Abstract
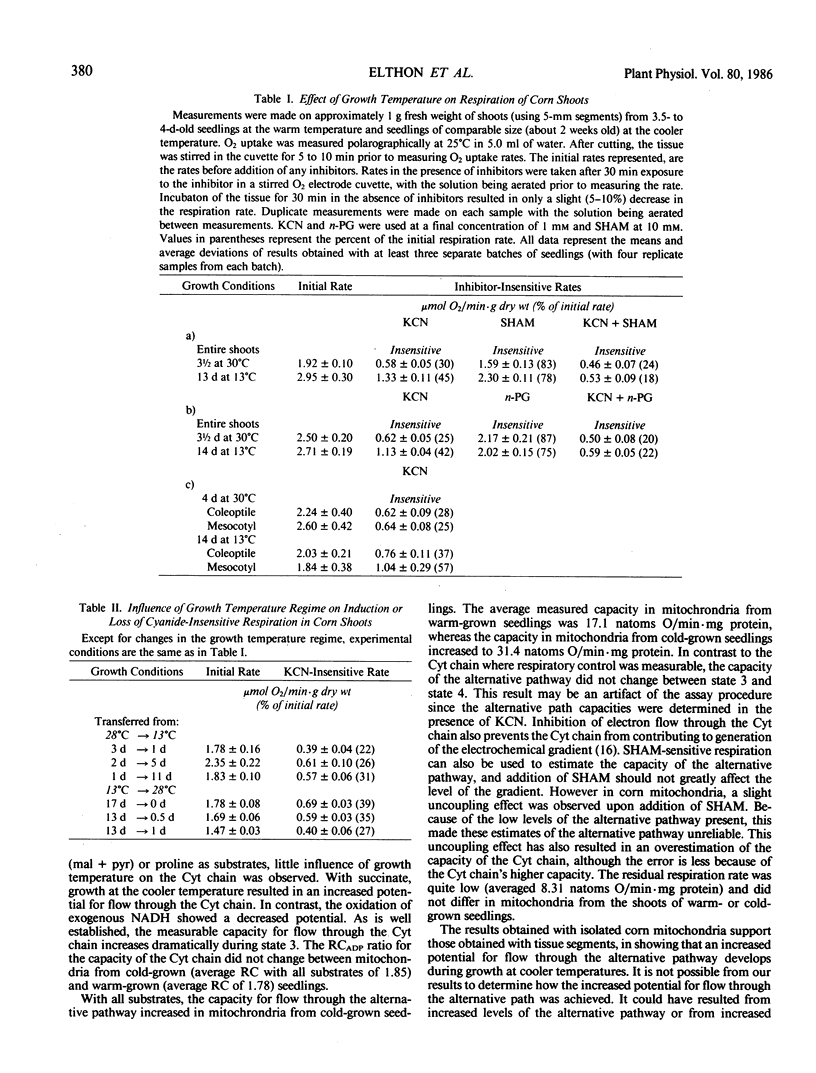
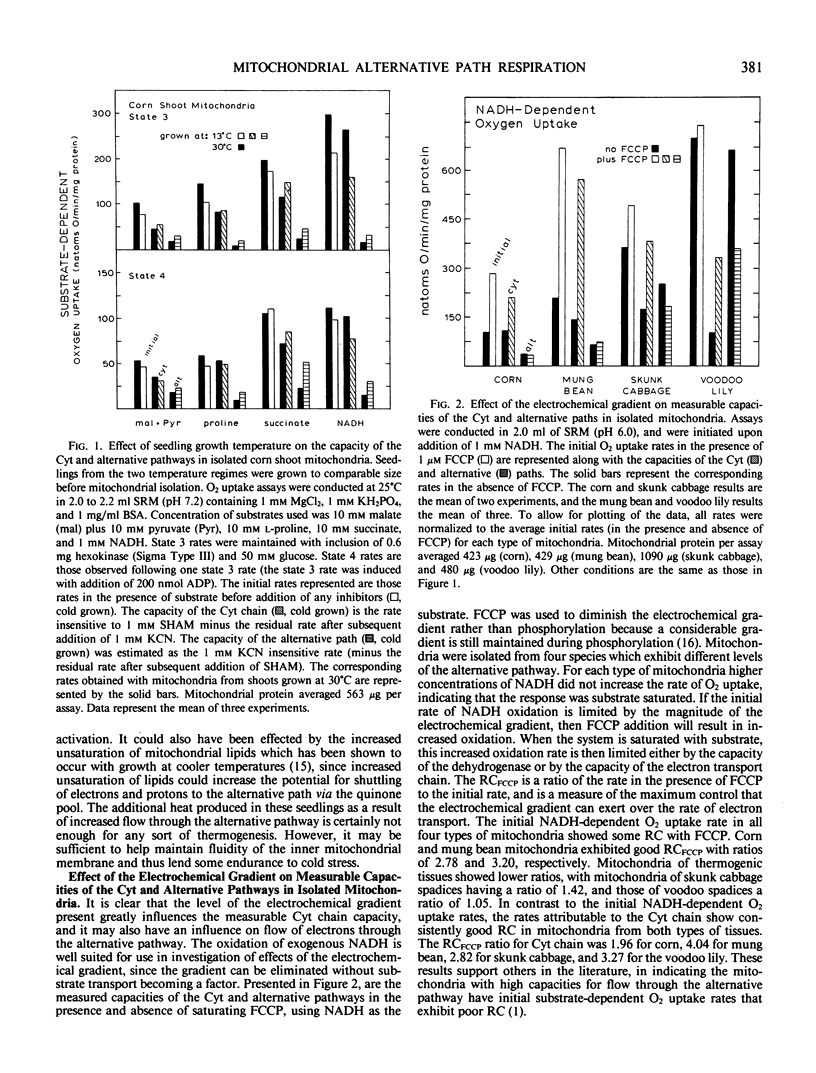
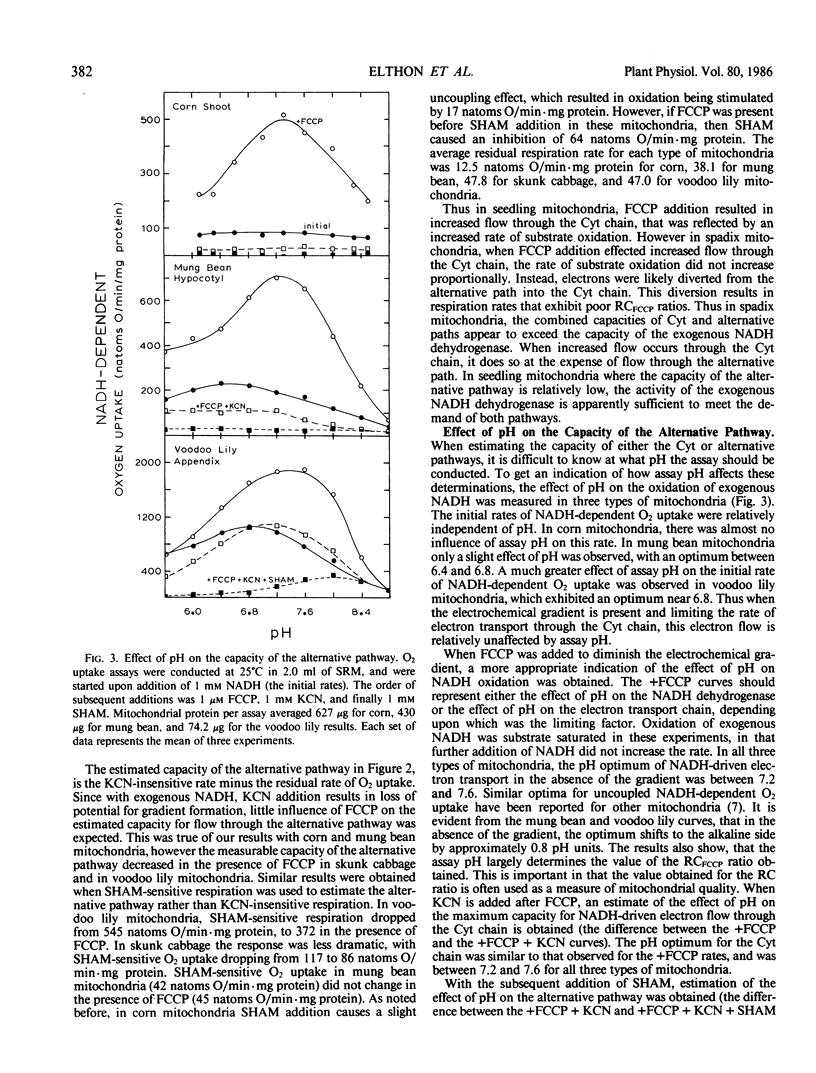
Influence of growth temperature on the capacity of the mitochondrial alternative pathway of electron transport was investigated using etiolated corn (Zea mays L.) seedlings. These seedlings were grown to comparable size in either a warm (30°C) or a cold (13°C) temperature regime, and then their respiration rates were measured as O2 uptake at 25°C. The capacity of the alternative pathway (KCN-insensitive O2 uptake) was found essentially to double in shoots of cold-grown seedlings. This increased capacity slowly developed over several days growth in the cold, but was lost within 1 day when the seedlings were exposed to a warm regime. When mitochondria were isolated from the shoots of these seedlings, a greater potential for flow through the alternative path was observed in mitochondria from the cold-grown seedlings with all substrates used (an average increase of 84%). Using exogenous NADH as the substrate, the effect of the electrochemical gradient on measurable capacities of the cytochrome and alternative pathways was investigated in mitochondria from both etiolated seedlings and thermogenic spadices. The uncoupler FCCP (p-trifluoromethoxycarbonylcyanide phenylhydrazone) was used to diminish the electrochemical gradient when desired. In corn (Zea mays L.) shoot and mung bean (Vigna radiata L.) hypocotyl mitochondria, which have relatively low capacities of the alternative pathway, increased flow through the cytochrome chain in the absence of the electrochemical gradient was found not to influence the potential for flow through the alternative path. However, in mitochondria from skunk cabbage (Symplocarpus foetidus L.) and voodoo lily (Sauromatum guttatum Schott) spadices, which have high capacities of the alternative pathway, increased flow through the cytochrome chain in the absence of the gradient occurred at the expense of flow through the alternative pathway. These results suggest that in mitochondria of thermogenic spadices, the combined capacities of the cytochrome and alternative paths exceed the capacity of the exogenous NADH dehydrogenase. The effect of assay pH on measurable capacities of the cytochrome and alternative paths was determined over a pH range of 5.6 to 8.8 using exogenous NADH as the mitochondrial substrate. When the electrochemical gradient was present, it limited the electron transport rate and little effect of assay pH was observed. However, when formation of the gradient was prevented through inclusion of FCCP, measurable capacities of the cytochrome and alternative paths were found to be greatly influenced by pH. This experiment also revealed that the potential for respiratory control is largely dependent upon the assay pH.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bahr J. T., Bonner W. D., Jr Cyanide-insensitive respiration. I. The steady states of skunk cabbage spadix and bean hypocotyl mitochondria. J Biol Chem. 1973 May 25;248(10):3441–3445. [PubMed] [Google Scholar]
- Douce R., Christensen E. L., Bonner W. D., Jr Preparation of intaintact plant mitochondria. Biochim Biophys Acta. 1972 Aug 17;275(2):148–160. doi: 10.1016/0005-2728(72)90035-7. [DOI] [PubMed] [Google Scholar]
- Knutson R. M. Heat production and temperature regulation in eastern skunk cabbage. Science. 1974 Nov 22;186(4165):746–747. doi: 10.1126/science.186.4165.746. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Miller R. W., de la Roche I., Pomeroy M. K. Structural and functional responses of wheat mitochondrial membranes to growth at low temperatures. Plant Physiol. 1974 Mar;53(3):426–433. doi: 10.1104/pp.53.3.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore A. L., Bonner W. D. Measurements of membrane potentials in plant mitochondria with the safranine method. Plant Physiol. 1982 Nov;70(5):1271–1276. doi: 10.1104/pp.70.5.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson O. P. Metabolism of Small Mammals, With Remarks on the Lower Limit of Mammalian Size. Science. 1948 Jul 9;108(2793):44–44. doi: 10.1126/science.108.2793.44. [DOI] [PubMed] [Google Scholar]


