Abstract
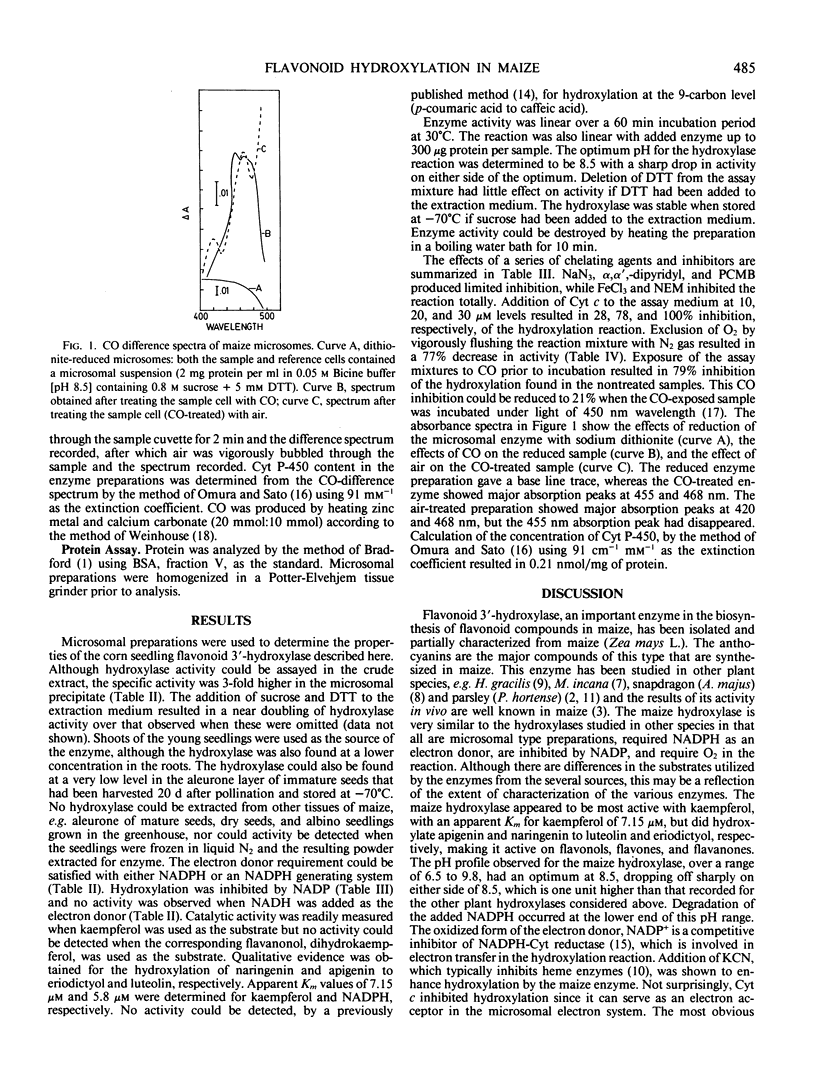
Identification of flavonoid 3′-monooxygenase establishes another reaction in the biosynthesis of flavonoid compounds in maize (Zea mays L.). The flavonoid 3′-hydroxylase was obtained as a microsomal enzyme preparation by buffer extraction of 5 day old maize seedlings and ultracentrifugation. Seedlings were exposed to light 24 hours prior to enzyme extraction. The extraction buffer required the addition of sucrose or glycerin and dithiothreitol to obtain an active hydroxylase that retained its activity on storage at −70°C. Enzymic activity required O2 and NADPH, was optimum at pH 8.5 and 30°C, and could be inhibited 79% by carbon monoxide. Carbon monoxide inhibition could be reduced to 21% by irradiation of the samples with 450 nanometer light during incubation. Kaempferol, a flavonol; naringenin, a flavanone; and apigenin, a flavone, all served as substrates for the hydroxylase. Treatment of the microsomal enzyme preparation, previously reduced with sodium dithionite, with carbon monoxide gave a 455 nanometer absorption peak which disappeared on oxidation of the preparation with the formation of a 420 nanometer peak. These results suggest a cytochrome P-450 type monooxygenase enzyme. The concentration of cytochrome P-450 was 0.21 nanomoles per milligram protein. Identification of the monooxygenase provides further biochemical information about a biosynthetic sequence for which the genetics have been studied intensely.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Diesperger H., Müller C. R., Sandermann H., Jr Rapid isolation of a plant microsomal fraction by Mg2+--precipitation. FEBS Lett. 1974 Jul 15;43(2):155–158. doi: 10.1016/0014-5793(74)80990-7. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Fujita M., Oba K., Uritani I. Properties of a Mixed Function Oxygenase Catalyzing Ipomeamarone 15-Hydroxylation in Microsomes from Cut-Injured and Ceratocystis fimbriata-Infected Sweet Potato Root Tissues. Plant Physiol. 1982 Aug;70(2):573–578. doi: 10.1104/pp.70.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagmann M. L., Heller W., Grisebach H. Induction and characterization of a microsomal flavonoid 3'-hydroxylase from parsley cell cultures. Eur J Biochem. 1983 Aug 15;134(3):547–554. doi: 10.1111/j.1432-1033.1983.tb07601.x. [DOI] [PubMed] [Google Scholar]
- Madyastha K. M., Coscia C. J. Detergent-solubilized NADPH-cytochrome c(P-450) reductase from the higher plant, Catharanthus roseus. Purification and characterization. J Biol Chem. 1979 Apr 10;254(7):2419–2427. [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. I. EVIDENCE FOR ITS HEMOPROTEIN NATURE. J Biol Chem. 1964 Jul;239:2370–2378. [PubMed] [Google Scholar]
- Potts J. R., Weklych R., Conn E. E., Rowell J. The 4-hydroxylation of cinnamic acid by sorghum microsomes and the requirement for cytochrome P-450. J Biol Chem. 1974 Aug 25;249(16):5019–5026. [PubMed] [Google Scholar]


