google Scholar]
- 8.Szmulewicz DJ, Roberts L, McLean CA, MacDougall HG, Halmagyi GM, Storey E. Proposed diagnostic criteria for cerebellar ataxia with neuropathy and vestibular areflexia syndrome (CANVAS). Neurol Clin Pract. 2016;6(1):61-68. doi: 10.1212/CPJ.0000000000000215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leigh RJ, Zee DS. The Neurology of Eye Movements. 5th ed. Oxford University Press; 2015. [Google Scholar]
- 10.Baets J, Deconinck T, Smets K, et al. Mutations in SACS cause atypical and late-onset forms of ARSACS. Neurology. 2010;75(13):1181-1188. doi: 10.1212/WNL.0b013e3181f4d86c [DOI] [PubMed] [Google Scholar]
- 11.Paulus-Andres JA, Burnett MS. Three adult-onset autosomal recessive ataxias: what adult neurologists need to know. Neurol Clin Pract. 2021;11(3):256-262. doi: 10.1212/CPJ.0000000000000947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fahey MC, Cremer PD, Aw ST, et al. Vestibular, saccadic and fixation abnormalities in genetically confirmed Friedreich ataxia. Brain. 2008;131(Pt 4):1035-1045. doi: 10.1093/brain/awm323 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Download Supplementary Video 1 (110.8MB, mov) via http://dx.doi.org/10.1212/200239_Video_1