Abstract
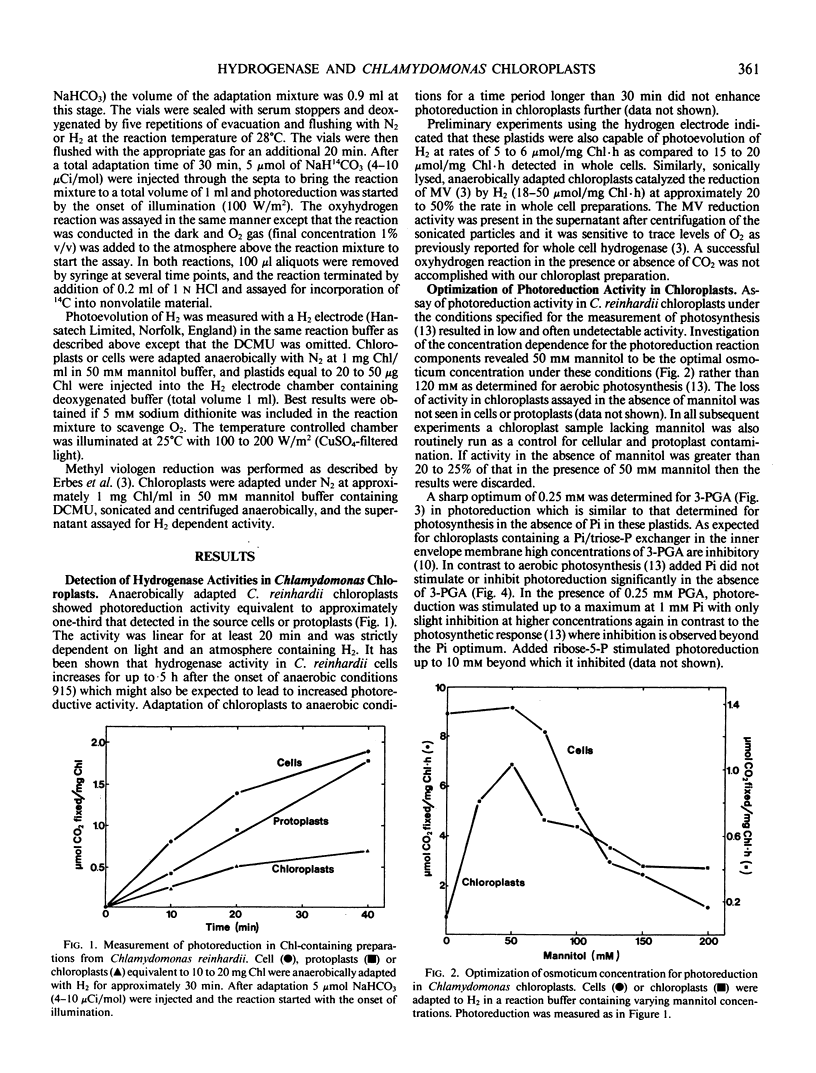
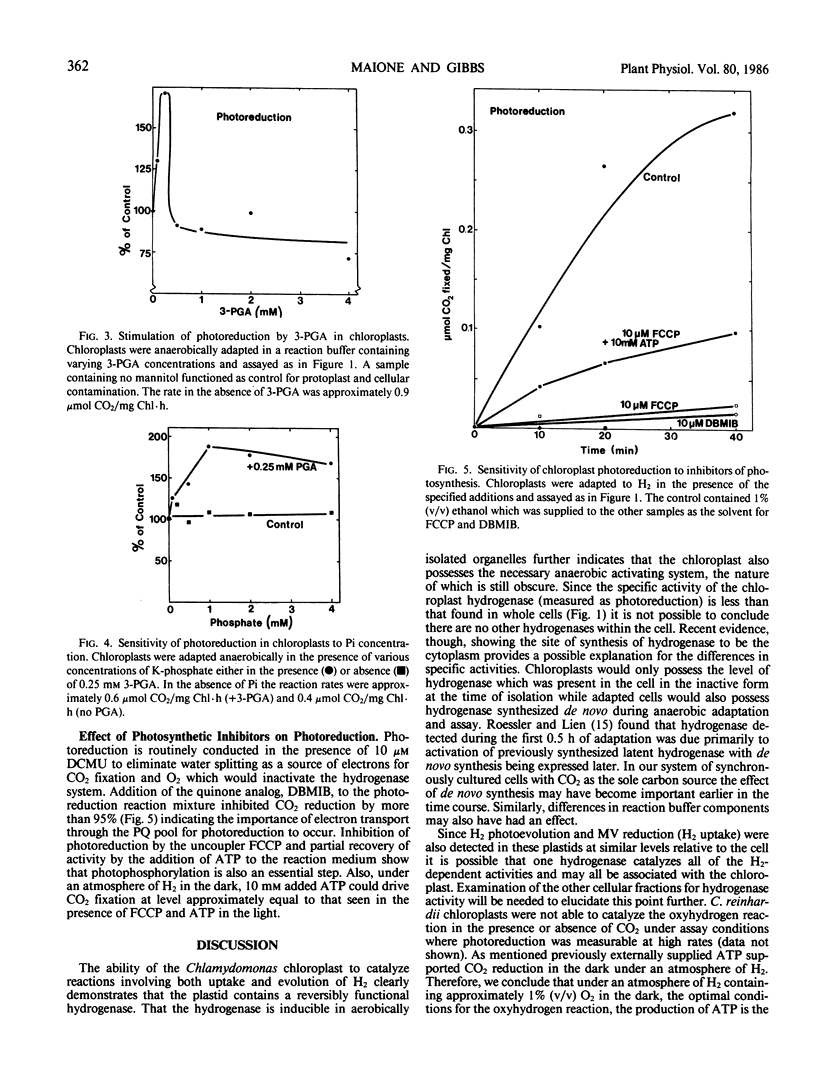
Isolated intact chloroplasts of Chlamydomonas reinhardii were found to catalyze photoreduction of CO2 in the presence of 3-(3,4-dichlorophenyl)-1,1-dimethylurea when adapted under an atmosphere of H2 demonstrating the association of a hydrogenase and anaerobic adaptation system with these plastids. The specific activity of photoreduction was approximately one third that detected in cells and protoplasts. Photoreduction was found to have a lower osmoticum optimum relative to aerobically maintained chloroplasts (50 millimolar versus 120 millimolar mannitol). 3-Phosphoglycerate (3-PGA) stimulated photoreduction up to a peak at 0.25 millimolar beyond which inhibition was observed. In the absence of 3-PGA, inorganic phosphate had no effect on photoreduction but in the presence of 3-PGA, inorganic phosphate also stimulated the reaction. Carbonyl cyanide-p-trifluoromethoxyphenylhydrazone and 2,5-dibromo-3-methyl-6-isopropyl-p-benzoquinone inhibited photoreduction but inhibition by the former could be partially overcome by exogenously added ATP. The intact plastid can also catalyze photoevolution of H2 while lysed chloroplast extracts catalyzed the reduction of methyl viologen by H2. Both reactions occurred at rates approximately one-third of those found in cells. The oxyhydrogen reaction in the presence or absence of CO2 was not detected.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Amotz A., Gibbs M. H2 metabolism in photosynthetic organisms. II. Light-dependent H2 evolution by preparations from Chlamydomonas, Scenedesmus and spinach. Biochem Biophys Res Commun. 1975 May 5;64(1):355–359. doi: 10.1016/0006-291x(75)90261-2. [DOI] [PubMed] [Google Scholar]
- Erbes D. L., King D., Gibbs M. Inactivation of Hydrogenase in Cell-free Extracts and Whole Cells of Chlamydomonas reinhardi by Oxygen. Plant Physiol. 1979 Jun;63(6):1138–1142. doi: 10.1104/pp.63.6.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRENKEL A. W. Hydrogen evolution by the flagellate green alga, Chlamydomonas moewusii. Arch Biochem Biophys. 1952 Jul;38:219–230. doi: 10.1016/0003-9861(52)90026-x. [DOI] [PubMed] [Google Scholar]
- Gfeller R. P., Gibbs M. Fermentative Metabolism of Chlamydomonas reinhardtii: I. Analysis of Fermentative Products from Starch in Dark and Light. Plant Physiol. 1984 May;75(1):212–218. doi: 10.1104/pp.75.1.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healey F. P. The Mechanism of Hydrogen Evolution by Chlamydomonas moewusii. Plant Physiol. 1970 Feb;45(2):153–159. doi: 10.1104/pp.45.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldt H. W., Rapley L. Specific transport of inorganic phosphate, 3-phosphoglycerate and dihydroxyacetonephosphate, and of dicarboxylates across the inner membrane of spinach chloroplasts. FEBS Lett. 1970 Oct 5;10(3):143–148. doi: 10.1016/0014-5793(70)80438-0. [DOI] [PubMed] [Google Scholar]
- Klein U., Chen C., Gibbs M. Photosynthetic Properties of Chloroplasts from Chlamydomonas reinhardii. Plant Physiol. 1983 Jun;72(2):488–491. doi: 10.1104/pp.72.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein U., Chen C., Gibbs M., Platt-Aloia K. A. Cellular Fractionation of Chlamydomonas reinhardii with Emphasis on the Isolation of the Chloroplast. Plant Physiol. 1983 Jun;72(2):481–487. doi: 10.1104/pp.72.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roessler P. G., Lien S. Activation and de novo synthesis of hydrogenase in chlamydomonas. Plant Physiol. 1984 Dec;76(4):1086–1089. doi: 10.1104/pp.76.4.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlösser U. G., Sachs H., Robinson D. G. Isolation of protoplasts by means of a "species-specific" autolysine in Chlamydomonas. Protoplasma. 1976;88(1):51–64. doi: 10.1007/BF01280359. [DOI] [PubMed] [Google Scholar]


