Abstract
Background and Objectives
Multiple sclerosis (MS) commonly affects women in their childbearing years, necessitating discussion between patients and their MS treatment team around the issues of family planning, pregnancy, and postpartum experiences. This study assessed the impact of a diagnosis of MS on women's reproductive decision-making and on their perception of counseling received surrounding pregnancy. It also sought to evaluate trends in pregnancy and postpartum experiences and determine whether experiences differed by race, ethnicity, and zip code.
Methods
Women with an MS diagnosis seen at the University of Virginia MS Clinic or at Virginia Commonwealth University (VCU) MS Clinic were invited to participate in a survey study. MS disease and pregnancy history, and, when appropriate, reasons for pregnancy avoidance were collected. Respondents who had >1 pregnancy following MS diagnosis were asked to evaluate the counseling they received from medical professionals and to share their pregnancy experiences including complications during pregnancy, delivery outcomes, and postpartum experience including breastfeeding.
Results
Of the 280 respondents, 76.6% were currently receiving MS specialty care. Most of them (79.3%) had not been pregnant following MS diagnosis. Of them, 20.1% indicated that this decision was driven by MS-related concerns: MS worsening with pregnancy (47%); ability to care for child secondary to MS (35%); passing MS onto child (19%); stopping disease-modifying therapies to attempt pregnancy (14%); lack of knowledge about options for pregnancy and MS (9%). Women with a more recent estimated decade of pregnancy were more likely to report neurologist counseling regarding MS and pregnancy (pregnancy before 2000: 40%, 2000–2010: 64.7%, 2010- present: 83.3%; χ2 0.020). Breastfeeding initiation was reported in 71.4% of postdiagnosis pregnancies (median duration 6 months, interquartile range 1.75–11).
Discussion
Over the past few decades, women with MS have received a wide range of evolving guidance surrounding family planning, pregnancy, and postpartum care. Survey data suggest improvements in MS/pregnancy counseling and medical management in recent years, which may be driven by an increase in research in the field. There remains an important need and opportunity to improve counseling of women with MS who are considering pregnancy.
Introduction
Multiple sclerosis (MS) is a chronic progressive neurologic disease with onset commonly between the ages of 20 and 40 years, affecting women in their childbearing years. Current evidence indicates that for most women with MS, pregnancy is safe, feasible, and without negative long-term consequences.1-5 The immunotolerant state of pregnancy is associated with reduced MS disease activity, but with increased relapse risk in the first 3 months postpartum.5-8 Pregnancy seems to have no effect on long-term accumulation of disability or risk of developing secondary progressive MS.2-5 MS is not associated with a significantly higher risk of obstetric or neonatal complications.4,5,9-11 Furthermore, breastfeeding does not worsen MS disease progression and may be protective against postpartum relapses.12-15
Despite these encouraging natural history studies, available research indicates that many women with MS modify their plans for pregnancy following diagnosis, suggesting an educational gap exists for patients regarding MS pregnancy topics. In a multinational survey of people with MS, most respondents said MS affected their family planning decision-making.16 Additional studies suggested 8%–28% of people with MS decide against having children due to MS.16-18 While some choose not to have children because of MS, many people with MS still desire to be parents, necessitating accurate information and counseling around family planning, pregnancy, and breastfeeding.19,20 Studies of people with MS desiring parenthood report that only 39% were counseled by their treating physician to plan pregnancy.18
The number of disease-modifying therapies (DMTs) for MS has markedly increased in the past 2 decades. Research on safety of common DMTs in pregnancy and breastfeeding shows a range of safety profiles, with limited data for some agents.6,7,21-25 The expansive growth of DMTs in a population that is often of childbearing age raises questions around the use of these medications and family planning. However, in a recent study, 42% of women with MS reported that they did not understand the teratogenic risk profile of their MS DMT, and almost half did not feel they were well informed about MS treatment and family planning.19
Where available, formal recommendations historically have been for women to stop DMTs before pregnancy and stay off until completion of breastfeeding, with DMT continuation only in the case that “MS activity during pregnancy outweighs the risk associated with the specific DMT.”12,14,26 However, recommendations are without consensus, and some studies suggest that the use of DMTs prepregnancy or in the first few weeks of pregnancy prevents relapses during pregnancy and the postpartum period.25,27-31 Lack of consensus in guidelines and limited safety data have likely resulted in inconsistent guidance for women with MS since the availability of the first DMTs in the mid-1990s.
The rapid advance of DMTs and limited data surrounding safety have resulted in variable and changing guidance for women with MS regarding pregnancy, breastfeeding, and DMT use. Research efforts to improve guidance and understanding have primarily occurred outside the United States,1,11,16,18-20 and importantly, evidence suggests that trends in family planning look different in the United States.2 Of importance, we have yet to elucidate how race, ethnicity, and socioeconomic status affect women's experiences with these issues. In this study, we sought to understand women's experiences, concerns, and choices surrounding MS, DMTs, pregnancy, and breastfeeding in a diverse, US-based sample of women with MS.
Methods
Standard Protocol Approvals, Registrations, and Patient Consents
This cross-sectional, survey-based cohort study was approved by the Institutional Review Boards at the University of Virginia (UVA) and Virginia Commonwealth University (VCU). All participants provided informed consent, written or electronic, before the start of any study-related procedures.
UVA Study
An administrative billing search identified 1,047 female patients, ages 18–65 years, seen by the UVA Neurology Department between 2011 and 2016 with an ICD-9/10 code for MS (including all MS subtypes). These patients were mailed a 10-page survey packet (eAppendix 1, links.lww.com/CPJ/A486: UVA Pregnancy Survey) with a return self-addressed, stamped envelope in August 2016. The packet included a cover letter explaining the study. All respondents were asked to provide information regarding MS history (age at diagnosis, treating neurologist type, and MS subtype), pregnancy history, and preferences and decision-making surrounding childbearing. Women with any pregnancy following MS diagnosis were asked additional information about MS history, current MS treatment (DMT), demographics (occupation, salary, and relationship status), and general questions surrounding pregnancy counseling received. For each pregnancy following MS diagnosis, women were also asked about intrapartum and postpartum complications, birth outcomes, and breastfeeding. Data were compiled on all surveys received by August 2017. Zip code data were used to derive the Child Opportunity Index (COI), a nationally normalized measure expressed as a percentile from 0 to 100 representing least to most opportunity.32 For example, children living in a zip code with a score of 57 would have better opportunities than 57% of other children nationwide. The COI was used as a proxy measure for socioeconomic status in this analysis.
VCU Study
An administrative database search (enterprise analytic) identified 1,517 adult female patients seen by the VCU Neurology Department between 2015 and 2021 with an ICD-9/10 code for MS (including all MS subtypes). These patients were mailed a letter with a description of the survey and link to an online RedCap survey in May 2022.33,34 The RedCap survey was formatted to resemble the UVA survey (eAppendix 1, links.lww.com/CPJ/A486: UVA Pregnancy Survey). The RedCap survey used branching logic, displaying appropriate fields based on the user's previous responses (e.g., if a pregnancy ended in spontaneous abortion, breastfeeding questions were not asked for that pregnancy). Data were compiled on all surveys completed and received by October 2022. Notable differences in the VCU survey include addition of a question regarding ethnicity (Hispanic vs non-Hispanic) and online survey format.
Statistical Analysis
Statistical analysis was completed using SPSS (version 28) software. All responses were deidentified and blinded to the researcher (E.E.K.) performing the analysis. General/demographic data from all respondents were collectively analyzed, with results reported as total and stratified by center (UVA vs VCU). Demographic traits were then compared between these 2 centers using ANOVA and the χ2 test as appropriate for continuous and ordinal variables. A 2-sided p value of <0.05 was defined as statistically significant, and intercohort demographic analyses were corrected for multiple comparisons through Bonferroni correction. MS/pregnancy counseling question responses were analyzed for all respondents reporting pregnancy with diagnosis of MS. Prepregnancy, intrapartum, and postpartum data were analyzed by individual pregnancy rather than by woman (some women reported multiple pregnancies with MS diagnosis). Selected response variables were analyzed through ANOVA or the χ2 test for associations with estimated decade of pregnancy or demographic variables.
Data Availability
Anonymized data not published within this article will be made available by request from any qualified investigator.
Results
Respondent Demographics
Of the 2,517 surveys sent out, 282 (11.2%) patients responded, with a higher response rate from the UVA at 17.4% compared with 7.12% from the VCU. Two UVA surveys were returned uncompleted by family members of the deceased recipient for an adjusted total of 280 valid responses. Because some participants did not respond to all questions, results were reported as the percent of responses divided by respondents to that particular question.
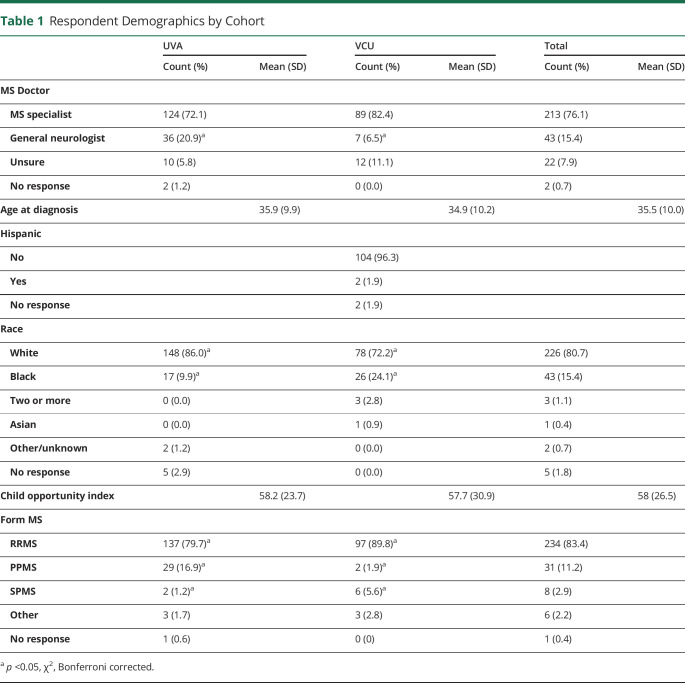
A total of 76.6% of respondents (213/278) were in the care of an MS specialist rather than a general neurologist during survey completion. MS subtype was as follows: 83.9% (234/279) relapsing-remitting MS, 11.1% (31/279) primary progressive MS, 2.9% (8/279) secondary progressive MS, and 2.2% (6/279) were unsure of their MS subtype. Compared with the VCU cohort, the UVA cohort reported a significantly higher percentage of patients with primary progressive MS (17.0% vs 1.9%) and a lower percentage of patients with relapsing-remitting MS (80.1% vs 89.8%) (χ2, p < 0.05); additional details are summarized in Table 1. The average age at diagnosis was 35.5 ± 10.0 years (mean ± SD). Self-reported race was as follows: 82.2% (226/275) White, 15.6% Black (43/275), and the remainder as Asian or 2 or more races. Data on ethnicity (Hispanic vs non-Hispanic) were collected from VCU respondents only (n = 108 respondents). Self-reported ethnicity in the VCU cohort included 1.9% being of Hispanic or Latino origin (2/108). The mean COI derived from zip codes was 58.0, with a range of 2–100. Rates of reported spontaneous and elective abortion were 28.0% (77/275) and 16.3% (44/270), respectively.
Table 1.
Respondent Demographics by Cohort
| UVA | VCU | Total | ||||
| Count (%) | Mean (SD) | Count (%) | Mean (SD) | Count (%) | Mean (SD) | |
| MS Doctor | ||||||
| MS specialist | 124 (72.1) | 89 (82.4) | 213 (76.1) | |||
| General neurologist | 36 (20.9)a | 7 (6.5)a | 43 (15.4) | |||
| Unsure | 10 (5.8) | 12 (11.1) | 22 (7.9) | |||
| No response | 2 (1.2) | 0 (0.0) | 2 (0.7) | |||
| Age at diagnosis | 35.9 (9.9) | 34.9 (10.2) | 35.5 (10.0) | |||
| Hispanic | ||||||
| No | 104 (96.3) | |||||
| Yes | 2 (1.9) | |||||
| No response | 2 (1.9) | |||||
| Race | ||||||
| White | 148 (86.0)a | 78 (72.2)a | 226 (80.7) | |||
| Black | 17 (9.9)a | 26 (24.1)a | 43 (15.4) | |||
| Two or more | 0 (0.0) | 3 (2.8) | 3 (1.1) | |||
| Asian | 0 (0.0) | 1 (0.9) | 1 (0.4) | |||
| Other/unknown | 2 (1.2) | 0 (0.0) | 2 (0.7) | |||
| No response | 5 (2.9) | 0 (0.0) | 5 (1.8) | |||
| Child opportunity index | 58.2 (23.7) | 57.7 (30.9) | 58 (26.5) | |||
| Form MS | ||||||
| RRMS | 137 (79.7)a | 97 (89.8)a | 234 (83.4) | |||
| PPMS | 29 (16.9)a | 2 (1.9)a | 31 (11.2) | |||
| SPMS | 2 (1.2)a | 6 (5.6)a | 8 (2.9) | |||
| Other | 3 (1.7) | 3 (2.8) | 6 (2.2) | |||
| No response | 1 (0.6) | 0 (0) | 1 (0.4) | |||
p <0.05, χ2, Bonferroni corrected.
MS-Related Pregnancy Avoidance
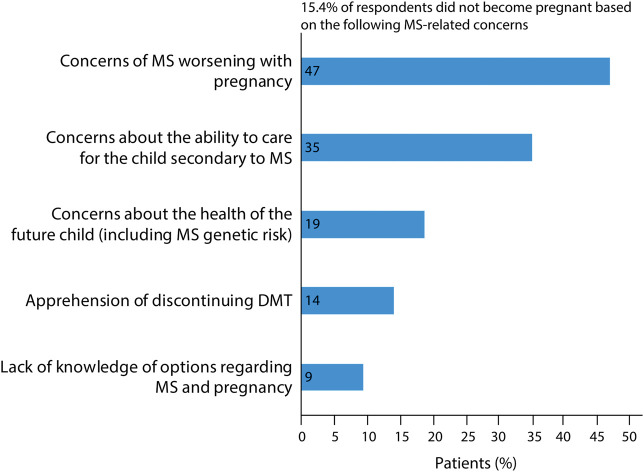
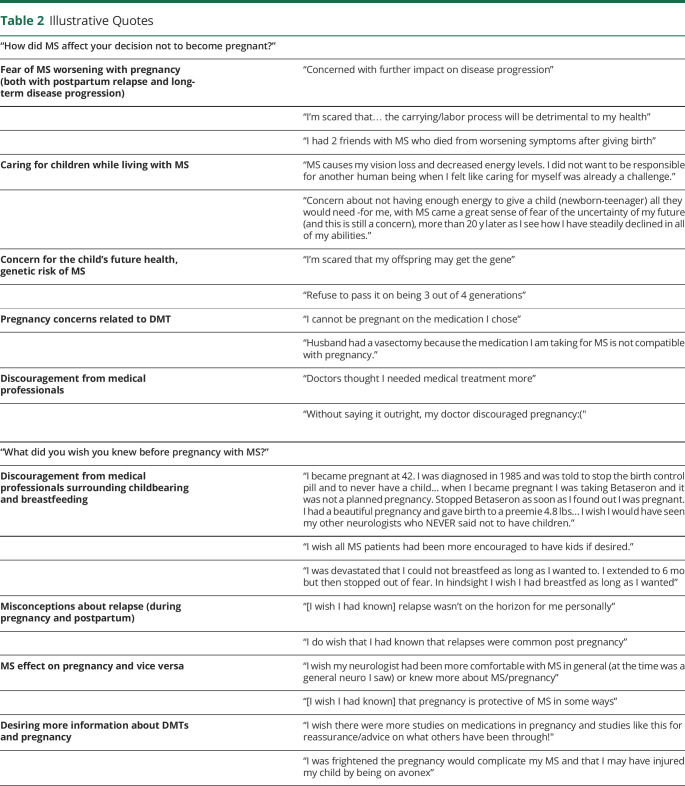
Approximately 79.3% of respondents reported no pregnancy after MS diagnosis (222/280). Of these individuals, the average age at diagnosis was 37.8 ± 9.8 years. For 20.1% (43/214), their decision not to become pregnant postdiagnosis was related to their MS, and they were diagnosed at a significantly younger age on average compared with respondents who had avoided pregnancy for non-MS reasons (30.3 ± 5.9 vs 39.8 ± 9.7 years; ANOVA p value <0.001). Patients reported several concerns that led to MS-related pregnancy avoidance including concern about MS worsening due to pregnancy (47%); concern of MS compromising ability to care for a child (35%); fear of health risks (including genetic risk of passing on MS) for their child (19%); apprehension of discontinuing MS DMT (14%); and lack of knowledge of options for carrying out a pregnancy with MS (9%) (Figure 1). In a free text section of the survey, 7% (3/43) of women who reported pregnancy avoidance stated this was due to medical professionals who discouraged them from having children or told them that they “needed to choose” between taking MS medications and childbearing (Table 2).
Figure 1. MS-Related Reasons for Pregnancy Avoidance Among Respondents.
Table 2.
Illustrative Quotes
| “How did MS affect your decision not to become pregnant?” | |
| Fear of MS worsening with pregnancy (both with postpartum relapse and long-term disease progression) | “Concerned with further impact on disease progression” |
| “I'm scared that… the carrying/labor process will be detrimental to my health” | |
| “I had 2 friends with MS who died from worsening symptoms after giving birth” | |
| Caring for children while living with MS | “MS causes my vision loss and decreased energy levels. I did not want to be responsible for another human being when I felt like caring for myself was already a challenge.” |
| “Concern about not having enough energy to give a child (newborn-teenager) all they would need -for me, with MS came a great sense of fear of the uncertainty of my future (and this is still a concern), more than 20 y later as I see how I have steadily declined in all of my abilities.” | |
| Concern for the child's future health, genetic risk of MS | “I'm scared that my offspring may get the gene” |
| “Refuse to pass it on being 3 out of 4 generations” | |
| Pregnancy concerns related to DMT | “I cannot be pregnant on the medication I chose” |
| “Husband had a vasectomy because the medication I am taking for MS is not compatible with pregnancy.” | |
| Discouragement from medical professionals | “Doctors thought I needed medical treatment more” |
| “Without saying it outright, my doctor discouraged pregnancy:(" | |
| “What did you wish you knew before pregnancy with MS?” | |
| Discouragement from medical professionals surrounding childbearing and breastfeeding | “I became pregnant at 42. I was diagnosed in 1985 and was told to stop the birth control pill and to never have a child... when I became pregnant I was taking Betaseron and it was not a planned pregnancy. Stopped Betaseron as soon as I found out I was pregnant. I had a beautiful pregnancy and gave birth to a preemie 4.8 lbs... I wish I would have seen my other neurologists who NEVER said not to have children.” |
| “I wish all MS patients had been more encouraged to have kids if desired.” | |
| “I was devastated that I could not breastfeed as long as I wanted to. I extended to 6 mo but then stopped out of fear. In hindsight I wish I had breastfed as long as I wanted” | |
| Misconceptions about relapse (during pregnancy and postpartum) | “[I wish I had known] relapse wasn't on the horizon for me personally” |
| “I do wish that I had known that relapses were common post pregnancy” | |
| MS effect on pregnancy and vice versa | “I wish my neurologist had been more comfortable with MS in general (at the time was a general neuro I saw) or knew more about MS/pregnancy” |
| “[I wish I had known] that pregnancy is protective of MS in some ways” | |
| Desiring more information about DMTs and pregnancy | “I wish there were more studies on medications in pregnancy and studies like this for reassurance/advice on what others have been through!" |
| “I was frightened the pregnancy would complicate my MS and that I may have injured my child by being on avonex” | |
When evaluating potential clinical and sociodemographic factors related to pregnancy avoidance, neither type of neurologist (general neurologist vs MS specialist), race, nor ethnicity were significantly associated with pregnancy avoidance due to MS. While not reaching significance in this small sample, the mean COI was lower for women with MS-related pregnancy avoidance vs those women who did not become pregnant for other reasons (50.4 vs 59.6; ANOVA, p = 0.051).
Women With Pregnancy After MS Diagnosis: Counseling and Prepregnancy
A minority of respondents (20.7%) reported pregnancy following MS diagnosis. Age at diagnosis was significantly younger (27.0 ± 5.0 years) compared with the respondents who did not become pregnant after diagnosis (37.8 ± 9.8 years; ANOVA, p < 0.001). Women in the VCU cohort were significantly more likely to have been pregnant before their MS diagnosis (60.9% at VCU vs 20% at UVA; χ2, p < 0.001) and had more children (60.9% at VCU vs 17.6% at UVA; χ2, p = 0.006) before MS diagnosis compared with the UVA cohort.
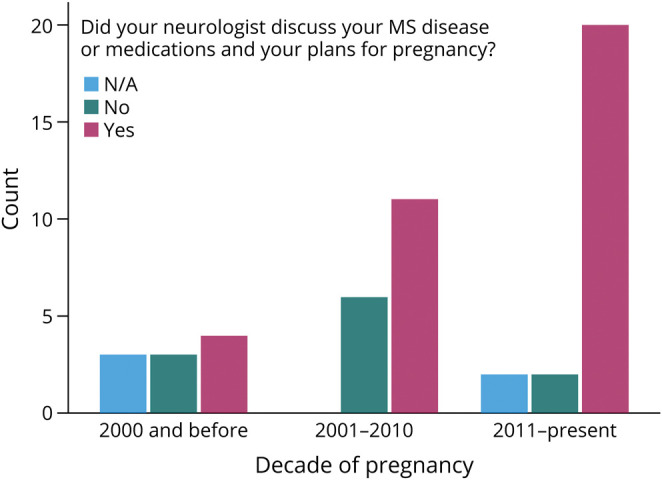
Among respondents with a pregnancy after MS diagnosis (n = 58), 69.1% (38/55) reported that their neurologist discussed pregnancy plans and MS disease or medications. When pooled by approximate decade of pregnancy (1985–2000, 2001–2010, 2011-present), the proportion of women who received counseling from their neurologist significantly increased over time (pregnancy before 2000: 40%, 2000–2010: 64.7%, 2010- present: 83.3%; χ2, p = 0.020) (Figure 2). Furthermore, although not statistically significant, a greater proportion of women treated by an MS specialist than by a general neurologist reported their neurologist counseled them regarding MS disease or medications and plans for pregnancy (71.4% vs 50%; χ2, p = 0.193).
Figure 2. Changes in MS and Pregnancy Counseling Over Time.
Collectively, 47.2% (25/53) of women chose their DMT based on their plans for subsequent pregnancy. Those with pregnancies in the past decade (2011-present) were most likely to choose their treatment plan based on their decision to get pregnant (58.3%), compared with the 2001–2010 group (41.2%) and the 2000 and earlier group (25%). Only 12.7% (7/55) of women with pregnancy after MS diagnosis were familiar with the medication pregnancy registry (referring to industry-sponsored medication pregnancy registries for individual DMT). When asked “what do you wish you knew before pregnancy with MS,” 42 respondents provided free-text responses. Approximately 52.3% were satisfied with the information they received from their physician; others identified the following gaps in education: 14% wished they knew more about the effects of pregnancy on MS (or vice versa), 10% reported misconceptions specifically about postpartum relapse (underestimating or overestimating postpartum relapse severity and incidence), 7% wished they had more complete safety information regarding DMTs, and 7% of women reported that they were discouraged by medical professionals from having pregnancies or breastfeeding for their desired duration (eFigure 1, links.lww.com/CPJ/A487).
The rate of unplanned pregnancy was 27.1% (23/85) among women pregnant after MS diagnosis, notably lower than the national average of 45%.35 The reported percentage of infertility (8.4%; 7/83) more closely approximated the national average of 11%.36 Furthermore, 27.4% of women reported they were on MS medication during conception (23/84), slightly higher than previously reported numbers ranging 14–25%.13,27 Of them, 41% were exposed to interferon beta products after conception, 27% were exposed to glatiramer acetate, and the remainder included natalizumab, rituximab, teriflunomide, fingolimod, and 1 unknown. DMT use at conception was associated with later pregnancy year (mean year of pregnancy, 2012 for those on DMT during conception vs 2006 for those not; ANOVA, p = 0.028). Of women who became pregnant after MS diagnosis, 76.8% (43/56) were not planning for additional children; among them, 18% cited MS-related concerns as the reason for this.
Pregnancy and Delivery
Women reported complications in 23.5% (20/85) of pregnancies after MS diagnosis; 70% of complications were presumed unrelated to MS, while 3 (15%) were attributed to MS, and 15% had unclear cause. Pregnancies with complications attributed to MS (n = 3) included 2 miscarriages while the mother was on DMT and 1 exacerbation of MS symptoms during pregnancy. Delivery outcomes were analyzed for all 75 live births reported after diagnosis of MS, i.e., excluding elective and spontaneous abortions and ongoing pregnancies during survey. Of the live births, 18.7% were reported to be premature, higher compared with the national average of 10%.37 For pregnancies with birth data available, 38.7% (29/75) of deliveries were by C-section, compared with national averages of 20%–32% over the past 3 decades.38 Furthermore, 82.4% (28/34) of respondents received an epidural, higher than national averages ranging approximately 40%–70% in the past 3 decades.38-40 Although limited in number of respondents, 21.2% (14/66) self-reported complications with delivery compared with an estimated 16.9% national average.41
Postpartum and Breastfeeding
Postpartum experience and breastfeeding-related questions were analyzed for women with pregnancies after MS diagnosis resulting in live births. Elective and spontaneous abortions, current pregnancies, or stillbirth/neonatal death were excluded. Postpartum issues (most commonly anxiety, depression, and infection) were reported in 26.5% (18/67) of these pregnancies, and the incidence of these complications approximated national averages.42-44
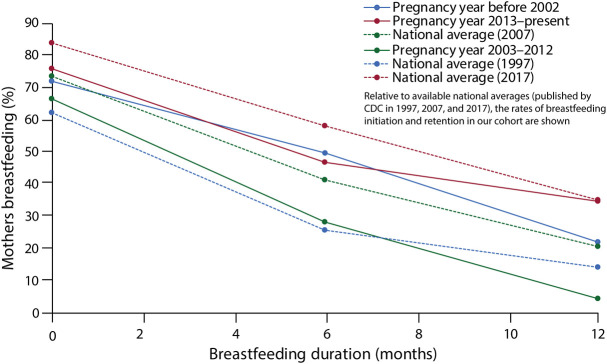
For the breastfeeding analyses, respondents (n = 5) who were currently breastfeeding during the survey were also excluded from breastfeeding duration data. In pregnancies with postpartum breastfeeding data (n = 61), 71.4% initiated breastfeeding overall, which is compared with corresponding national averages in Figure 3. The median duration of breastfeeding was 6.0 months (first quartile 1.75 months, third quartile 11.0 months). Respondents with live births after diagnosis of MS were asked whether they initiated breastfeeding; those who did not initiate breastfeeding were asked, “Why did you choose not to breastfeed?,” and free-text responses were categorized thematically. In 53% (9/17) of cases in which breastfeeding was not initiated, MS was the primary reason, with 41% specifically reporting a decision not to breastfeed to start MS medications (eFigure 2, links.lww.com/CPJ/A488). When breastfeeding was initiated, 23% reported their primary reason for cessation was to start MS treatment (among those who gave a reason n = 26, eFigure 2). The median breastfeeding duration was 5.5 months when the reason for cessation was to start MS treatment vs 7.5 months for all other reasons. Among the 8.9% (n = 4) who breastfed while taking DMTs, 3 were on glatiramer acetate and 1 on interferon beta.
Figure 3. Breastfeeding—Changes by Decade.
Postpartum relapse data were available for 73 pregnancies: 15.1% (11/73) reported MS relapse within the first 3 months postpartum and 22.9% (16/70) within the first year postpartum. Approximately 81.8% were treated (most commonly with IV steroids). Among those who reported outcomes for relapses in the first 3 months postpartum, 33% reported full recovery, 44% reported partial recovery, and 11% reported no recovery. Postpartum administration of steroids was given to 11.9% (5/42) preemptively, as a relapse prevention strategy. Comparing those women who received preventative steroids with those who did not, the actual relapse occurrence was greater in the steroid prevention group (60% vs 10.8% reported a relapse; χ2, p = 0.006). This relationship remained significant for relapses at 1 year postpartum.
Discussion
This study explores choices and attitudes surrounding family planning, pregnancy, and postpartum experiences in a racially and socioeconomically diverse cohort of US-based women with MS. Most surveys on reproductive decision-making among people with MS have been conducted in most European cohorts, with only 1 study conducted in a North American cohort, over a decade ago.17 Our results expand the current available literature with the highest percentage of participants self-identifying as Black (15.6%). This study offers an updated perspective on evolving attitudes toward family planning in women with MS, representative of the changing demographics of women with MS in the United States and of the changing scientific landscape and counseling strategies over 4 decades (1980–2020s).
Similar to other studies,17 we found that the top MS-related reasons for avoiding pregnancy remain concerns of MS worsening with pregnancy, decreased ability to care for a child secondary to MS, and concern about genetic risk of passing MS to child. This finding highlights the need for improved counseling among women of reproductive age with MS, with evidence-based information found in the literature to provide assurance to these women. Studies show that pregnancy is not associated with long-term increased disability accumulation in MS.2-5 Patients who express a fear of passing MS to their child often do so in the absence of genetic counseling. Notably, the absolute risk of developing MS in a first-degree relative, such as parent to child, is approximately 3%–5%.45
Furthermore, the analysis also evaluated possible demographic factors associated with MS-related pregnancy avoidance. Age at diagnosis was the strongest predictor of MS-related pregnancy avoidance, with women diagnosed at younger ages being more likely to avoid pregnancy. Older women may have achieved their planned completed family size before diagnosis. An interesting finding was an apparently higher, but nonsignificant, percentage of women from zip codes with low COI reporting pregnancy avoidance due to MS. Future studies in a larger sample size may offer more definitive evidence regarding demographic factors associated with MS-related pregnancy avoidance.
Overall, the most interesting observation from this study is that counseling and medical management for women with MS during and after pregnancy is remarkably variable and has shifted over the past 3 decades of advancing and growing MS therapeutics. Based on free response data, women with MS have heard a wide range of guidance in the past 4 decades regarding safety and feasibility of pregnancy, MS DMT continuation vs discontinuation, pregnancy effects on MS, and safety of breastfeeding (Table 2). Some reported upsetting experiences of being discouraged from pregnancy or breastfeeding based on disease progression risk, and many reported that they “wish they knew” more before pregnancy with MS.
Reassuringly, those respondents with pregnancies in the most recent decade were more likely to receive counseling about pregnancy and MS from their neurologist. This is likely, partly, due to the increasing number of relevant publications related to reproductive issues in MS. This is particularly evident in the data surrounding breastfeeding (Figure 3). During the pre-DMT era, breastfeeding rates were higher in women with MS compared with those in the general population (before 2001). However, following wider availability of DMTs, breastfeeding rates fell in 2002–2012 relative. In the most recent decade (2013-present), an increasing number of respondents chose to breastfeed. This notable change coincided historically with several first-time publications evaluating the relationship between breastfeeding and postpartum relapse published between 2009 and 2013.31,46,47 Additional studies in 2012 and 2014 demonstrated a lack of adverse effects in infants of lactating mothers taking interferon or glatiramer acetate.28,48 Reported rate of first trimester relapse in our cohort was 15.5%, which approximates that reported in other recent studies.30 This contrasts with historical rates of postpartum relapse reaching closer to 30% and suggests a trend in reduction in postpartum relapse, as noted by Yeh et al. (2021),30 which is postulated to result from improved preconception disease control.7 The abovementioned examples show the impact of research in MS medical management with pregnancy: it guides clinical practice as evidenced by these temporal relationships.
Our work provides an updated perspective of the patient experience surrounding MS and family planning, pregnancy, and postpartum care in a diverse, US-based cohort of women with MS. Our combined cohort's average age at diagnosis and proportion of MS subtypes closely approximate national averages.49 Additional strengths include the inclusion criteria of database-confirmed diagnosis of MS (as opposed to self-reported MS diagnosis used in other studies on this topic) and recruitment of participants from multiple centers. One limitation of this survey-based study is the use of self-reported data from patients, which is susceptible to recall bias. The use of self-reported data limits a rigorous assessment of pregnancy outcomes in our cohort because objective data points such as postpartum relapses and delivery complications may not be accurately reported. Another limitation is nonresponse bias because our study had a low response rate of 11.2%, and certain participant characteristics (such as housing stability or employment status) may have influenced survey completion. In addition, we note that the difference in format between the paper-based UVA survey and online VCU survey may have differentially affected responses in these groups. A difference in the response collection period between the UVA and VCU (2016 vs 2022) may also have affected our findings.
Appendix. Authors
| Name | Location | Contribution |
| Erin E. Kelly, BA | Virginia Commonwealth University School of Medicine, Richmond | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; and analysis or interpretation of data |
| Casey Engel, BA | Virginia Tech Carilion School of Medicine, Roanoke | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; and study concept or design |
| Rylan Pearsall | College of Arts and Sciences, University of Virginia, Charlottesville | Drafting/revision of the article for content, including medical writing for content; additional contributions (in addition to 1 or more of the abovementioned criteria) |
| J. Nicholas Brenton, MD | Division of Child Neurology, Department of Neurology, University of Virginia, Charlottesville | Drafting/revision of the article for content, including medical writing for content; additional contributions (in addition to 1 or more of the abovementioned criteria) |
| Riley Bove, MD, MMSc | UCSF Weill Institute for Neurosciences, University of California San Francisco | Drafting/revision of the article for content, including medical writing for content |
| Unsong Oh, MD | Virginia Commonwealth University | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data |
| Myla D. Goldman, MD, MSc | Virginia Commonwealth University | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; study concept or design; and analysis or interpretation of data |
Study Funding
This study was supported by philanthropic funds provided by the ziMS Foundation. Grant funding by the VCU School of Medicine Dean's Fund for Summer Research 2022.
Disclosure
J.N. Brenton served as a consultant for Cycle Pharmaceuticals and is funded by the NIH/NINDS grant #K23NS116225; R. Bove receives research support from the NMSS Harry Weaver Award, NIH, DOD and from Biogen, Novartis, and Roche Genentech. She has received fees for consulting or advisory boards from Alexion, Horizon, Janssen, and TG Therapeutics; M.D. Goldman has received consulting fees from Alexion, Genentech, Horizon, and Novartis. M.D. Goldman serves on DSMB for Anokion and Immunic. All other authors report no disclosures relevant to the manuscript. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
References
- 1.Roux T, Courtillot C, Debs R, Touraine P, Lubetzki C, Papeix C. Fecundity in women with multiple sclerosis: an observational mono-centric study. J Neurol. 2015;262(4):957-960. doi: 10.1007/s00415-015-7663-1 [DOI] [PubMed] [Google Scholar]
- 2.Koch M, Uyttenboogaart M, Heersema D, Steen C, De Keyser J. Parity and secondary progression in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2009;80(6):676-678. doi: 10.1136/jnnp.2008.160911 [DOI] [PubMed] [Google Scholar]
- 3.Ramagopalan S, Yee I, Byrnes J, Guimond C, Ebers G, Sadovnick D. Term pregnancies and the clinical characteristics of multiple sclerosis: a population based study. J Neurol Neurosurg Psychiatry. 2012;83(8):793-795. doi: 10.1136/jnnp-2012-302848 [DOI] [PubMed] [Google Scholar]
- 4.Thöne J, Thiel S, Gold R, Hellwig K. Treatment of multiple sclerosis during pregnancy—safety considerations. Expert Opin Drug Saf. 2017;16(5):523-534. doi: 10.1080/14740338.2017.1311321 [DOI] [PubMed] [Google Scholar]
- 5.Canibaño B, Deleu D, Mesraoua B, Melikyan G, Ibrahim F, Hanssens Y. Pregnancy-related issues in women with multiple sclerosis: an evidence-based review with practical recommendations. J Drug Assess. 2020;9(1):20-36. doi: 10.1080/21556660.2020.1721507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Voskuhl R, Momtazee C. Pregnancy: effect on multiple sclerosis, treatment considerations, and breastfeeding. Neurotherapeutics. 2017;14(4):974-984. doi: 10.1007/s13311-017-0562-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsui A, Lee MA. Multiple sclerosis and pregnancy. Curr Opin Obstet Gynecol. 2011;23(6):435-439. doi: 10.1097/GCO.0b013e32834cef8f [DOI] [PubMed] [Google Scholar]
- 8.Confavreux C, Hutchinson M, Hours MM, Cortinovis-Tourniaire P, Moreau T. Rate of pregnancy-related relapse in multiple sclerosis. Pregnancy in Multiple Sclerosis Group. N Engl J Med. 1998;339(5):285-291. doi: 10.1056/NEJM199807303390501 [DOI] [PubMed] [Google Scholar]
- 9.Finkelsztejn A, Brooks JB, Paschoal FM Jr., Fragoso YD. What can we really tell women with multiple sclerosis regarding pregnancy? A systematic review and meta-analysis of the literature. BJOG. 2011;118(7):790-797. doi: 10.1111/j.1471-0528.2011.02931.x [DOI] [PubMed] [Google Scholar]
- 10.MacDonald SC, McElrath TF, Hernández-Díaz S. Pregnancy outcomes in women with multiple sclerosis. Am J Epidemiol. 2019;188(1):57-66. doi: 10.1093/aje/kwy197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soler B, Ciampi E, Uribe-San-Martín R, et al. Pregnancy outcomes in women with multiple sclerosis. Mult Scler Relat Disord. 2021;48:102682. doi: 10.1016/j.msard.2020.102682 [DOI] [PubMed] [Google Scholar]
- 12.Alroughani R, Inshasi J, Al-Asmi A, et al. Disease-modifying drugs and family planning in people with multiple sclerosis: a consensus narrative review from the Gulf Region. Neurol Ther. 2020;9(2):265-280. doi: 10.1007/s40120-020-00201-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Langer-Gould AM. Pregnancy and family planning in multiple sclerosis. Continuum (Minneap Minn). 2019;25(3):773-792. doi: 10.1212/CON.0000000000000745 [DOI] [PubMed] [Google Scholar]
- 14.Hellwig K, Rockhoff M, Herbstritt S, et al. Exclusive breastfeeding and the effect on postpartum multiple sclerosis relapses. JAMA Neurol. 2015;72(10):1132-1138. doi: 10.1001/jamaneurol.2015.1806 [DOI] [PubMed] [Google Scholar]
- 15.Langer-Gould A, Smith JB, Albers KB, et al. Pregnancy-related relapses and breastfeeding in a contemporary multiple sclerosis cohort. Neurology. 2020;94(18):e1939-e1949. doi: 10.1212/WNL.0000000000009374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonavita S, Lavorgna L, Worton H, Russell S, Jack D. Family planning decision making in people with multiple sclerosis. Front Neurol. 2021;12:620772. doi: 10.3389/fneur.2021.620772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alwan S, Yee IM, Dybalski M, et al. Reproductive decision making after the diagnosis of multiple sclerosis (MS). Mult Scler. 2013;19(3):351-358. doi: 10.1177/1352458512452920 [DOI] [PubMed] [Google Scholar]
- 18.Lavorgna L, Esposito S, Lanzillo R, et al. Factors interfering with parenthood decision-making in an Italian sample of people with multiple sclerosis: an exploratory online survey. J Neurol. 2019;266(3):707-716. doi: 10.1007/s00415-019-09193-4 [DOI] [PubMed] [Google Scholar]
- 19.Rasmussen PV, Magyari M, Moberg JY, Bøgelund M, Jensen UFA, Madsen KG. Patient awareness about family planning represents a major knowledge gap in multiple sclerosis. Mult Scler Relat Disord. 2018;24:129-134. doi: 10.1016/j.msard.2018.06.006 [DOI] [PubMed] [Google Scholar]
- 20.Kamm CP, Muehl S, Mircsof D, et al. Role of family planning in women with multiple sclerosis in Switzerland: results of the women with multiple sclerosis patient survey. Front Neurol. 2018;9:821. doi: 10.3389/fneur.2018.00821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Villaverde-González R. Updated perspectives on the challenges of managing multiple sclerosis during pregnancy. Degener Neurol Neuromuscul Dis. 2022;12:1-21. doi: 10.2147/DNND.S203406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salminen HJ, Leggett H, Boggild M. Glatiramer acetate exposure in pregnancy: preliminary safter and birth outcomes. J Neurol. 2010;257(12):2020-2023. doi: 10.1007/s00415-010-5652-y [DOI] [PubMed] [Google Scholar]
- 23.Dobson R, Dassan P, Roberts M, Giovannoni G, Nelson-Piercy C, Brex PA. UK consensus on pregnancy in multiple sclerosis: 'Association of British Neurologists' guidelines. Pract Neurol. 2019;19(2):106-114. doi: 10.1136/practneurol-2018-002060 [DOI] [PubMed] [Google Scholar]
- 24.Amato MP, Portaccio E. Fertility, pregnancy and childbirth in patients with multiple sclerosis: impact of disease-modifying drugs. CNS Drugs. 2015;29(3):207-220. doi: 10.1007/s40263-015-0238-y [DOI] [PubMed] [Google Scholar]
- 25.Thiel S, Langer-Gould A, Rockhoff M, et al. Interferon-beta exposure during first trimester is safe in women with multiple sclerosis-A prospective cohort study from the German Multiple Sclerosis and Pregnancy Registry. Mult Scler. 2016;22(6):801-809. doi: 10.1177/1352458516634872 [DOI] [PubMed] [Google Scholar]
- 26.Rae-Grant A, Day GS, Marrie RA, et al. Practice guideline recommendations summary: disease-modifying therapies for adults with multiple sclerosis: report of the guideline development, dissemination, and implementation Subcommittee of the American Academy of Neurology. Neurology. 2018;90(17):777-788. doi: 10.1212/WNL.0000000000005347 [DOI] [PubMed] [Google Scholar]
- 27.Herbstritt S, Langer-Gould A, Rockhoff M, et al. Glatiramer acetate during early pregnancy: a prospective cohort study. Mult Scler. 2016;22(6):810-816. doi: 10.1177/1352458515623366 [DOI] [PubMed] [Google Scholar]
- 28.Fragoso YD, Boggild M, Macias-Islas MA, et al. The effects of long-term exposure to disease-modifying drugs during pregnancy in multiple sclerosis. Clin Neurol Neurosurg. 2013;115(2):154-159. doi: 10.1016/j.clineuro.2012.04.024 [DOI] [PubMed] [Google Scholar]
- 29.Hughes SE, Spelman T, Gray OM, et al. Predictors and dynamics of postpartum relapses in women with multiple sclerosis. Mult Scler. 2014;20(6):739-746. doi: 10.1177/1352458513507816 [DOI] [PubMed] [Google Scholar]
- 30.Yeh WZ, Widyastuti PA, Van der Walt A, et al. Natalizumab, fingolimod and dimethyl fumarate use and pregnancy-related relapse and disability in women with multiple sclerosis. Neurology. 2021;96(24):e2989-e3002. doi: 10.1212/WNL.0000000000012084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hellwig K, Haghikia A, Rockhoff M, Gold R. Multiple sclerosis and pregnancy: experience from a nationwide database in Germany. Ther Adv Neurol Disord. 2012;5:247-253. doi: 10.1177/1756285612453192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.diversitydatakids.org. Child Opportunity Index 2.0 Database [online]. Accessed August 26.
- 33.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Unintended Pregnancy in the United States [online]. Accessed July 31, 2022. guttmacher.org/sites/default/files/factsheet/fb-unintended-pregnancy-us.pdf [Google Scholar]
- 36.Chandra A, Copen CE, Stephen EH. Infertility and impaired fecundity in the United States, 1982-2010: data from the national survey of family growth. Natl Health Stat Rep. 2013;67:1-18, 1 p following 19. [PubMed] [Google Scholar]
- 37.Births and Natality [online]. cdc.gov/nchs/fastats/births.htm [Google Scholar]
- 38.Silva M, Halpern SH. Epidural analgesia for labor: current techniques. Local Reg Anesth. 2010;3:143-153. doi: 10.2147/LRA.S10237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Osterman M, Hamilton B, Martin JA, Driscoll AK, Valenzuela CP. Births: final data for 2020. Natl Vital Stat Rep. 2021;70(17):1-50. [PubMed] [Google Scholar]
- 40.White T. Epidurals Increase in Popularity, Stanford Study Finds [online]. Accessed July 31, 2022. scopeblog.stanford.edu/2018/06/26/epidurals-increase-in-popularity-stanford-study-finds/ [Google Scholar]
- 41.Trends in Pregnancy and Childbirth Complications in the U.S. [online]. Accessed July 31, 2022. bcbs.com/the-health-of-america/reports/trends-in-pregnancy-and-childbirth-complications-in-the-us [Google Scholar]
- 42.Mughal S, Azhar Y, Siddiqui W. Postpartum Depression. StatPearls Publishing Copyright 2022, StatPearls Publishing LLC; 2022. [Google Scholar]
- 43.Axelsson D, Blomberg M. Prevalence of postpartum infections: a population-based observational study. Acta Obstet Gynecol Scand. 2014;93(10):1065-1068. doi: 10.1111/aogs.12455 [DOI] [PubMed] [Google Scholar]
- 44.Nakić Radoš S, Tadinac M, Herman R. Anxiety during pregnancy and postpartum: course, predictors and comorbidity with postpartum depression. Acta Clin Croat. 2018;57(1):39-51. doi: 10.20471/acc.2017.56.04.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nielsen NM, Westergaard T, Rostgaard K, et al. Familial risk of multiple sclerosis: a nationwide cohort study. Am J Epidemiol. 2005;162(8):774-778. doi: 10.1093/aje/kwi280 [DOI] [PubMed] [Google Scholar]
- 46.Langer-Gould A. Exclusive breastfeeding and the risk of postpartum relapses in women with multiple sclerosis. Arch Neurol. 2009;66(8):958-963. doi: 10.1001/archneurol.2009.132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Langer-Gould A, Hellwig K. One can prevent post-partum MS relapses by exclusive breast feeding: yes. Mult Scler. 2013;19(12):1567-1568. doi: 10.1177/1352458513505161 [DOI] [PubMed] [Google Scholar]
- 48.Hale TW, Siddiqui AA, Baker TE. Transfer of interferon β-1a into human breastmilk. Breastfeed Med. 2012;7(2):123-125. doi: 10.1089/bfm.2011.0044 [DOI] [PubMed] [Google Scholar]
- 49.Types of MS [online]. Accessed July 31, 2022. nationalmssociety.org/What-is-MS/Types-of-MS [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data not published within this article will be made available by request from any qualified investigator.