Abstract
The effects of exposure to low temperature on photosynthesis and protein phosphorylation in chilling-sensitive and cold-tolerant plant species were compared. Chilling temperatures resulted in light-dependent loss of photosynthetic electron transport in chilling-sensitive rice (Oryza sativa L.) but not in cold-tolerant barley (Hordeum vulgare L.). Brief exposure to chilling temperatures (0-15°C, 10 min) did not cause a significant difference in photosynthetic O2 evolution capacity in vivo between rice and barley. Analysis of in vivo chlorophyll fluorescence in chilling-sensitive rice suggests that low temperatures cause an increased reduction of the plastoquinone pool that could result in photoinhibitory damage to the photosystem II reaction centers. Analysis of 32P incorporation into thylakoid proteins both in vivo and in vitro demonstrated that chilling temperature inhibited protein phosphorylation in rice, but not in barley. Low temperature (77 K) fluorescence analysis of isolated thylakoid membranes indicated that state I to state II transitions occurred in barley, but not in rice subjected to chilling temperatures. These observations suggest that protein phosphorylation may play an important role in protection against photoinhibition caused by exposure to chilling temperatures.
Full text
PDF

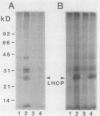
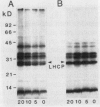
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bamberger E. S., Rottenberg H., Avron M. Internal pH, pH, and the kinetics of electron transport in chloroplasts. Eur J Biochem. 1973 May 2;34(3):557–563. doi: 10.1111/j.1432-1033.1973.tb02795.x. [DOI] [PubMed] [Google Scholar]
- Bennett J. Chloroplast phosphoproteins. Evidence for a thylakoid-bound phosphoprotein phosphatase. Eur J Biochem. 1980 Feb;104(1):85–89. doi: 10.1111/j.1432-1033.1980.tb04403.x. [DOI] [PubMed] [Google Scholar]
- Bennett J. Chloroplast phosphoproteins. The protein kinase of thylakoid membranes is light-dependent. FEBS Lett. 1979 Jul 15;103(2):342–344. doi: 10.1016/0014-5793(79)81358-7. [DOI] [PubMed] [Google Scholar]
- Bennett J., Steinback K. E., Arntzen C. J. Chloroplast phosphoproteins: regulation of excitation energy transfer by phosphorylation of thylakoid membrane polypeptides. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5253–5257. doi: 10.1073/pnas.77.9.5253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyle D. J., Ohad I., Arntzen C. J. Membrane protein damage and repair: Selective loss of a quinone-protein function in chloroplast membranes. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4070–4074. doi: 10.1073/pnas.81.13.4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mullet J. E. The amino acid sequence of the polypeptide segment which regulates membrane adhesion (grana stacking) in chloroplasts. J Biol Chem. 1983 Aug 25;258(16):9941–9948. [PubMed] [Google Scholar]
- Murata N. Control of excitation transfer in photosynthesis. II. Magnesium ion-dependent distribution of excitation energy between two pigment systems in spinach chloroplasts. Biochim Biophys Acta. 1969 Oct 21;189(2):171–181. doi: 10.1016/0005-2728(69)90045-0. [DOI] [PubMed] [Google Scholar]
- Nolan W. G. Effect of temperature on electron transport activities of isolated chloroplasts. Plant Physiol. 1980 Aug;66(2):234–237. doi: 10.1104/pp.66.2.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan W. G. Effect of temperature on proton efflux from isolated chloroplast thylakoids. Plant Physiol. 1981 Jun;67(6):1259–1263. doi: 10.1104/pp.67.6.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottenberg H., Grunwald T., Avron M. Direct determination of DeltapH in chloroplasts, and its relation to the mechanisms of photoinduced reactions. FEBS Lett. 1971 Feb 12;13(1):41–44. doi: 10.1016/0014-5793(71)80659-2. [DOI] [PubMed] [Google Scholar]
- Steinback K. E., Burke J. J., Arntzen C. J. Evidence for the role of surface-exposed segments of the light-harvesting complex in cation-mediated control of chloroplast structure and function. Arch Biochem Biophys. 1979 Jul;195(2):546–557. doi: 10.1016/0003-9861(79)90381-3. [DOI] [PubMed] [Google Scholar]