Summary
Patients with sickle cell disease (SCD) are considered to be immunocompromised, yet data on the antibody response to SARS-CoV-2 vaccination in SCD is limited. We investigated anti-SARS-CoV-2 IgG titers and overall neutralizing activity in 201 adults with SCD and demographically matched non-SCD controls. Unexpectedly, patients with SCD generate a more robust and durable COVID-19 vaccine IgG response compared to matched controls, though the neutralizing activity remained similar across both cohorts. These findings suggest that patients with SCD achieve a similar antibody response following COVID-19 vaccination compared to the general population, with implications for optimal vaccination strategies for patients with SCD.
Keywords: SICKLE CELL DISEASE, VACCINES, IMMUNOLOGY, SICKLE CELL ANAEMIA, HAEMOGLOBINOPATHIES, ANTIBODIES
Introduction
Adults with sickle cell disease (SCD) are considered to be immunocompromised due to functional asplenia with impaired humoral immunity as evidenced by reduced IgM memory B cells, IgM secretion, and increased susceptibility to microbial sepsis (1,2). Given these data, several reports have suggested that patients with SCD are at increased risk of severe COVID-19 infection and may warrant the distinct vaccination schedules recommended for immunocompromised patients (3). However, efficacy and safety data for COVID-19 vaccination in SCD is limited (4–6). Furthermore, studies have failed to detect different outcomes for COVID-19 infection in patients with SCD when controlled for co-morbidities and end-organ damage (7), suggesting that the overall response to COVID-19 vaccination may not significantly differ from the general population. Indeed, some have suggested similar COVID-19 IgG and neutralizing antibody responses after vaccination in patients with SCD but these studies are limited by small sample size, short follow up period, and lack of matched controls (8–10). Consequently, the extent to which the immunocompromised state in SCD affects antibody responses after COVID-19 vaccination remains incompletely defined. To address this, we compared the antibody response to COVID-19 vaccination in 201 adults with SCD to demographically matched controls.
Patients and Methods
Residual clinical serum samples were collected from patients in the Grady Health System (Atlanta, GA, USA) between March and September 2022. Demographic data (age, sex, race and ethnicity) was obtained from electronic health record. Race and ethnicity categories were as follows: American Indian and Alaskan Native, Asian, Black or African American, Hispanic, Multiracial, Other, Patient Refused, Unknown, White or Caucasian. Chart review was performed to obtain vaccination data (number of doses, vaccination type and date). For patients with SCD, the sickle genotype, presence of RBC alloantibodies, history of RBC transfusions, hydroxyurea use, and surgical splenectomy status were also recorded. RBC transfusion was categorized as “minimal” for patients never or rarely transfused, “chronic” for patients on chronic transfusion programs (current or past), or “episodic” for all others. RBC alloantibodies were enumerated from the patient record; autoantibodies were excluded. COVID-19 vaccinated patients with SCD and non-SCD control patients were matched algorithmically in priority order of age, race and ethnicity, and sex. Asian and White or Caucasian patients were excluded from matched controls due to their absence in the SCD cohort. Serum aliquots were stored at −80°C prior to analysis.
Anti-SARS-CoV-2 receptor-binding domain (RBD)- and nucleocapsid (NC)-specific IgG titer was determined by enzyme-linked immunosorbent assay (ELISA) as previously described (11). The Pfizer-BioNTech, Moderna, and Janssen (Johnson & Johnson) vaccines contain nucleic acid sequence encoding for the SARS-CoV-2 spike protein, but not the NC. Therefore, the presence of NC-specific IgG was used as marker of SARS-CoV-2 infection. Surrogate neutralization activity was measured by high-throughput blockade of hACE-2 binding (BoAb) as previously described (12), except with two-point dilution at 1:50 and 1:200 to improve throughput (Supplementary Data). Recombinant viral proteins were derived from wildtype Wuhan-Hu-1 (GenBank: MN908947.3). Statistical analysis was performed using GraphPad Prism software 9.5.0. Statistical difference was assessed by chi-square test, Mann-Whitney test, ANCOVA or Kruskal-Wallis test followed by Dunn’s test as applicable. Correction for multiple comparisons was done by Benjamini-Krieger-Yekutieli two-stage step-up method with 5% false discovery rate.
Results
Serum samples from 201 vaccinated adults with SCD and 201 matched non-SCD controls were obtained over the six-month study period. Overall characteristics were similar between the two cohorts (Table 1), except for gender (56.2% females in SCD vs 68.2% females in control) and Hispanic ethnicity (2.0% in SCD vs 9.5% in control)). Nearly all participants received the Pfizer-BioNTech (78.6% in SCD vs 80.6% in control) or Moderna (19.4% in SCD vs 16.9% in control) vaccine and a similar percentage of each group received two (50.7% in SCD vs 52.7% in control) or three (34.8% in SCD vs 35.8% in control) vaccine doses. Time since last vaccine dose was increased in SCD relative to control, though statistically significant difference was observed only for those that received three vaccine doses (154 days in SCD vs 108 days in control, p = 0.0071).
Table 1.
Descriptive Statistics of Study Cohort Characteristics
| SCD (n = 201) |
Control (n = 201) |
P value | |
|---|---|---|---|
| Age, years | 0.2590a | ||
| Median (SD) | 34 (14) | 35 (14) | |
| Minimum-Maximum | 18–80 | 18–81 | |
| Sex | 0.0179b | ||
| Female | 56.2% (n = 113) | 68.2% (n = 137) | |
| Male | 43.8% (n = 88) | 31.8% (n = 64) | |
| Race and Ethnicity | 0.0012b | ||
| Black or African American | 95.0% (n = 191) | 87.1% (n = 175) | |
| Hispanic | 2.0% (n = 4) | 9.5% (n = 19) | |
| Non-Black or African American, Non-Hispanic | 3.0% (n = 6) | 3.5% (n = 7) | |
| COVID vaccine type | 0.5963b | ||
| Pfizer-BioNTech | 78.6% (n = 158) | 80.6% (n = 162) | |
| Moderna | 19.4% (n = 39) | 16.9% (n = 34) | |
| Janssen (Johnson & Johnson) | 2.0% (n = 4) | 2.5% (n = 5) | |
| Number of vaccine dose Time since last dose, mean days (SD) | 0.6210b | ||
| 1 dose | 11.9% (n = 24) | 9.5% (n = 19) | |
| 254 days (96) | 225 days (135) | 0.9373a | |
| 2 dose | 50.7% (n = 102) | 52.7% (n = 106) | |
| 293 days (121) | 267 days (120) | 0.3793a | |
| 3 dose | 34.8% (n = 70) | 35.8% (n = 72) | |
| 154 days (81) | 108 days (103) | 0.0071a | |
| 4 dose | 2.5% (n = 5) | 2.0% (n = 4) | |
| 17 days (86) | 64 days (49) | 0.9762a |
Mann-Whitney test
Chi-square test
To assess immune response to COVID-19 vaccination, the SARS-CoV-2 RBD-specific IgG titer was measured by ELISA (Fig 1A). SARS-CoV-2 RBD-specific IgG level was higher in SCD vs controls with median endpoint titer of 1:54572 in SCD vs 1:31131 in controls (p = 0.0137). Comparison of neutralization activity by BoAb assay showed similar proportion of subjects in the SCD and control groups with strong (79.6% vs 78.1%), average (8.5% vs 8.0%), and weak (11.9% vs 13.9%) neutralizing activity, respectively (p = 0.7078) (Fig 1B). This suggests that both cohorts achieve equivalent levels of neutralizing antibody production even with higher IgG level in the SCD group. To examine for possible differences in exposure to natural infection, the SARS-CoV-2 NC-specific IgG level was measured by ELISA (Fig 1C). The two cohorts showed no statistical difference in SARS-CoV-2 NC-specific IgG level with median NC-specific IgG endpoint titer of 835.5 in SCD vs 741.4 in controls (p = 0.8779) suggesting comparable rates of natural COVID-19 infection. Potential correlations between the NC-specific IgG level and the magnitude of RBD-specific IgG level was assessed by simple linear regression and compared between SCD and controls (Fig 1D). The low r2 (0.1073 in SCD vs 0.1401 in controls) and nearly equivalent slope (0.3848 in SCD vs 0.3895 in controls, p = 0.9641) suggest minor but comparable correlation of NC-specific IgG levels with RBD-specific IgG levels in both groups.
Figure 1.
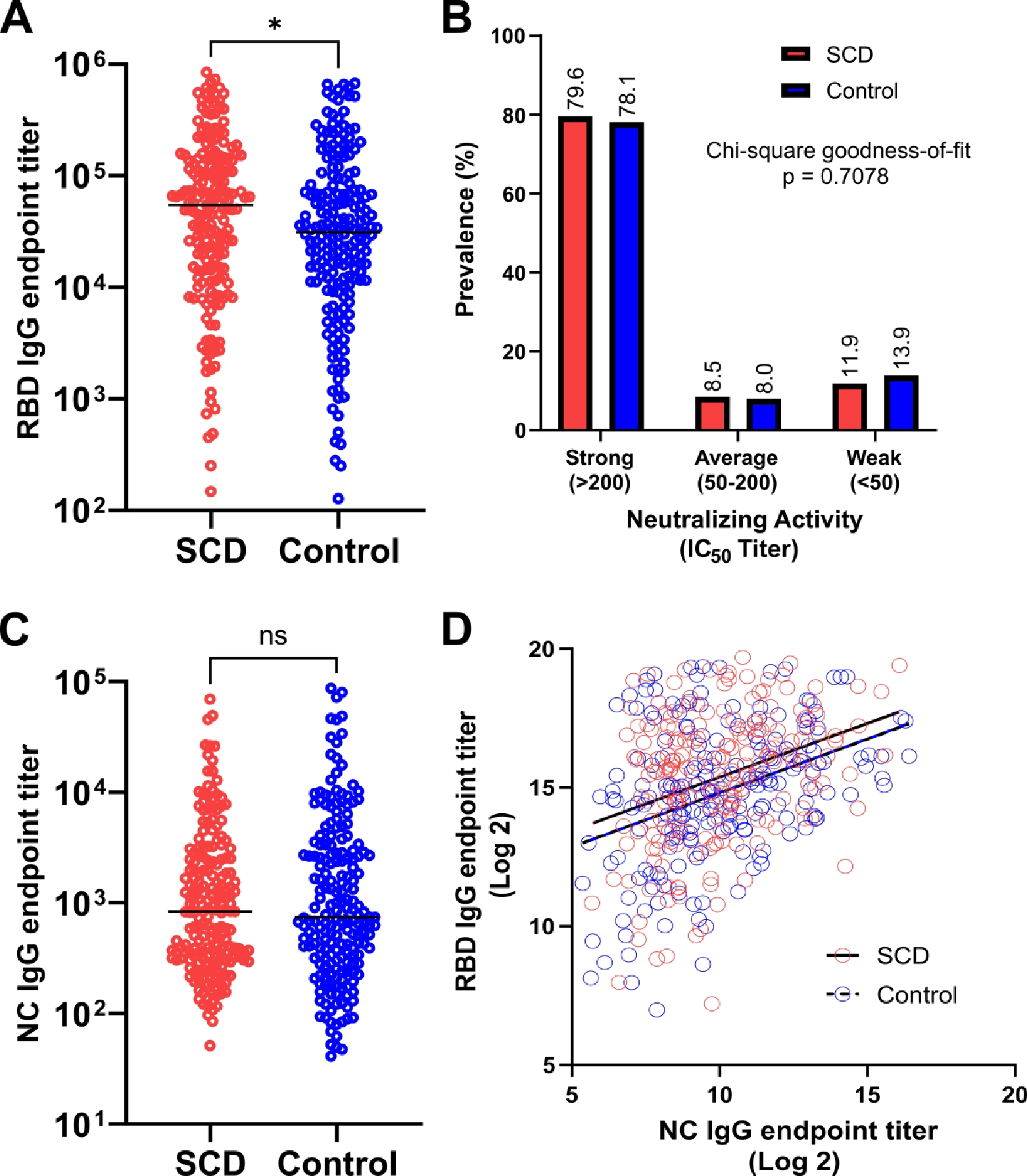
Comparison of COVID-19 vaccine immune response in SCD (red) and matched non-SCD controls (blue). (A) SARS-CoV-2 RBD-specific IgG endpoint titer. Lines indicate median. (B) Prevalence of neutralizing activity, by strength. (C) SARS-CoV-2 NC-specific IgG endpoint titer. Lines indicate median. (D) Simple linear regression of NC- and RBD-specific IgG endpoint titer after log2 transformation with best-fit lines for SCD (solid) and controls (dashed). Statistical difference measured by Mann-Whitney test, chi-square goodness-of-fit test or ANCOVA test as applicable. * = P ≤ 0.05, ns = P > 0.05. IC50, 50% inhibitory concentration; NC, nucleocapsid; RBD, receptor binding domain; SCD, sickle cell disease.
Subgroup analysis was performed to account for differences in demographic and vaccination-related variables (Table 1), and to identify potential associations for the increased anti-RBD IgG response in patients with SCD (Fig S1A–F). Overall trend consistently showed higher titer of RBD-specific IgG levels in SCD vs controls, with statistically significant difference (P ≤ 0.05) observed for the following subgroup comparisons: female, Black or African American, 2 vaccine doses, and >365 days since last vaccine dose. However, correction for multiple comparisons did not yield any true “discovery” based on insufficiently small P values (Table S1). The SCD cohort was further characterized by variables expected to correlate with SCD disease severity (sickle genotype, RBC transfusion burden), potential immunomodulatory effects (hydroxyurea, splenectomy), and markers of alloimmune hyper-responsiveness (presence and number of RBC alloantibodies) (Table S2). Most were HbSS genotype (75.9%) or HbSC (17.2%) with nearly half with history of episodic RBC transfusions (48.8%), followed by minimal (35.5%) or chronic RBC transfusion (15.9%). Over half (53.7%) were currently prescribed hydroxyurea at a median dose of 1000 mg/day at time of initial vaccination, and a minority (11.8%) had a history of total surgical splenectomy. RBC alloantibody records were available for 146 of 201 patients with SCD in this study and 26% had history of at least one alloantibody. Subgroup analysis of RBD-specific IgG levels adjusted for select SCD-related characteristics as defined above was unable to detect any statistically significant differences (Fig S2A–E).
Discussion
Despite their immunocompromised state, patients with SCD generate a more robust (Fig 1A) IgG response to COVID-19 vaccines compared to the general population, with similar prevalence of strong neutralizing antibodies (Fig 1B) even after examining natural COVID-19 infection (Fig 1C–D). Based on correlation between RBD IgG level and BoAb neutralization activity (12), patients with SCD may also generate a more robust neutralizing antibody response than control. The overall trend of higher IgG level in SCD compared to controls held across demographic and COVID-19 vaccine-related characteristics (Fig S1A–F), which suggests that the difference in COVID-19 vaccine IgG response may reflect SCD-related characteristics not present in the controls. Given that time since last vaccine dose was greater in SCD vs controls (Table 1), the IgG levels in SCD should have been lower than controls based on expected COVID-19 vaccine antibody response kinetics (13). While longitudinal samples were not available for confirmation, this suggests comparable, if not more durable IgG response in SCD compared to the general population.
Concurrent with the immune suppression from splenic hypofunction/asplenia, individuals with SCD are in a pro-inflammatory state at baseline, likely due to chronic hemolysis, TLR4 signaling through free heme and complement activation, all of which can alter innate and adaptive immunity (2,14,15). Future studies to examine cytokine production, complement activation and other changes in baseline immune activity at the time of vaccination may shed light on the possibility that the pro-inflammatory background offsets the immune suppression from splenic hypofunction/asplenia resulting in enhanced magnitude and kinetics of the IgG response in SCD (1,2). Additional interactions between mRNA vaccine, SARS-CoV-2 virus and SCD pathophysiology may also play a role and deserves more mechanistic investigation.
Limitations to this study include cross-sectional design and restriction of the analysis to humoral IgG and neutralization assessment to the original wildtype Wuhan-Hu-1 strain. The strength of this study is in the large sample size, adequate follow up period, and direct comparison with demographically matched controls (8–10). Overall, our findings suggest that the same COVID-19 vaccination regimen as the general population will confer a similar antibody response in patients with SCD. Combining high-throughput sero-survey of residual clinical samples with electronic health record data extraction may be a useful approach to evaluate humoral responses after vaccination in patients with SCD or other patient populations with similar potentially immunocompromised disease states.
Supplementary Material
Funding Information
This study was supported by NIH National Cancer Institute 1 U54 CA260563 01: “Immune Regulation of COVID-19 Infection in Cancer and Autoimmunity”.
Footnotes
Conflict of Interest
The authors declare no competing interests.
Patient Consent and Ethics Approval Statement
Collection of residual clinical samples and retrospective chart review was approved with waiver of consent by the Grady Health System institutional review board.
Data Availability Statement
De-identified data available upon requests to the corresponding author.
References
- 1.Lenti MV, Luu S, Carsetti R, Osier F, Ogwang R, Nnodu OE, et al. Asplenia and spleen hypofunction. Nat Rev Dis Prim. 2022;8(1):1–18. [DOI] [PubMed] [Google Scholar]
- 2.Balandya E, Reynolds T, Obaro S, Makani J. Alteration of lymphocyte phenotype and function in sickle cell anemia: Implications for vaccine responses. Am J Hematol. 2016;91(9):938–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mbaeyi S, Oliver SE, Collins JP, Godfrey M, Goswami ND, Hadler SC, et al. The Advisory Committee on Immunization Practices’ Interim Recommendations for Additional Primary and Booster Doses of COVID-19 Vaccines — United States, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(44):1545–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han J, Zhang X, Molokie RE, Njoku FU, Hussain FA, Farooqui M, et al. COVID-19 vaccination status and disease burden in patients with sickle cell disease. Br J Haematol. 2022;199(4):e21–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friedman E, Minniti C, Campbell S, Curtis S. COVID19 vaccination in adults with sickle cell disease is not associated with increases in rates of pain crisis. Hematol (United Kingdom). 2022;27(1):742–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joseph L, Corbasson A, Manceau S, Khimoud D, Meunier B, Cheminet G, et al. Safety of coronavirus disease 2019 vaccines in 213 adult patients with sickle cell disease. Br J Haematol. 2022;(July):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoogenboom WS, Alamuri TT, McMahon DM, Balanchivadze N, Dabak V, Mitchell WB, et al. Clinical outcomes of COVID-19 in patients with sickle cell disease and sickle cell trait: A critical appraisal of the literature. Blood Rev [Internet]. 2022;53(November):100911. Available from: 10.1016/j.blre.2021.100911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Varelas C, Gavriilaki E, Sakellari I, Klonizakis P, Koravou EE, Christodoulou I, et al. Immune Response of Adult Sickle Cell Disease Patients after COVID-19 Vaccination: The Experience of a Greek Center. J Clin Med. 2022;11(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Radhwi OO, Jan H, Waheeb A, Alamri SS, Alahwal HM, Denetiu I, et al. Immunogenicity of The BNT162b2 COVID-19 mRNA and ChAdOx1 nCoV-19 Vaccines in Patients with Hemoglobinopathies. Vaccines. 2022;10(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haggenburg S, Lissenberg-Witte BI, van Binnendijk RS, den Hartog G, Bhoekhan MS, Haverkate NJE, et al. Quantitative analysis of mRNA-1273 COVID-19 vaccination response in immunocompromised adult hematology patients. Blood Adv. 2022;6(5):1537–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verkerke H, Horwath M, Saeedi B, Boyer D, Allen JW, Owens J, et al. Comparison of Antibody Class-Specific SARS-CoV-2 Serologies for the Diagnosis of Acute COVID-19. J Clin Microbiol. 2021;59(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheedarla N, Verkerke HP, Potlapalli S, McLendon KB, Patel A, Frank F, et al. Rapid, high throughput, automated detection of SARS-CoV-2 neutralizing antibodies against native-like vaccine and delta variant spike trimers. medRxiv [Internet]. 2022;2022.02.01.22270279. Available from: http://medrxiv.org/content/early/2022/02/02/2022.02.01.22270279.abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang A, Akhtar A, Linderman SL, Lai L, Orellana-Noia VM, Valanparambil R, et al. Humoral Responses Against SARS-CoV-2 and Variants of Concern after mRNA Vaccines in Patients with Non-Hodgkin Lymphoma and Chronic Lymphocytic Leukemia. J Clin Oncol. 2022;10:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allali S, Maciel TT, Hermine O, De Montalembert M. Innate immune cells, major protagonists of sickle cell disease pathophysiology. Haematologica. 2020;105(2):273–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arthur CM, Stowell SR. The Development and Consequences of Red Blood Cell Alloimmunization. Annu Rev Pathol Mech Dis. 2023;18:537–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
De-identified data available upon requests to the corresponding author.


