Abstract
Mutations in the tol-pal genes induce pleiotropic effects such as release of periplasmic proteins into the extracellular medium and hypersensitivity to drugs and detergents. Other outer membrane defective strains such as tolC, lpp, and rfa mutations are also altered in their outer membrane permeability. In this study, electron microscopy and Western blot analyses were used to show that strains with mutations in each of the tol-pal genes formed outer membrane vesicles after growth in standard liquid or solid media. This phenotype was not observed in tolC and rfaD cells in the same conditions. A tolA deletion in three different Escherichia coli strains was shown to lead to elevated amounts of vesicles. These results, together with plasmid complementation experiments, indicated that the formation of vesicles resulted from the defect of any of the Tol-Pal proteins. The vesicles contained outer membrane trimeric porins correctly exposed at the cell surface. Pal outer membrane lipoprotein was also immunodetected in the vesicle fraction of tol strains. The results are discussed in view of the role of the Tol-Pal transenvelope proteins in maintaining outer membrane integrity by contributing to target or integrate newly synthesized components of this structure.
Different cell envelope proteins have been proposed to link the inner and the outer membranes of gram-negative bacteria. TonB inner membrane protein, involved in the active transport of iron siderophores and vitamin B12, interacts with outer membrane receptors (7, 34, 51). Export systems in Escherichia coli contain membrane fusion proteins which seem to bring a cytoplasmic membrane transporter (belonging to either the ATP-binding cassette family, the major facilitator family, or the multidrug resistance family) into contact with outer membrane proteins such as TolC (16, 38). The TolA inner membrane protein containing a long alpha-helical domain is also thought to cross the periplasmic space (61). TolA belongs to the multiprotein Tol-Pal complex. The topologies of the Tol-Pal proteins have been previously characterized (25, 27, 30, 35, 44, 45, 57), and these proteins have been shown to form two complexes in the cell envelope. The TolA, TolQ, and TolR inner membrane proteins are associated via their transmembrane segments (13, 33). The TolB periplasmic protein interacts with the Pal outer membrane lipoprotein (5). The function of these proteins is not known, but a mutation in any of the tol-pal genes confers a defect in outer membrane integrity resulting in hypersensitivity to drugs and detergents and in leakage of periplasmic proteins to the medium (32, 61). The tol-pal gene cluster encodes two other proteins, Orf1 and Orf2, which are localized in the cytoplasm and in the periplasm, respectively (58). The Tol-Pal system is exploited for the entry of group A colicins and single-stranded DNA phages, the TolB and TolR proteins being required only for entry of the enzymatic E colicins and some pore-forming colicins such as colicin A (1, 9, 12, 61). The TonB system, which is involved in active transport, is used by group B pore-forming colicins (1, 11). The ExbB, ExbD, and TonB inner membrane proteins show sequence similarity with TolQ, TolR, and TolA proteins (17, 28). However, outer membrane integrity defects observed in tol-pal strains are not found in tonB cells.
Other cell envelope mutants have been reported to have outer membrane defects. Mutations deleting the inner core region of the lipopolysaccharide in rfa cells (23, 43, 50), the major lipoprotein (56, 62), or blocking the maturation process of porins (8) also induce hypersensitivity to detergents and drugs. Cells devoided of the periplasmic peptidyl prolyl isomerase, SurA, which seems to be involved in the folding of porins, also exhibit altered membrane properties (43). A strain with another outer membrane mutation, tolC, which confers reduced OmpF synthesis, is hypersensitive to various antibacterial agents (20, 42). Efflux mutations such as acrA or acrE cells confer drug hypersusceptibility (37). Unlike the outer membrane integrity mutants, AcrA and AcrE are inner membrane lipoproteins involved in the AcrAB-TolC (19) or AcrEF-outer membrane protein efflux pumps (38). However, only the tol-pal and lpp strains have been reported to release periplasmic proteins into the medium. A more pronounced outer membrane defect has been observed with lpp ompA cells, which form vesicles under normal growth conditions (52). Outer membrane vesicles were observed in lpp strains overproducing periplasmic β-lactamase (3). lpp strains, lacking the structural link between the outer membrane and the peptidoglycan, also form vesicles upon Mg2+ starvation (56, 62). In this study, different strains affected in outer membrane integrity were analyzed by electron microscopy (EM) and Western blotting. Each tol-pal strain was found to form outer membrane vesicles containing native outer membrane proteins. tol-pal cells were classified into two groups based on the amount of vesicles formed.
MATERIALS AND METHODS
Strains and plasmids.
The strains and plasmids used in this study are listed in Table 1. Strain SC44tolA contains a Tn10 transposon which maps between min 16 and 17 of E. coli chromosome and the tolA deletion of JC7782. P1 lysate of SC44tolA cells was used to cotransduce the tolA deletion and the tetracycline resistance in SR1458, giving strain LG10. This strain transformed with a plasmid encoding TolA is sensitive to colicins A and E3, indicating that Tn10 is inserted after the tol-pal gene cluster.
TABLE 1.
E. coli strains and plasmids used in this study
| Strain or plasmid | Relevant characteristics | Reference or source |
|---|---|---|
| Strains | ||
| A5922 | C600 tolA1 (stop after codon 400) recA | 54 |
| JC207 | P4X tolA207 (stop after codon 126) | 18 |
| JC864 | P4X tolB864 (stop after codon 22) | J. C. Lazzaroni |
| JC3417 | 1292 tolB (stop after codon 329) | 25 |
| JC7752 | 1292 tolB (stop after codon363)Δpal | 5 |
| JC7782 | 1292 tolA (stop after codon 40) | J. C. Lazzaroni |
| JC8031 | 1292 ΔtolRA | 13 |
| JC8931 | 1292 ompA::TnlacZ | J. C. Lazzaroni |
| JC8963 | JE5505 ompA::TnlacZ | 9 |
| JE5505 | JE5506 lpp5508 | 56 |
| KS303 | KS272 lpp5508 | 53 |
| LG10 | SR1458 tolA | This study |
| SR22 | CA8000 htrM378::Tn5 | 47 |
| SR1458 | MC4100φ (htrA::lacZ) | 48 |
| SR3205 | MC4100 surA::ΩKan | 43 |
| SC44 | tolC::Tn5 | 59 |
| SC44tolA | tolC::Tn5 tolA | C. Wandersman |
| TPS13 | GM1 tolQ13 (stop after codon 36) | 54 |
| TPS300 | GM1 tolR::ΩCm | 55 |
| Plasmids | ||
| pAmpB | pBR328 vector, tolB Ampr | This study |
| pARTolA | pACYC184 vector, tolA His6 Cmr | This study |
| pAX617 | pACYC184 vector, tolC Cmr | 24 |
| pBP | pBR328 vector, tolB pal Tetr | 5 |
| pNN | pUC9 vector, tolA Ampr | 2 |
| pQRA | pT7-5 vector, orf1 tolQRA Ampr | 13 |
| pTPS304 | pUC9 vector, tolQR Ampr | 54 |
| pTPS306 | pUC9 vector, tolRA Ampr | 54 |
Plasmid pARTolA was constructed by PCR amplification of the DNA fragment corresponding to the N-terminal region of TolA (from residues 2 to 65, which contain the TolA inner membrane sequence). PCR was carried out with pTPS306 as the template, using the following oligonucleotides (where the SphI restriction sites are underlined, and N correspond to A, C, G, or T): 5′-NNNNNNGCATGC TGAGC TCAAAGGCAACCGAACAAAACGACAAGC-3′ and 5′-CCTGGCTTTGCATGCGTTTGTACTGC-3′. The PCR DNA fragment was digested with SphI and purified on an acrylamide gel. Then, this 208-bp purified fragment was inserted into the unique SphI site of pARTolAII-III, giving pARTolA. Plasmid pARTolAII-III encodes an N-terminally His6-tagged soluble TolA derivative corresponding to the entire C-terminal periplasmic domain of TolA from residues 66 to 421 (15). Plasmid pARTolA was selected after transformation into JC7782 (tolA) cells grown on LB agar containing 2% deoxycholate and by DNA sequencing. Plasmid pAmpB was constructed from pBR328 derivative containing the EcoRI-PvuII DNA fragment of the tol gene cluster (4,645 bp) encoding Orf1, TolQRAB. This plasmid was digested with NruI in order to keep the tolB and bla genes and ori and to remove the tet, orf1, and tolQRA genes. Ampicillin-resistant plasmids were tested for complementation of a tolB strain by using colicin sensitivity tests, and expression of TolB was checked by immunodetection.
EM analyses.
Negative staining of colonies grown overnight on LB agar medium (containing 10 g of NaCl per liter) was routinely done. Cells were suspended in Tris-buffered saline (10 mM Tris-HCl [pH 8.0], 150 mM NaCl). Droplets were deposited onto freshly ionized Formvar-carbon-coated grids for 1 min. Grids were negatively stained with 1% aqueous uranyl acetate. Immunolabeling of LamB was performed with cells grown on M9 minimum medium agar containing 0.4% maltose as the sole carbon source (and 0.4% glycerol for the negative control). Colonies, reisolated on the same medium, were suspended in phosphate-buffered saline (PBS), and droplets were deposited onto coated grids. The samples were fixed with 2% paraformaldehyde in PBS (5 min) and incubated for 60 min with monoclonal antibody E302 (1/200 dilution). The samples were incubated with 10-nm-gold-conjugated anti-mouse immunoglobulin G (Biocell) for 30 min. After extensive washes in water, the grids were negatively stained with 0.5% aqueous uranyl acetate (1 min).
Ultrathin sections were obtained from cells grown overnight on disc filters (Millipore type VC; 0.1-μm pore size) placed on LB agar plates. Cell colonies were fixed with glutaraldehyde 2.5% in PBS (60 min), postfixed with 1% osmium tetroxide (60 min), dehydrated in ethanol, and embedded in EMbed-812 (E.M.S.). Ultrathin sections were stained with uranyl acetate and lead citrate.
RNase I periplasmic leakage and colicin tests.
Cells were streaked on LB agar containing 1.5% RNA. After overnight incubation, the addition of 10% trichloroacetic acid precipitated RNA with an opaque zone, while a halo indicated that the RNA was degraded by released periplasmic RNase I (18). Cells grown on LB medium at the exponential growth phase were adsorbed on LB agar. Lawns were spotted with 1-μl dilutions of purified colicins A and E1 and then incubated overnight. Formation of a halo indicated sensitivity to colicin. The incubation temperature was 37°C for all strains except SR22 (30°C).
Immunodetection of vesicles.
Cells, grown either in LB medium (containing 10 g of NaCl per liter) or in M9 minimal medium supplemented with glycerol and amino acids, were collected at the early exponential growth phase. Cells were pelleted by centrifugation at 3,000 × g for 5 min. The supernatants were either centrifuged at 20,000 × g for 15 min to remove remaining cells or filtered through 0.2-μm-pore-size filters (Gelman HT). This supernatant was either analyzed directly or ultracentrifuged at 250,000 × g for 45 min, yielding the vesicle pellet and final supernatant. Supernatants were trichloroacetic acid precipitated before analyses. For EM analyses, the vesicle pellets were suspended in Tris-buffered saline and further centrifuged at 20,000 × g to remove vesicles not resuspended. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting were performed as previously described (14).
Antibodies.
Polyclonal antibodies raised against TolA (anti-TolAIII or anti-TolAII-III, corresponding to the C-terminal region or the whole periplasmic soluble form, respectively), TolB, Pal proteins, porins (5, 13, 14), and TolC (60), and anti-LamB monoclonal antibody E302 (29), have been previously described. Anti-Lpp and anti β-lactamase polyclonal antibodies were generous gifts of Danièle Cavard.
RESULTS
tolA and tolB-pal mutants release outer membrane.
E. coli K-12 strains 1292, JC7782 (tolA), and JC7752 (tolB pal), all containing plasmid pBR322 (4), were grown on LB medium to early exponential growth phase. Cells and supernatants were analyzed for the release of β-lactamase. It has been previously shown that in tol-pal strains, periplasmic proteins such as β-lactamase, alkaline phosphatase, and RNase I leak into the extracellular medium (32, 61). Coomassie blue-stained gels revealed that outer membrane proteins OmpFC and OmpA were recovered in the supernatant as major proteins without any detection of major cytoplasmic proteins such as EF-Tu (not shown). β-Lactamase was also detected by Western blotting. The inner membrane TolA protein was immunodetected in JC7752 cells but not in the supernatant fraction. These results were obtained by removing cells from culture supernatants either by low-speed centrifugation (Fig. 1) or by filtration through 0.2-μm-pore-size filters (not shown). When the supernatants were ultracentrifuged (250,000 × g), only the β-lactamase was recovered in the soluble fraction (Fig. 1). Because immunodetection with an anti-LexA antibody gave negative results for the supernatant fractions, as determined by Coomassie blue staining, (not shown), we assumed that the cells were not lysed. We also observed that the isogenic strain 1292 did not release periplasmic or outer membrane proteins. Furthermore, 1292 cells transformed with a multicopy plasmid (either pBR322 [Fig. 1] or pUC9 [not shown]) and overexpressing β-lactamase did not show outer membrane leakage. These results suggest that tolA and tolB-pal cells release outer membrane.
FIG. 1.
Immunoblot analyses of tolA (JC7782), tolB pal (JC7752), and wild-type (wt) parent (1292) strains. About 108 cells and the supernatant of 5 × 108 cells were analyzed after heat denaturation. S1 and S2 correspond to the supernatants resulting from centrifugation (20,000 × g) and ultracentrifugation (250,000 × g), respectively. Three immunodetections were carried out sequentially with antiporin (cross-reacting with OmpA), anti-β-lactamase (β-Lac), and anti-TolAIII antibodies.
Direct evidence of the release of outer membrane vesicles.
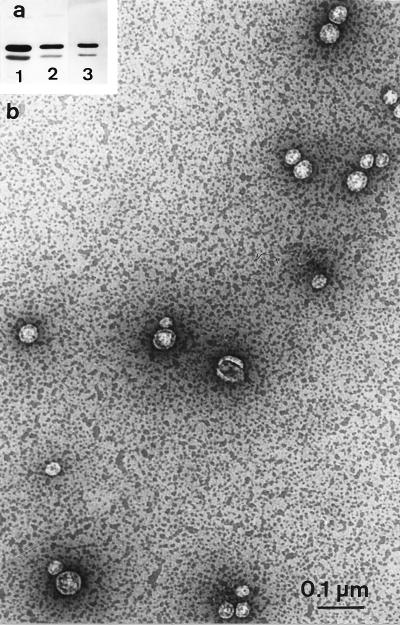
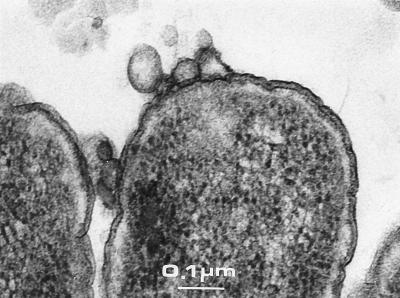
Supernatant fractions of JC8031 (tolRA), JC7782 (tolA), and LG10 (tolA) cells grown in LB medium were ultracentrifuged and analyzed by Western blotting followed by antiporin immunodetection. The outer membrane proteins found in the supernatant (after centrifugation at 20,000 × g) were recovered in the pellet obtained after ultracentrifugation (Fig. 2a). This pellet was resuspended and adsorbed on a glow-discharged carbon-coated grid. After negative staining and EM analyses, we observed that the samples contained small vesicles with an average size of about 40 ± 20 nm (Fig. 2b). The pellet obtained after centrifugation at 20,000 × g was also analyzed and found to contain low amounts of larger vesicles (up to 200 nm) and few lysed cells (not shown). To analyze the vesicle envelope and cell localization of the vesicles, we used another approach. Cell colonies grown on a disc filter placed on LB agar plates were fixed in situ and resin embedded. Morphological analyses were performed on ultrathin sections. Vesicles could clearly be seen on the surface of cells without preferential pole or septum localization. These sections demonstrated that the vesicles are enclosed by only one membrane. Except for vesicles, tol cell envelopes do not show any peculiar characteristic (Fig. 3).
FIG. 2.
(a) Immunodetection of OmpFC and OmpA in tolRA (JC8031) cells, supernatants, and vesicles. Cells (lane 1), supernatant (lane 2), and resuspended vesicle pellet of ultracentrifugation (lane 3) of 5 × 108 cells are shown. (b) Electron micrograph showing negative staining of the vesicle suspension (same sample as analyzed in lane 3).
FIG. 3.
Thin sections of Epon-embedded tolA cells (LG10).
All tol-pal strains form vesicles.
Supernatant fractions of tolA, tolB, tolQ, tolR, and pal strains (either nonsense, missense, or deletion mutants), grown in LB medium, were checked by Western blotting with antiporin antibodies. Given the polar effect of tolQ13 mutation on TolR and TolA expression (58), we used strain TPS13(pTPS306) to check only the tolQ mutation. In each mutant, we observed that the supernatant contained outer membrane proteins as previously observed in tolRA, tolA, or tolB pal cells, thus indicating that all of the tol-pal strains had the same outer membrane defect (Table 2). Complementation experiments were carried out to confirm that the effect was the result of tol-pal mutations. Cells were transformed with plasmids carrying tol-pal genes able to complement the corresponding chromosomal mutations, and supernatant fractions were immunodetected with antiporin antibodies. We observed that the outer membrane defects of tol-pal strains were plasmids complemented for RNase I leakage and for the absence of outer membrane porins in the supernatants (Table 2). Plasmids pTPS304 (encoding TolR and TolQ) and pQRA complement the tolQ13 mutation regardless of the level of overexpression of TolA. However, it appeared that plasmid complementations of TPS13, TPS300, and JC7782 were not total since faint immunoreactions could be detected in the supernatants (Table 2).
TABLE 2.
Porin and vesicle detections in the supernatants of tol-pal and outer membrane hypersensitive strains
| Strain type | Detection of:
|
||
|---|---|---|---|
| Porinsa | Vesiclesb | RNase I/ colicinc | |
| tol | |||
| A5922 (tolA) | + | LL | +/R |
| JC7782 LG10, and JC207 (all tolA) | + | HL | +/R |
| JC3417 and JC864 (both tolB) | + | LL | +/R |
| JC7752 (tolB pal) | + | LL | +/R |
| JC7752(pAmpB) (pal) | + | LL | +/R |
| JC8031(tolRA) | + | HL | +/R |
| SC44tolA(pAX617) (tolA) | + | HL | +/R |
| TPS13 (tolQR) | + | HL | +/R |
| TPS13(pTPS306) (tolQ) | + | HL | +/R |
| TPS300 (tolR) | + | HL | +/R |
| Outer membrane altered | |||
| JC8931 (ompA) | − | N | −/S |
| JC8963 (lpp ompA) | + | HL | +/S |
| JE5505 (lpp) | −/+ | N | +/S |
| KS303 (lpp) | + | HL | +/S |
| SC44 (tolC) | − | N | −/Sd |
| SR22 (htrM) | − | N | −/S |
| SR3205 (surA) | − | N | −/Se |
| Isogenic and complemented | |||
| 1292, GM1, CA8000, KS272, and JE5506 | − | N | −/S |
| JC3417(pAmpB) | − | N | −/S |
| JC7752(pBP) | − | N | −/S |
| JC7782(pNN/pARTolA/pQRA) | −/+ | LL | −/S |
| TPS13(pQRA/pTPS304) | −/+ | N | −/S |
| TPS300(pQRA/pTPS304) | −/+ | N | −/S |
Supernatant fractions were immunodetected with antiporin antibodies. +, −/+, and − indicate strong, faint, and no immunodetection, respectively.
Amount of vesicles visualized by EM, indicated as HL (high level [many vesicles on all cells]), LL (low level [some vesicles on most cells]), or N (no vesicle on most cells).
RNase I leakage (+) or no release (−) and colicin A and E1 sensitivity tests (R or S [resistant or sensitive, respectively]).
Cells are less sensitive than parent cells to colicin A and are resistant to colicin E1.
Cells are less sensitive to colicin A than, and as sensitive to colicin E1 as, parent cells.
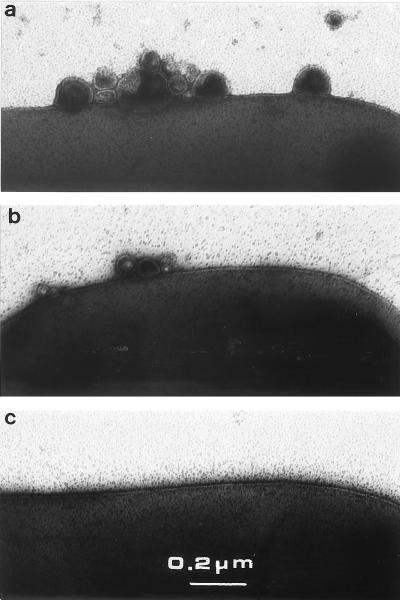
EM analyses were carried out essentially by negative staining to visualize directly a cell population without a centrifugation step, which might introduce size exclusion. As a preliminary control, we analyzed the supernatant fractions of tol cells grown on solid or liquid culture medium by Western blotting. Porins were equally immunodetected in cell supernatants under these conditions (not shown). EM observations revealed small vesicles. On the basis of multiple EM observations, the tol-pal cells were classified into two groups corresponding to the different amounts of vesicles (Table 2 and Fig. 4). Numerous vesicles were recovered from tolQ and tolR strains as well as from all tolA strains except A5922. Low amounts of vesicles were detected in tolB and pal strains. To confirm that the amount of vesicles was not dependent on the isogenic strain 1292 background, the tolA deletion from JC7782 was transferred by P1 transduction in strains C600 and MC4100 and further analyzed. Numerous vesicles were observed in the supernatants of the three tolA strains [JC7782, LG10, and SC44tolA(pAX617)], indicating no dependence on the genetic context for vesicle formation. The size of the vesicles was found to be 20 to 200 nm in all tol-pal mutants, without preferential localization (Fig. 4). tol-pal strains complemented by plasmids were also observed, and a good correlation of vesicles (from cells growing on LB plates) with supernatant immunodetection (from liquid culture) was found except for strains TPS13 and TPS300 (Table 2). Faint immunodetection could be correlated with low amounts of vesicles in the case of complemented tolA strains, while in complemented tolQ and tolR strains, no vesicles were observed. Immunodetection with anti-LexA antibody indicated that after growth in liquid medium, few cells overexpressing inner membrane Tol proteins, by multicopy plasmids, were lysed. However, EM results showed few vesicles in the complemented tolA strain, indicating a partial restoration of outer membrane integrity. In conclusion, tolA, tolQ, and tolR strains were shown to form numerous vesicles, while tolB, pal, and tolB-pal strains formed few vesicles. The low level of vesicles detected in A5922 may be due to the expression of a 400-residue TolA derivative which was immunodetected (not shown).
FIG. 4.
Negative staining of tol cells and vesicles. Electron micrographs are representative of the vesicle amounts: high (a; JC7782 cells), low (b; JC7752 cells), and none (c; 1292 cells).
Outer membrane hypersensitive mutants and cell vesicles.
Some mutants defective in outer membrane or periplasmic protein content showed outer membrane alterations similar to those found in tol strains, such as hypersensitivity to drugs and detergents. Only the lpp (56) and the tol-pal (18) cells were found to release periplasmic proteins such as RNase I into the extracellular medium (Table 2). We analyzed the supernatant fractions of SC44 (tolC), SR22 (htrM or rfaD), and SR3205 (surA) mutants after growth in LB or M9 minimal medium. No vesicle or outer membrane protein was detected in the supernatant by EM experiments or by Western blot analyses (Table 2). JE5506 and JC8963 (lpp ompA) strains were checked by the two approaches, and only the latter strain was found to form high amounts of vesicles. Interestingly, KS303 (lpp) cells were found to form numerous vesicles, while in JE5505 (lpp) no or few vesicles were detected regardless of the medium used (LB rich or M9 minimum medium with or without the addition of Mg2+). Therefore, the two lpp strains (JE5505 and KS303) may differ by having accumulated a suppressor mutation(s) which renders JE5505 devoid of vesicles. All of the lpp strains used were checked for the absence of Lpp by immunoblotting and periplasmic RNase I leakage. We also verified that the lpp strains expressed the TolQRAB-Pal proteins by testing for sensitivity to colicins A and E1 and by immunoblotting.
tol mutants and outer membrane proteins.
In earlier studies, the only difference between tol and isogenic strains with respect to the membrane defect was the diminution of the expression levels of the outer membrane proteins OmpF and LamB (31). These variations were observed in cell pellets and with protein or operon fusions. Thus, it was of interest to compare the relative amounts of porins in tol cells and in the vesicles. Cells were grown in LB medium devoid of NaCl (since low osmolarity induced OmpF expression). We found that OmpF was not recovered preferentially in the vesicles of tolA, tolB, and tolRA strains. Similar results were obtained for LamB (not shown), demonstrating low-level expression of OmpF and LamB in the tol-pal mutants. Thus, the lower amounts of OmpF and LamB might be an indirect effect relative to osmoregulation (9). High amounts of vesicles were also detected when tolA cells were grown at a low temperature (Table 2) which was previously shown to induce capsular polysaccharide synthesis (10), indicating that mucoidy does not prevent the formation of vesicles in tolA cells.
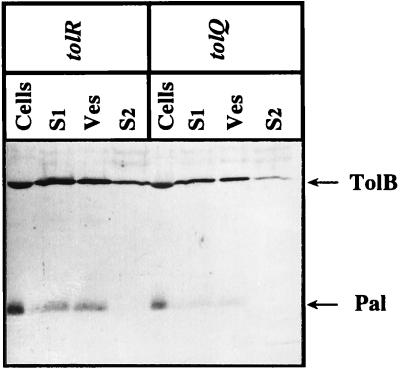
The oligomeric state of the porins recovered in the vesicle fractions of tol cells was checked by Western blot analyses using samples heat denatured or not. Monomeric porins OmpF and OmpC could be immunodetected only at 96°C. OmpA was immunodetected at its native or denatured electrophoretic mobility, as demonstrated by sample heating at 37 or 96°C, respectively (not shown). The exposure of LamB porin at the surface of the vesicles was investigated by EM immunodetection using monoclonal antibody E302, raised against the external loop L9 of LamB (29). LG10 cells, grown on minimal medium plates, were adsorbed on grids and immunogold labeled. Gold particles on the surface of cells and vesicles were observed only after maltose induction (not shown). These results indicated correct exposure of the LamB L9 loop and correct assembly of trimeric porins present in the vesicles of the tol strains. In addition to LamB, OmpFC, and OmpA, TolC was immunodetected in the outer membrane vesicles (not shown). Using an anti-TolB-Pal antibody, we detected the Pal lipoprotein in the vesicle fraction of tol strains and found TolB in the extracellular medium and in the vesicle pellets (Fig. 5). Periplasmic TolB is then released, and its presence in the vesicles probably reflects its interaction with Pal.
FIG. 5.
Immunodetection of TolB and Pal in the supernatants and vesicle fractions of tolQ (TPS13) and tolR (TPS300) cells. Cells, vesicles (Ves), cell supernatant (S1), and ultracentrifuged supernatant (S2) were loaded in the same amounts as for Fig. 1 and subjected to immunodetection with anti-TolB-Pal antibodies.
DISCUSSION
In this work, we showed that tol-pal strains form vesicles containing outer membrane proteins such as trimeric porins. The EM assays facilitate observations of cells by virtue of their rapid and simple approach without any size exclusion. tol-pal or lpp cells from single bacteria were appeared different from other cells. However, some vesicles could be observed in nearly all of the E. coli cells that we analyzed. The level of vesicle formation was found to be high in tolA, tolQ, and tolR strains and low in tolB and pal strains. Thus, a major outer membrane defect should be attributed to alteration of the TolAQR complex rather than the TolB-Pal complex. Aside from the EM classification data obtained with adsorbed material, we have not yet precisely quantified the differences between tolABQR and pal cells. However, densitometry scannings of blue-stained outer membrane porins of tol-pal cell membranes and supernatant filtrates indicated that between 10 and 20% of porins were found in vesicles.
Recent results indicated that the TolB-Pal complex interacted in vivo with the Lpp and OmpA proteins and that the expression of Pal was increased in an lpp ompA strain (9). Together with our data, these results suggest that the Tol-Pal proteins may be involved in a structural cell envelope network. Hence, the TolB-Pal outer membrane complex interacted with the peptidoglycan and might be linked to the TolAQR inner membrane complex through direct or indirect interaction. Then, a defect in any of the Tol protein might disrupt the stoichiometry of the Tol-Pal transenvelope complex, inducing vesicle formation. However, no experimental evidence for the interaction between the two complexes TolAQR and TolB-Pal-peptidoglycan has been demonstrated. The Tol-Pal complex may also be involved in the integration of newly synthesized outer membrane components. Indeed, these two potential roles are not incompatible. Because porins were found to interact in vitro with TolA and TolB (14, 49), the absence of a functional Tol-Pal complex of definite stoichiometry (22) would lead to the release rather than correct integration of outer membrane components. The induction of cell hypersensitivity and colicin tolerance had been previously shown for wild-type cells overexpressing periplasmic TolA derivatives (36) or periplasmic N-terminal domains of group A colicins (6). These results indicated that the Tol-Pal complex can be destabilized even when present in the cell envelope. Preliminary experiments revealed that vesicle formation was stopped upon carbon starvation of exponentially growing tolA cells, indicating that this process was dependent on cell growth. All of these results suggest a role of the Tol-Pal proteins in assuming a transient link between the two membranes and the peptidoglycan. Further analyses of vesicles and outer membrane fractions after pulse-chase labeling experiments will indicate if the Tol-Pal proteins contribute in the dynamic integration of outer membrane components.
An interesting aspect concerns the relationship between (i) hypersensitivity to drugs and detergents and (ii) vesicle detection. We observed that tolC, surA, and rfa cells did not form vesicles. Efficient release of outer membrane in rfa cells treated with EDTA has been demonstrated (39). In accord with this observation, in the absence of EDTA, no vesicles were detected in the rfaD strain. lpp strains were previously shown to form vesicles under low concentrations of Mg2+ (21, 52, 56) or overexpression of periplasmic proteins (3). In the two lpp strains used, we observed that KS303 formed numerous vesicles whereas JE5505 did not. However, JE5505ompA (lpp ompA double mutant) was the strain which formed the greatest number of vesicles, while ompA strain JC8931 had no obvious outer membrane defect. All of these lpp strains contain the Tol-Pal proteins. Hence, it appears that an independent genetic event may differentiate the two lpp strains. As for the lpp strains, we suspected that vesicles recovered in the tol-pal strains may be a consequence of the absence of Pal (or its association with the peptidoglycan). Because the pal mutants formed lower amounts of vesicles compared to the tolAQR strains, this possibility did not seem likely. Furthermore, we observed that each of the tolABQR mutations did not prevent the localization of Pal in the vesicle fraction, which probably reflects its correct outer membrane localization. Thus, sorting and transport of Pal to the outer membrane, driven by the periplasmic LolA (40) and outer membrane LolB (41) proteins, is not prevented by the periplasmic leaky phenotype of tol cells.
The last point of relevance is the detection in other gram-negative bacteria such as Pseudomonas aeruginosa (26) or Neisseria gonorrhoeae (46) of outer membrane vesicles which were suspected to interfere with bacterial pathogenesis. As homologs of the Tol-Pal proteins have been found in the genomes of all gram-negative bacteria sequenced so far, a better understanding of their regulation and involvement in vesicle formation is of interest.
ACKNOWLEDGMENTS
We are grateful to Cécile Wandersman for the tolC and tolA tolC strains and the TolC plasmid and antiserum, Alain Charbit and Danièle Cavard for antibodies, Dominique Missiakas for genetic techniques, Emmanuelle Bouveret and Hélène Bénédetti for careful reading of the manuscript, Alain Rigal for figures, and Claude Lazdunski for encouragements.
This work was supported by the CNRS.
REFERENCES
- 1.Bénédetti H, Géli V. Colicin transport, channel formation and inhibition. In: Konings W N, Kaback H R, Lolkema J S, editors. Handbook of biological physics. Vol. 2. Amsterdam, The Netherlands: Elsevier Science Publishers; 1996. pp. 665–691. [Google Scholar]
- 2.Bénédetti H, Lazdunski C, Lloubès R. Protein import into E. coli: colicins A and E1 interact with a component of their translocation system. EMBO J. 1991;10:1989–1995. doi: 10.1002/j.1460-2075.1991.tb07728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernadac A, Bolla J M, Inouye M, Pagès J M. Precise localization of an overproduced periplasmic protein in E. coli: use of a double immuno-gold labelling. Biol Cell. 1987;61:141–147. doi: 10.1111/j.1768-322x.1987.tb00580.x. [DOI] [PubMed] [Google Scholar]
- 4.Bolivar F, Rodriguez R, Greene P, Betlach M, Heyneker H, Boyer H. Construction and characterization of new cloning vehicles. Gene. 1977;2:95–113. [PubMed] [Google Scholar]
- 5.Bouveret E, Derouiche R, Rigal A, Lloubès R, Lazdunski C, Bénédetti H. Peptidoglycan-associated lipoprotein-TolB interaction. J Biol Chem. 1995;270:11071–11077. doi: 10.1074/jbc.270.19.11071. [DOI] [PubMed] [Google Scholar]
- 6.Bouveret E, Rigal A, Lazdunski C, Bénédetti H. Distinct regions of the colicin A translocation domain are involved in the interaction with TolA and TolB proteins upon import into E. coli. Mol Microbiol. 1998;27:143–157. doi: 10.1046/j.1365-2958.1998.00667.x. [DOI] [PubMed] [Google Scholar]
- 7.Braun V. Energy-coupled transport and signal transduction through the Gram-negative outer membrane via TonB-ExbB-ExbD-dependent receptors proteins. FEMS Microbiol Rev. 1995;16:295–307. doi: 10.1111/j.1574-6976.1995.tb00177.x. [DOI] [PubMed] [Google Scholar]
- 8.Carlson J, Silhavy T. Signal sequence processing is required for the assembly of LamB trimers in the outer membrane of Escherichia coli. J Bacteriol. 1993;175:3327–3334. doi: 10.1128/jb.175.11.3327-3334.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clavel T, Germon P, Vianney A, Portalier R, Lazzaroni J C. TolB protein of Escherichia coli K12 interacts with the outer membrane peptidoglycan-associated proteins Pal, Lpp and OmpA. Mol Microbiol. 1998;29:359–367. doi: 10.1046/j.1365-2958.1998.00945.x. [DOI] [PubMed] [Google Scholar]
- 10.Clavel T, Lazzaroni J C, Vianney A, Portalier R. Expression of the tolQRA genes of E. coli K-12 is controlled by the RcsC sensor protein involved in capsule synthesis. Mol Microbiol. 1996;19:19–25. doi: 10.1046/j.1365-2958.1996.343880.x. [DOI] [PubMed] [Google Scholar]
- 11.Davies J, Reeves P. Genetics of resistance to colicins in Escherichia coli K-12: cross-resistance among colicins of group B. J Bacteriol. 1975;123:96–101. doi: 10.1128/jb.123.1.96-101.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davies J, Reeves P. Genetics of resistance to colicins in Escherichia coli K-12: cross-resistance among colicins of group A. J Bacteriol. 1975;123:102–117. doi: 10.1128/jb.123.1.102-117.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Derouiche R, Bénédetti H, Lazzaroni J C, Lazdunski C, Lloubès R. Protein complex within E. coli inner membrane. J Biol Chem. 1995;270:11078–11084. doi: 10.1074/jbc.270.19.11078. [DOI] [PubMed] [Google Scholar]
- 14.Derouiche R, Gavioli M, Bénédetti H, Prilipov A, Lazdunski C, Lloubès R. TolA central domain interacts with E. coli porins. EMBO J. 1997;15:6408–6415. [PMC free article] [PubMed] [Google Scholar]
- 15.Derouiche R, Zeder-Lutz G, Bénédetti H, Gavioli M, Rigal A, Lazdunski C, Lloubès R. Binding of colicins A and E1 to purified TolA domains. Microbiology. 1997;143:3185–3192. doi: 10.1099/00221287-143-10-3185. [DOI] [PubMed] [Google Scholar]
- 16.Dinh T, Paulsen I, Saier M. A family of extracytoplasmic protein that allow transport of large molecules across the outer membrane of gram-negative bacteria. J Bacteriol. 1994;176:3825–3831. doi: 10.1128/jb.176.13.3825-3831.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eick-Helmerich K, Braun V. Import of biopolymers into Escherichia coli: nucleotide sequences of exbB and exbD genes are homologous to those of the tolQ and tolR genes, respectively. J Bacteriol. 1989;171:5117–5127. doi: 10.1128/jb.171.9.5117-5126.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fognini-Lefebvre N, Lazzaroni J C, Portalier R. tolA, tolB and excC, three cistrons involved in the control of pleiotropic release of periplasmic proteins by E. coli K12. Mol Gen Genet. 1987;209:391–395. doi: 10.1007/BF00329670. [DOI] [PubMed] [Google Scholar]
- 19.Fralick J. Evidence that TolC is required for functioning of the Mar/AcrAB efflux pump of Escherichia coli. J Bacteriol. 1996;178:5803–5805. doi: 10.1128/jb.178.19.5803-5805.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fralick J, Burns-Keliher L. Additive effect of tolC and rfA mutations on the hydrophobic barrier of the outer membrane of Escherichia coli K-12. J Bacteriol. 1994;176:6404–6406. doi: 10.1128/jb.176.20.6404-6406.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fung T, MacLister T, Rothfield L. Role of murein lipoprotein in morphogenesis of the bacterial division septum: phenotype similarity of lkyD and lpo mutants. J Bacteriol. 1978;133:1467–1471. doi: 10.1128/jb.133.3.1467-1471.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guihard G, Boulanger P, Bénédetti H, Lloubès R, Besnard M, Letellier L. Colicin A and the Tol proteins involved in its translocation are preferentially located in the contact sites between the inner and outer membranes of E. coli cells. J Biol Chem. 1994;269:5874–5880. [PubMed] [Google Scholar]
- 23.Hancock R. The bacterial outer membrane as a drug barrier. Trends Microbiol. 1997;7:37–42. doi: 10.1016/S0966-842X(97)81773-8. [DOI] [PubMed] [Google Scholar]
- 24.Hiraga S, Niki H, Ogura T, Ichinose C, Mori H, Ezaki B, Jaffé A. Chromosome partitioning in Escherichia coli novel mutants producing anucleate cells. J Bacteriol. 1989;171:1496–1505. doi: 10.1128/jb.171.3.1496-1505.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Isnard M, Rigal A, Lazzaroni J C, Lazdunski C, Lloubès R. Maturation and localization of the TolB protein required for colicin import. J Bacteriol. 1994;176:6392–6396. doi: 10.1128/jb.176.20.6392-6396.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kadurugamuwa J, Beveridge T. Virulence factors released from Pseudomonas aeruginosa in association with membrane vesicles during normal growth and exposure to gentamicin: a novel mechanism of enzyme secretion. J Bacteriol. 1995;177:3998–4008. doi: 10.1128/jb.177.14.3998-4008.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kampfenkel K, Braun V. Membrane topologies of the TolQ and TolR proteins of Escherichia coli: inactivation of TolQ by a missense mutation in the proposed first transmembrane segment. J Bacteriol. 1993;175:4485–4491. doi: 10.1128/jb.175.14.4485-4491.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karlsson M, Hannavy K, Higgins C. A sequence-specific function for the N-terminal signal-like sequence of the TonB protein. Mol Microbiol. 1993;8:379–388. doi: 10.1111/j.1365-2958.1993.tb01581.x. [DOI] [PubMed] [Google Scholar]
- 29.Klebba P E, Newton S M C, Charbit A, Michel V, Perrin D, Hofnung M. Further genetic analysis of the C-terminal external loop region in E. coli maltoporin. Res Microbiol. 1997;148:375–387. doi: 10.1016/S0923-2508(97)83868-5. [DOI] [PubMed] [Google Scholar]
- 30.Lazzaroni J C, Portalier R. The excC gene of E. coli K-12 required for cell envelope integrity encodes the peptidoglycan-associated lipoprotein (PAL) Mol Microbiol. 1992;6:735–742. doi: 10.1111/j.1365-2958.1992.tb01523.x. [DOI] [PubMed] [Google Scholar]
- 31.Lazzaroni J C, Fognini-Lefebvre N, Portalier R. Effects of IkyB mutations on the expression of ompF, ompC and lamB porin structural genes in E. coli K-12. FEMS Microbiol Lett. 1986;33:235–239. [Google Scholar]
- 32.Lazzaroni J C, Fognini-Lefebvre N, Portalier R. Cloning of excC and excD genes involved in the release of periplasmic proteins by E. coli K-12. Mol Gen Genet. 1989;218:460–464. doi: 10.1007/BF00332410. [DOI] [PubMed] [Google Scholar]
- 33.Lazzaroni J C, Vianney A, Popot J L, Benedetti H, Samatey F, Lazdunski C, Portalier R, Geli V. Transmembrane alpha-helix interactions are required for the functional assembly of the E. coli Tol complex. J Mol Biol. 1995;246:1–7. doi: 10.1006/jmbi.1994.0058. [DOI] [PubMed] [Google Scholar]
- 34.Letain T, Postle K. TonB protein appears to transduce energy by shuttling between the cytoplasmic membrane and the outer membrane in E. coli. Mol Microbiol. 1997;24:271–283. doi: 10.1046/j.1365-2958.1997.3331703.x. [DOI] [PubMed] [Google Scholar]
- 35.Levengood S, Beyer W, Webster R. TolA: a membrane protein involved in colicin uptake contains an extended helical region. Proc Natl Acad Sci USA. 1991;88:5939–5943. doi: 10.1073/pnas.88.14.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levengood-Freyermuth S, Click E, Webster R. Role of the carboxy-terminal domain of TolA in protein import and integrity of the outer membrane. J Bacteriol. 1993;175:222–228. doi: 10.1128/jb.175.1.222-228.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ma D, Cook D, Alberti M, Pon N, Nikaido H, Hearst J. Molecular cloning and characterization of acrA and acrE genes of Escherichia coli. J Bacteriol. 1993;175:6299–6313. doi: 10.1128/jb.175.19.6299-6313.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma D, Cook D, Hearst J, Nikaido H. Efflux pumps and drug resistance in Gram-negative bacteria. Trends Microbiol. 1994;2:489–493. doi: 10.1016/0966-842x(94)90654-8. [DOI] [PubMed] [Google Scholar]
- 39.Marvin H, Ter Beest M, Witholt B. Release of outer membrane fragments from wild-type Escherichia coli and from several E. coli lipopolysaccharide mutants by EDTA and heat shock treatments. J Bacteriol. 1989;171:5262–5267. doi: 10.1128/jb.171.10.5262-5267.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matsuyama S, Tajima T, Tokuda H. A novel periplasmic carrier protein involved in the sorting and transport of E. coli lipoproteins destined for the outer membrane. EMBO J. 1995;14:3365–3372. doi: 10.1002/j.1460-2075.1995.tb07342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matsuyama S, Yokota N, Tokuda H. A novel outer membrane lipoprotein, LolB, involved in the LolA (p20)-dependent localization of lipoproteins to the outer membrane of E. coli. EMBO J. 1997;16:6947–6955. doi: 10.1093/emboj/16.23.6947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Misra R, Reeves P. Role of micF in the tolC-mediated regulation of OmpF, a major outer membrane protein of Escherichia coli K-12. J Bacteriol. 1987;169:4722–4730. doi: 10.1128/jb.169.10.4722-4730.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Missiakas D, Betton J M, Raina S. New components of protein folding in extracytoplasmic compartments of E. coli SurA, FkpA, and Skp/OmpH. Mol Microbiol. 1996;21:871–884. doi: 10.1046/j.1365-2958.1996.561412.x. [DOI] [PubMed] [Google Scholar]
- 44.Mizuno T. A novel peptidoglycan-associated lipoprotein found in the cell envelopes of Pseudomonas aeruginosa and Escherichia coli. J Biochem. 1979;86:991–1000. doi: 10.1093/oxfordjournals.jbchem.a132631. [DOI] [PubMed] [Google Scholar]
- 45.Müller M M, Vianney A, Lazzaroni J C, Webster R E, Portalier R. Membrane topology of the Escherichia coli TolR protein required for cell envelope integrity. J Bacteriol. 1993;175:6059–6061. doi: 10.1128/jb.175.18.6059-6061.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pettit R, Judd R. Characterization of naturally elaborated blebs from serum-susceptible and serum-resistant strains of Neisseria gonorrhoeae. Mol Microbiol. 1992;6:723–728. doi: 10.1111/j.1365-2958.1992.tb01521.x. [DOI] [PubMed] [Google Scholar]
- 47.Raina S, Georgopoulos C. The htrM gene, whose product is essential for E. coli viability only at elevated temperatures, is identical to the rfaD gene. Nucleic Acids Res. 1991;19:3811–3819. doi: 10.1093/nar/19.14.3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Raina S, Missiakas D, Georgopoulos C. The rpoE gene encoding the ςE heat shock sigma factor of E. coli. EMBO J. 1995;14:1043–1055. doi: 10.1002/j.1460-2075.1995.tb07085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rigal A, Bouveret E, Lloubès R, Lazdunski C, Bénédetti H. The TolB protein interacts with the porins of Escherichia coli. J Bacteriol. 1997;179:7274–7279. doi: 10.1128/jb.179.23.7274-7279.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schnaitman C, Klena J. Genetic of lipopolysaccharide biosynthesis in enteric bacteria. Microbiol Rev. 1993;57:655–682. doi: 10.1128/mr.57.3.655-682.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Skare J, Ahmer B, Seachord C, Darveau R, Postle K. Energy transduction between membranes. TonB, a cytoplasmic membrane protein, can be chemically cross-linked in vitro to the outer membrane receptor FepA. J Biol Chem. 1993;268:16302–16308. [PubMed] [Google Scholar]
- 52.Sonntag I, Schwarz H, Hirota Y, Henning U. Cell envelope and shape of Escherichia coli: multiple mutants missing the outer membrane lipoprotein and other major outer membrane proteins. J Bacteriol. 1978;136:280–285. doi: 10.1128/jb.136.1.280-285.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Strauch K, Beckwith J. An E. coli mutation preventing degradation of abnormal periplasmic proteins. Proc Natl Acad Sci USA. 1988;85:1576–1580. doi: 10.1073/pnas.85.5.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sun T, Webster R. fii, a bacterial locus required for filamentous phage infection and its relation to colicin-tolerant tolA and tolB. J Bacteriol. 1986;165:107–115. doi: 10.1128/jb.165.1.107-115.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sun T, Webster R. Nucleotide sequence of a gene cluster involved in entry of E colicins and single-stranded DNA of infecting filamentous bacteriophages into Escherichia coli. J Bacteriol. 1987;169:2667–2674. doi: 10.1128/jb.169.6.2667-2674.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Susuki H, Nishimura Y, Yasuda S, Nishimura A, Yamada M, Hirota Y. Murein-lipoprotein of E. coli: a protein involved in the stabilization of bacterial envelope. Mol Gen Genet. 1978;167:1–9. doi: 10.1007/BF00270315. [DOI] [PubMed] [Google Scholar]
- 57.Vianney A, Lewin T, Bayer W, Lazzaroni J C, Portalier R, Webster R. Membrane topology and mutational analysis of the TolQ protein of Escherichia coli required for the uptake of macromolecules and cell envelope integrity. J Bacteriol. 1994;176:822–829. doi: 10.1128/jb.176.3.822-829.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vianney A, Müller M, Clavel T, Lazzaroni J C, Portalier R, Webster R. Characterization of the tol-pal region of Escherichia coli K-12: translational control of tolR expression by TolQ and identification of a new open reading frame downstream of pal encoding a periplasmic protein. J Bacteriol. 1996;178:4031–4038. doi: 10.1128/jb.178.14.4031-4038.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wandersmann C, Delepelaire P. TolC, an E. coli outer membrane protein required for haemolysin secretion. Proc Natl Acad Sci USA. 1990;87:4776–4780. doi: 10.1073/pnas.87.12.4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wandersmann C, Létoffé S. Involvement of lipopolysaccharide in the secretion of Escherichia coli α-haemolysin and Erwinia chrysanthemi proteases. Mol Microbiol. 1993;7:141–150. doi: 10.1111/j.1365-2958.1993.tb01105.x. [DOI] [PubMed] [Google Scholar]
- 61.Webster R. The tol gene products and the import of macromolecules into E. coli. Mol Microbiol. 1991;5:1005–1011. doi: 10.1111/j.1365-2958.1991.tb01873.x. [DOI] [PubMed] [Google Scholar]
- 62.Yem W, Wu H. Physiological characterization of an Escherichia coli mutant altered in the structure of murein lipoprotein. J Bacteriol. 1978;133:1419–1426. doi: 10.1128/jb.133.3.1419-1426.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]