Abstract
Background
The obesity paradox in patients with advanced non‐small cell lung cancer receiving immune checkpoint inhibitor therapy has been observed, but its underlying mechanism is not fully understood. We aimed to investigate whether body composition affects the prognostic impact of obesity, as determined by body mass index (BMI), on survival.
Methods
This retrospective study evaluated the data collected from Asian patients who were treated with immune checkpoint inhibitors for advanced non‐small cell lung cancer between October 2015 and October 2021. We used abdominal cross‐sectional imaging to calculate the skeletal muscle and visceral fat indices (cm2/m2) by dividing the cross‐sectional areas of the skeletal muscle and visceral fat by the height squared. Cox proportional‐hazards regression was performed to determine the correlation between BMI according to the Asia‐Pacific classification, body composition metrics and overall survival.
Results
We analysed the data of 820 patients (630 men and 190 women, with a mean age of 64.3 years [standard deviation: 10.4 years]) and observed 572 (69.8%) deaths with the 1‐year mortality rate of 0.58 (95% confidence interval, 0.55–0.62). Obese BMI was associated with longer overall survival, independent of clinical covariates (hazard ratio, 0.64; 95% confidence interval: 0.52–0.80). The prognostic value of obese BMI remained after additional adjustments for skeletal muscle index (hazard ratio, 0.68; 95% confidence interval, 0.53–0.87) or visceral fat index (hazard ratio, 0.54; 95% confidence interval: 0.41–0.70). No association was observed between sex and the impact of BMI on overall survival (P‐value for interaction >0.05).
Conclusions
In Asian patients with advanced non‐small cell lung cancer who received immune checkpoint inhibitors, obese BMI was associated with favourable overall survival independent of skeletal muscle or visceral fat mass.
Keywords: Lung neoplasms, Non‐small cell lung cancer, Immune checkpoint inhibitors, Immunotherapy, Tumour biomarkers
Introduction
The obesity paradox refers to the unexpected positive correlation between obesity and improved survival in patients with cancer and other chronic diseases, as reported in various observational studies. 1 , 2 While it was primarily reported in surgical cohorts of lung cancer, 3 recent evidence indicates that a high body mass index (BMI) is independently associated with improved survival in non‐small cell lung cancer (NSCLC) patients undergoing immune checkpoint inhibitor (ICI) therapy. 4 Furthermore, this effect was only observed in patients treated with ICI therapy, as opposed to the docetaxel group, implying that the relationship with the obesity paradox may be solid in ICI therapy. Because ICI therapy targets T cells to activate the tumouricidal potential of the adaptive immune system, rather than directly attacking cancer cells, 5 it is reasonable to suggest that host factors may impact immune cell function and cancer response, as evidenced by recent studies. 6 One such host factor is obesity, which has been speculated to alter immune cell activity, 7 thereby making the relationship between the obesity paradox and ICI therapy more plausible. With the increasing administration of ICI in NSCLC, unravelling the underlying biology of this phenomenon and exploring its prognostic value should be prioritized to improve risk stratification and enhance prognosis.
The mechanism underlying the obesity paradox remains poorly understood; however, several hypotheses have been proposed. One such hypothesis is the imprecise measurement of body composition using BMI, which fails to distinguish between skeletal muscles and adipose tissue. 1 , 2 Specifically, some authors have proposed that the mechanism underlying the obesity paradox relies on the fact that patients with an obese BMI have higher levels of protective muscles, 8 , 9 which aligns with the widely recognized poor prognostic factor of sarcopenia. 10 , 11 Additionally, accumulating evidence indicates that adipose tissue may be a potential biomarker, as it has been positively associated with improved survival in patients with cancer. 12 , 13 However, it was unclear whether the obesity paradox was primarily influenced by specific components of body composition.
Cross‐sectional imaging, including computed tomography (CT), provides accurate quantification and distinction of skeletal muscle and adipose tissue, which may be crucial in understanding the obesity paradox. In this study, we aimed to investigate whether body composition affected the association between obesity, as determined by BMI, and survival in patients with advanced NSCLC who underwent ICI therapy.
Methods
This retrospective study was approved by the Institutional Review Board of Samsung Medical Center (IRB file no. 2022‐01‐155), and the requirement for informed consent was waived. This study was performed in accordance with the principles of the Declaration of Helsinki.
Patients
Data for this study was extracted from the Clinical Data Warehouse Darwin‐C of the Samsung Medical Center for 2,247 consecutive patients who received palliative programmed death receptor 1 (PD‐1)/programmed death ligand 1 (PD‐L1) blockade therapy for advanced NSCLC between October 2015 and October 2021. Among them, 991 patients with baseline abdominal cross‐sectional imaging within 90 days prior to ICI treatment initiation were included. Patients who had received more than four lines of previous treatments (n = 100) or had insufficient baseline clinical data (e.g., no body weight) (n = 44) were excluded.
Image analysis
Baseline abdominal CT images were analysed using an artificial intelligence‐driven fully automated segmentation technique (AID‐U™, iAID Inc., Seoul, Republic of Korea) developed based on a fully convolutional network segmentation method. 14 From the single‐slice image at the level of the third lumbar vertebra, which was automatically selected, specific tissue demarcation was performed based on Hounsfield units (HU) as follows: The skeletal muscle area (cm2), including all muscles on the selected axial images (i.e., psoas, paraspinal, transversus abdominis, rectus abdominis, quadratus lumborum and internal and external obliques), was demarcated using predetermined thresholds ([−29]‐[+150] HU). The subcutaneous fat area (cm2) and visceral fat area (cm2) were demarcated using fat tissue thresholds of [−190] to [−30] HU. 15 A board‐certified radiologist with 7 years of experience in musculoskeletal imaging confirmed the appropriateness of the level selection and segmentation while being blinded to patient information. The cross‐sectional areas of each body composition were normalized by dividing by the square of the height in meters to generate the skeletal muscle index (SMI, cm2/m2), visceral fat index (VFI, cm2/m2) and subcutaneous fat index (SFI, cm2/m2).
Clinical variables and study outcomes
We extracted baseline demographic and clinical data from the Clinical Data Warehouse Darwin‐C and electronic medical records. It included age, sex, treatment agent, line of treatment, Eastern Cooperative Oncology Group performance status (ECOG PS), smoking status, histology, PD‐L1 expression status, gene mutation status, Charlson co‐morbidity index (CCI), 16 blood neutrophil‐to‐lymphocyte ratio (NLR), height and body weight. BMI was calculated as the weight divided by height squared (kg/m2) and classified as underweight (<18.5 kg/m2), normal (18.5–22.9 kg/m2), overweight (23.0–24.9 kg/m2), or obese (≥25 kg/m2) according to the Asia‐Pacific classification. 17 Tumours with a tumour proportion score of ≥50% were classified as PD‐L1‐positive using the Dako PD‐L1 IHC 22C3 pharmDx kit (Agilent Technologies, Santa Clara, CA, USA). 18 The primary endpoint was overall survival (OS), calculated from the treatment initiation to death from any cause. Vital status and date of death until 25 January 2022, were obtained from death certification collected from the Ministry of the Interior and Safety. The secondary endpoint was progression‐free survival (PFS), determined from the treatment initiation to disease progression or death from any cause, whichever occurs first, according to the RECIST 1.1 criteria. 19 Patients without events were censored at the last follow‐up visit.
Statistical analysis
Descriptive statistics were used to summarize patient characteristics according to BMI categories. Continuous and categorical variables were analysed using ANOVA and chi‐square tests, respectively, to compare the baseline characteristics of each BMI group.
The Kaplan–Meier method with a log‐rank test was used to analyse event‐time distributions and evaluate OS according to BMI groups. Multivariable Cox regression was used to determine the association between BMI and OS/PFS, with multivariable‐adjusted hazard ratios (HR) and 95% confidence intervals (CI) calculated for the underweight, overweight and obese BMI groups compared to the normal weight group. BMI was also treated continuously per 1 standard deviation (SD). We calculated Harrell's C‐statistic, which allows for censored data. 20 The Cox regression model evaluated whether the association between BMI groups and survival differed between men and women using an interaction term. BMI was modelled as a continuous variable using restricted cubic splines with knots at the 5th, 35th, 65th and 95th percentiles of the sample distribution to provide a flexible estimate of the dose–response relationship between BMI and OS. Three models were used: the first was adjusted for age, sex, smoking status, ECOG PS, CCI, NLR, histology, PD‐L1 expression status, treatment agents, and line of treatment (Model 1); to assess the additional impact of body composition on OS, we employed Models 2SMI and 2VFI, which incorporated adjustments for SMI and VFI, respectively.
The association between body composition and OS/PFS was assessed using multivariable Cox regression analysis without (Model 1) and after adjusting for BMI (Model 2BMI). The dose–response relationship between body composition and OS was estimated using restricted cubic splines with and without additional adjustment for BMI in the same manner.
Pearson's correlation analysis was used to examine the relationship between BMI and body composition metrics. Multicollinearity was assessed using the variance inflation factor, with values greater than four indicating multicollinearity. 21 P < 0.05 was considered statistically significant. All analyses were performed using STATA version 16 (StataCorp LP, College Station, TX, USA) and R 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
After excluding patients with inappropriately segmented body composition (n = 20) and those with metallic instrumentation in the lumbar spine (n = 7), 820 patients (417 treated with pembrolizumab, 271 treated with atezolizumab and 132 treated with nivolumab) were included in the analyses (Figure 1). The mean (standard deviation) age of participants was 64.3 (10.4) years, and 76.8% of the patients were men. The mean (standard deviation) BMI values of men and women were 23.0 (3.3) kg/m2 and 22.6 (3.4) kg/m2, respectively. Among men, the proportions of patients who had underweight, normal, overweight and obese BMI were 8.1%, 40.6%, 25.6% and 25.7%, respectively, while among women, the corresponding proportions were 10.5%, 45.8%, 20.5% and 23.2%, respectively. Overall, 18 patients (2.2%) had a BMI of ≥30 kg/m2. The median interval between abdominal CT and treatment initiation was 14 days (interquartile range, 5–44 days). Of 641 patients for whom PD‐L1 expression status was available, 299 patients (46.6%) had PD‐L1‐positive tumours. EGFR and ALK gene mutation test results were available for 707 and 693 patients, respectively, of whom 79 (11.2%) and 29 (4.2%) had genetic mutations, respectively. Although the obese BMI group was the least likely to score ≥2 on ECOG PS and highest NLR (P‐values < 0.001), other clinical factors were similar among the groups (Table 1).
Figure 1.
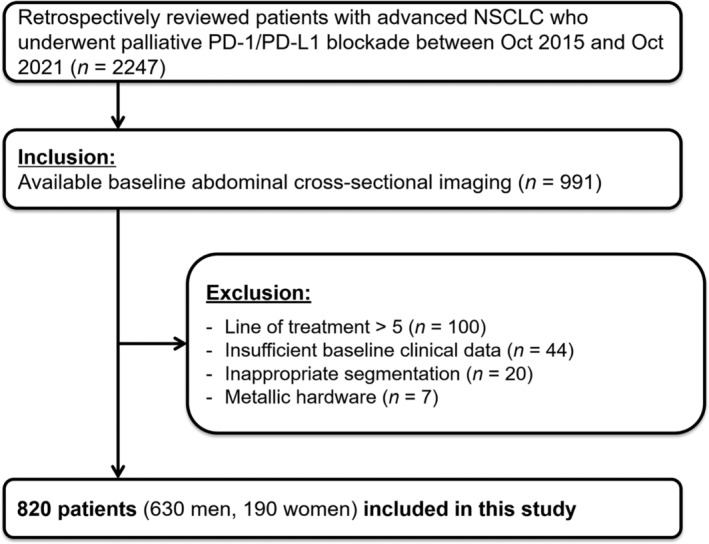
Flow diagram showing selection of the study population. PD‐1, programmed death receptor 1; PD‐L1, programmed death ligand 1; NSCLC, non‐small cell lung cancer.
Table 1.
Characteristics of the study population distinguished by body mass index
| Characteristic | Total (N = 820) | Body mass index categories | ||||
|---|---|---|---|---|---|---|
| Underweight (N = 71) | Normal (N = 343) | Overweight (N = 200) | Obese (N = 206) | P | ||
| Age, years | 64.3 (10.4) | 63.4 (11.5) | 64.3 (10.8) | 65.2 (10.2) | 63.6 (9.7) | 0.404 |
| Sex | 0.282 | |||||
| Men | 630 (76.8) | 51 (71.8) | 256 (74.6) | 161 (80.5) | 162 (78.6) | |
| Women | 190 (23.2) | 20 (28.2) | 87 (25.4) | 39 (19.5) | 44 (21.4) | |
| Smoking status | 0.289 | |||||
| Never | 213 (26.0) | 20 (28.2) | 100 (29.2) | 43 (21.5) | 50 (24.3) | |
| Ever | 606 (73.9) | 51 (71.8) | 243 (70.8) | 156 (78.0) | 156 (75.7) | |
| Unknown | 1 (0.1) | 0 (0.0) | 0 (0.0) | 1 (0.5) | 0 (0.0) | |
| ECOG PS, ≥2 | 93 (11.3) | 17 (23.9) | 49 (14.3) | 14 (7.0) | 13 (6.3) | <0.001 |
| Charlson comorbidity index, ≥2 | 95 (11.6) | 8 (11.3) | 39 (11.4) | 22 (11.0) | 26 (12.6) | 0.959 |
| Neutrophil‐to‐lymphocyte ratio | 5.39 (6.65) | 7.82 (8.79) | 6.05 (7.48) | 4.71 (5.16) | 4.13 (5.10) | <0.001 |
| Histology, squamous cell carcinoma | 218 (26.6) | 19 (26.8) | 84 (24.5) | 53 (26.5) | 62 (30.1) | 0.557 |
| PD‐L1 expression status | 0.956 | |||||
| Tumour proportion score < 50% | 342 (41.7) | 30 (42.3) | 146 (42.6) | 79 (39.5) | 87 (42.2) | |
| Tumour proportion score ≥ 50% | 299 (36.5) | 27 (38.0) | 127 (37.0) | 74 (37.0) | 71 (34.5) | |
| Unknown | 179 (21.8) | 14 (19.7) | 70 (20.4) | 47 (23.5) | 48 (23.3) | |
| Treatment agents | 0.126 | |||||
| Atezolizumab | 271 (33.0) | 20 (28.2) | 112 (32.7) | 61 (30.5) | 78 (37.9) | |
| Pembrolizumab | 417 (50.9) | 40 (56.3) | 165 (48.1) | 115 (57.5) | 97 (47.1) | |
| Nivolumab | 132 (16.1) | 11 (15.5) | 66 (19.2) | 24 (12.0) | 31 (15.0) | |
| Line of treatment | 0.152 | |||||
| 1 | 153 (18.7) | 15 (21.1) | 68 (19.8) | 45 (22.5) | 25 (12.1) | |
| 2 | 359 (43.8) | 28 (39.4) | 156 (45.5) | 80 (40.0) | 95 (46.1) | |
| 3 | 197 (24.0) | 16 (22.5) | 72 (21.0) | 54 (27.0) | 55 (26.7) | |
| ≥4 | 111 (13.5) | 12 (16.9) | 47 (13.7) | 21 (10.5) | 31 (15.0) | |
Values are presented as n (%) or mean (standard deviation).
ECOG PS, European Cooperative Oncology Group Performance Status; PD‐L1, programmed death ligand 1.
During 740 person‐years of follow‐up (median follow‐up, 6.9 months), we observed 572 (69.8%) deaths. The median OS was 6.9 months (interquartile range, 2.4–14.8 months) with the 1‐year mortality rate of 0.58 (95% CI 0.55, 0.62). OS differed significantly between the BMI categories (log‐rank P < 0.01), with better OS in the obese BMI group (Model 1; HR, 0.64; 95% CI 0.52, 0.80; P < 0.001) than in patients with normal BMI, independent of clinical covariates (Figure 2 and Table 2). The risk of death significantly decreased as BMI increased, demonstrating a 20% lower risk of death as BMI increased by 1 SD (P < 0.001). The associations between obese BMI and OS, as well as between BMI and OS, remained significant after additional adjustments for SMI (Model 2SMI) or VFI (Model 2VFI) (P‐values < 0.05). The C index of Model 1, Model 2SMI, and Model 2VFI were 0.67 (95% CI 0.64, 0.69), 0.67 (95% CI 0.64, 0.69) and 0.67 (95% CI 0.65, 0.70), respectively. The association between BMI and OS was consistent between men and women (P‐value for interaction > 0.05). The restricted cubic splines demonstrated that the risk of death decreased in a linear manner as BMI increases in both men and women, regardless of additional adjustment for SMI or VFI (Figure 3).
Figure 2.
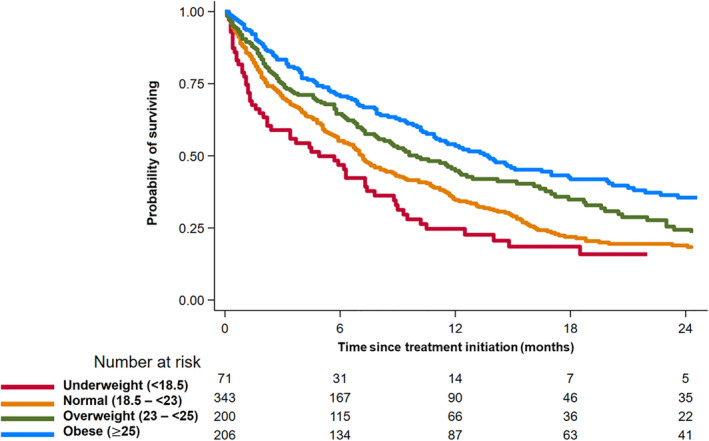
Kaplan–Meier curve for overall survival by body mass index.
Table 2.
Hazard ratios for clinical outcomes by body mass index
| Model 1 | P‐value | Model 2SMI | P‐value | Model 2VFI | P‐value | |
|---|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | ||||
| Overall survival | ||||||
| BMI* | 0.80 (0.74, 0.88) | <0.001 | 0.81 (0.73, 0.91) | <0.001 | 0.73 (0.64, 0.83) | <0.001 |
| BMI categories | ||||||
| Underweight (<18.5 kg/m2) | 1.31 (0.98, 1.75) | 0.065 | 1.26 (0.94, 1.70) | 0.125 | 1.42 (1.05, 1.91) | 0.038 |
| Normal (18.5 to <23 kg/m2) | Reference | Reference | Reference | |||
| Overweight (23 to <25 kg/m2) | 0.89 (0.71, 1.11) | 0.304 | 0.92 (0.73, 1.16) | 0.497 | 0.77 (0.61, 0.97) | 0.185 |
| Obese (≥25 kg/m2) | 0.64 (0.52, 0.80) | <0.001 | 0.68 (0.53, 0.87) | 0.002 | 0.54 (0.41, 0.70) | <0.001 |
| Progression‐free survival | ||||||
| BMI* | 0.88 (0.81, 0.95) | 0.001 | 0.91 (0.82, 0.99) | 0.048 | 0.78 (0.70, 0.88) | <0.001 |
| BMI categories | ||||||
| Underweight (<18.5 kg/m2) | 1.04 (0.78, 1.37) | 0.794 | 0.99 (0.74, 1.32) | 0.949 | 1.11 (0.83, 1.49) | 0.482 |
| Normal (18.5 to <23 kg/m2) | Reference | Reference | Reference | |||
| Overweight (23 to <25 kg/m2) | 0.90 (0.74, 1.10) | 0.307 | 0.94 (0.76, 1.16) | 0.562 | 0.84 (0.69, 1.05) | 0.128 |
| Obese (≥25 kg/m2) | 0.75 (0.62, 0.92) | 0.005 | 0.81 (0.65, 1.01) | 0.064 | 0.66 (0.52, 0.85) | 0.001 |
Per 1 standard deviation increasing.
Model 1: Adjusted for age, smoking status, ECOG PS, Charlson co‐morbidity index, neutrophil–to–lymphocyte ratio, histology, PD‐L1 expression status, treatment agents, and line of treatment. Model 2SMI: Model 1 + SMI; Model 2VFI: Model 1 + VFI.
BMI, body mass index; CI, confidence interval; SD, standard deviation; SMI, skeletal muscle index; VFI, visceral fat index.
Figure 3.
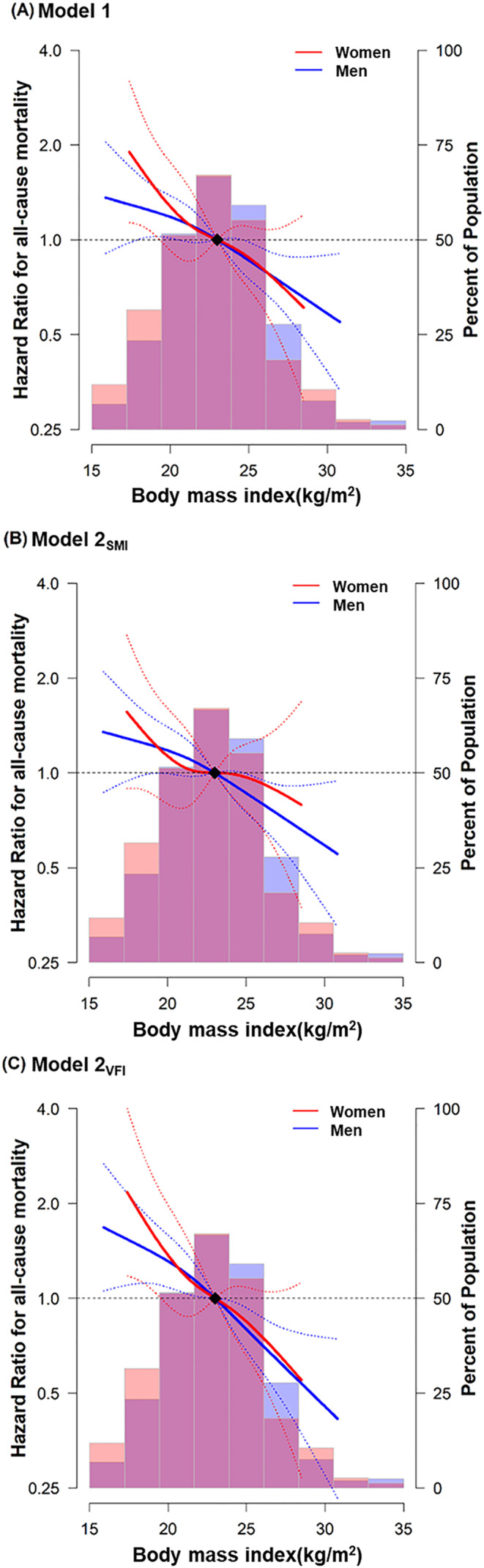
Multivariable‐adjusted hazard ratios for all‐cause death by body mass index in men and women. The curves represent the adjusted hazard ratios (solid lines) and their 95% confidence intervals (dashed lines) for all‐cause death based on restricted cubic splines for body mass index with knots at the 5th, 35th, 65th, and 95th percentiles of their sample distributions. The distribution of body mass index was visually represented using blue histogram for men and red histogram for women. The reference value (diamond dot) was set at the 50th percentile (body mass index of 23 kg/m2). (A) Model 1: Adjusted for age, sex, smoking status, ECOG PS, CCI, NLR, histology, PD‐L1 expression status, treatment agents, and line of treatment. (B) Model 2SMI: Model 1 + SMI. (C) Model 2VFI: Model 1 + VFI. CCI, Charlson co‐morbidity index; ECOG PS, Eastern Cooperative Oncology Group performance status; NLR, neutrophil‐to‐lymphocyte ratio; SFI, subcutaneous fat index; SMI, skeletal muscle index; VFI, visceral fat index.
The number of events for PFS was 669 (81.6%), including 355 patients (43.3%) with disease progression. The median PFS was 2.7 months (interquartile range, 1.4–8.6 months). Patients with obese BMI had a 25% lower risk of progression compared to patients with normal BMI (Model 1; HR, 0.75; 95% CI 0.62, 0.92; P = 0.005). The risk of progression significantly decreased as BMI increased, with a 12% lower risk of progression as BMI increased by 1 SD (P = 0.001). The association observed with BMI as a continuous variable remained significant after additional adjustments for SMI (Model 2SMI) and VFI (Model 2VFI) (P‐values < 0.05) (Table 2). The association between BMI and PFS did not significantly differ between men and women (P‐values for interaction > 0.05).
Before adjusting for BMI, all three body composition metrics were significantly associated with OS and PFS, indicating that higher values of these variables were associated with lower risk of death or progression (P‐values < 0.05), with the exception of the association between SFI and PFS, and that between VFI and PFS (P‐values > 0.05) (Model 1). However, upon further adjustment for BMI, the associations between the increases in these body composition metrics and favourable OS and PFS became statistically insignificant (P‐values > 0.05). In contrast, following this additional adjustment for BMI, we observed a tendency for the risk of death and progression to increase in higher VFI, and this association between higher VFI and poorer PFS reached statistical significance (P = 0.008) (Model 2BMI) (Table 3). The restricted cubic splines also showed that, before adjusting for BMI, there was a tendency for the risk of death to decrease as SMI, SFI and VFI increased in both men and women. These trends underwent significant changes after additional adjustment for BMI, transitioning to either a flat or opposing pattern. Specifically, an increase in VFI was associated with higher risk of death in both men and women following BMI adjustment (Figure 4).
Table 3.
Body mass index‐adjusted hazard ratios for clinical outcomes depending on body composition
| Model 1 | P‐value | Model 2BMI | P‐value | |
|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | |||
| Overall survival | ||||
| SMI* | 0.84 (0.77, 0.93) | 0.001 | 0.97 (0.86, 1.10) | 0.624 |
| SFI* | 0.84 (0.76, 0.93) | 0.001 | 1.09 (0.92, 1.28) | 0.318 |
| VFI* | 0.89 (0.82, 0.97) | 0.015 | 1.14 (1.00, 1.30) | 0.054 |
| Progression‐free survival | ||||
| SMI* | 0.89 (0.81, 0.97) | 0.008 | 0.95 (0.85, 1.07) | 0.392 |
| SFI* | 0.92 (0.84, 1.00) | 0.058 | 1.08 (0.94, 1.25) | 0.270 |
| VFI* | 0.97 (0.90, 1.06) | 0.505 | 1.18 (1.04, 1.33) | 0.008 |
Per 1 standard deviation increasing.
Model 1: adjusted for age, smoking status, ECOG PS, Charlson co‐morbidity index, neutrophil‐to‐lymphocyte ratio, histology, PD‐L1 expression status, treatment agents, and line of treatment. Model 2BMI: Model 1 + BMI.
BMI, body mass index; ECOG PS, European Cooperative Oncology Group Performance Status; SFI, subcutaneous fat index; SMI, skeletal muscle index; VFI, visceral fat index.
Figure 4.
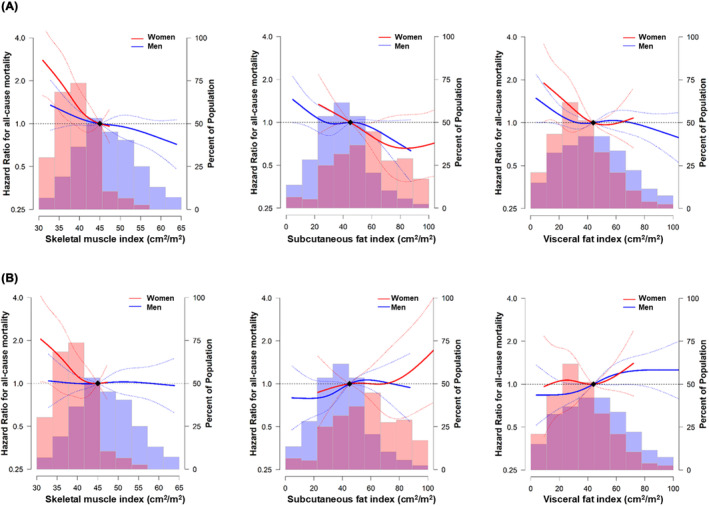
Multivariable‐adjusted hazard ratios for all‐cause death by skeletal muscle index, subcutaneous fat index, and visceral fat index in men and women. The curves represent the adjusted hazard ratios (solid line) and 95% confidence intervals (dashed lines) for all‐cause death based on restricted cubic splines for skeletal muscle (left row), subcutaneous fat (middle row), and visceral fat indices (right row) with knots at the 5th, 35th, 65th, and 95th percentiles of their sample distributions. The distribution of each body composition was visually represented using blue histogram for men and red histogram for women. The reference value (diamond dots) is set to the 50th percentile. (A) Model 1: Adjusted for age, sex, smoking status, ECOG PS, CCI, NLR, histology, PD‐L1 expression status, treatment agents, and line of treatment. (B) Model 2BMI: Additionally adjusted for BMI. BMI, body mass index; CCI, Charlson co‐morbidity index; ECOG PS, Eastern Cooperative Oncology Group Performance Status; NLR, blood neutrophil‐to‐lymphocyte ratio; SFI, subcutaneous fat index; SMI, skeletal muscle index; VFI, visceral fat index.
Although SMI (r = 0.56, P < 0.01), VFI (r = 0.73, P < 0.01) and SFI (r = 0.70, P < 0.01) were significantly and positively correlated with BMI, there was no evidence of multicollinearity (variance inflation factor values < 4).
Discussion
Our study found that obese BMI was a significant prognostic factor for improved OS in patients who received ICI therapy for advanced NSCLC, independent of body composition as well as clinical covariates. Given that the relationship between obese BMI and decreased risk of death persisted even after additional adjustments for body composition characteristics, we also discovered that the obesity paradox was not solely driven by the quantity of skeletal muscle or adipose tissue. The protective effect of excessive skeletal muscle mass did not fully explain the survival advantage of obese BMI. Thus, our findings suggest that factors other than body composition may be responsible for the obesity paradox, requiring additional exploration.
Although patients having an obese BMI may have a higher skeletal muscle mass, our study challenges the previous notion that greater quantities of muscle may confer protective effects on survival. This differs from the previous suggestion that skeletal muscle mass may be the missing link between obese BMI and improved survival. A previous study, reporting that the association between reduced mortality and obese BMI only existed when skeletal muscle mass was normal, was one of the studies to propose this theory. 22 Similarly, a BMI range of 25–30 kg/m2 was reported to be associated with the lowest mortality, likely because patients in this range have higher levels of protective muscles without morbid adiposity. 8 , 9 However, in a retrospective analysis of patients treated with surgical resection for NSCLC, the survival benefit of obese BMI was independent of skeletal muscle mass measured on baseline abdominal CT. 23 Our results also augment that of this previous study by finding independent prognostic significance of obese BMI on favourable OS, and contrariwise that the relationship between having greater than normal amounts of skeletal muscle and improved OS depended on BMI. This underscores the need for further investigation into the underlying mechanisms of the obesity paradox beyond skeletal muscle mass.
An alternative explanation for the obesity paradox, other than body composition, involves a different gene expression pattern related to fatty acid metabolism genes. FASN (fatty acid synthase) is a metabolic oncogene that regulates the de novo biosynthesis of fatty acids essential for tumour growth. It is overexpressed in several cancers and has been associated with unfavourable survival outcomes. 24 , 25 Notably, the downregulation of FASN pathway in patients with renal cell carcinoma whose BMI is ≥25 kg/m2 indicates that this potential metabolic oncogene may contribute to the paradoxical survival benefit observed in this patient population. 26 Different transcriptomic profiles between individuals with normal and obese BMI also has been suggested. In another study on patients with renal cell carcinoma, patients having BMI ≥ 30 kg/m2 harboured tumours with greater angiogenesis, hypoxia and epithelial‐mesenchymal transition. 27 The upregulation of angiogenesis in this population could potentially account for these tumours' heightened vulnerability to tyrosine kinase inhibitor therapy. In contrast, a study on patients with NSCLC who underwent lobectomy found that visceral adiposity is associated with reduced recurrence‐free and overall survival and argued that alterations in inflammatory transcriptomic signature in the tumour microenvironment could link the adverse survival outcomes with visceral adiposity. 28 Given the contrasting results presented by these studies, further research is warranted to comprehensively investigate the complex mechanisms underlying the obesity paradox in different cancer types from the perspective of gene expression and transcriptomic signatures.
The obesity paradox has been partly explained by obesity‐induced inflammation and immune alterations, identified as underlying biological factors. One theory suggests that upregulation of the PD‐1 receptor and increased secretion of leptin from adipose tissues plays a key role. 29 As blood levels of leptin increase in proportion to total body fat mass, 30 the role of adipose tissue in the obesity paradox seems reasonable. In line with this, a recent study comprising patients with various cancer types treated with immunotherapy found that a higher ratio of visceral fat to subcutaneous fat was associated with favourable OS. 31 Another study involving patients with advanced melanoma who received ICI therapy also reported a positive association between visceral adiposity and improved OS, which was interestingly influenced by systemic inflammation. 13 These findings supported the previous theory that the heightened efficacy of ICI therapy in individuals with obese BMI and more visceral adipose tissue might be related to their chronic inflammatory state and dysregulated immune response. 29 , 32 However, our findings are in contrast to those of these studies, showing that the apparent protective effect of obese BMI on survival was not dependent on visceral adiposity nor systemic inflammation represented by NLR. These discrepancies may suggest that the effect of obese BMI and visceral adiposity on prognosis and its mechanisms differ depending on the type of malignancy.
While our study further supports the obesity paradox, several methodological issues should be addressed, including unmeasured factors, residual confounding and reverse causation. Most importantly, the favourable baseline characteristics of the obese BMI group and the opposite in the underweight group imply that these issues cannot be fully ruled out despite adjustment. Of note, the tendency observed in NLR may raise questions about the potential role of the systemic inflammatory response in the association with the obesity paradox, as recent evidence links it to cancer cachexia and, conversely, the obesity paradox. 13 , 33 Considering that our analysis still yielded statistically significant results despite adjusting for NLR, it appears unlikely that systemic inflammation had a substantial impact on the survival outcomes in our study for individuals with an obese BMI. Nevertheless, the connection between systemic inflammation and the obesity paradox warrants additional inspection in this context. Weight loss prior to ICI treatment which was not available in this study, should be considered as another potentially influential confounding factor, given its prognostic significance in patients with NSCLC. 34 Further investigation is necessary to explore this relationship in more detail and specificity.
We recognize the importance of considering the BMI distribution of our Asian study subjects, who had a lower BMI than patients in previous studies conducted in Western countries, 4 , 28 , 35 as well as a small number of patients having a BMI in the morbid obesity range. Given that most patients with obese BMI in our study had a BMI in the 25–30 kg/m2 range and that mortality curves for BMI follow a U‐shaped pattern with increasing mortality at both extremes, 36 this group of patients may belong to the so‐called survival sweet spot for BMI. 8 , 37 , 38 In this context, the prognostic value of BMI, independent of body composition, may differ in patients with even higher BMI, as does the positive relationship between increased BMI and improved survival. Therefore, future research is needed to determine if the association between BMI, body composition, and patient prognosis is similar in patients of other races and those with morbidly obese BMI.
The strength of our study lies in the fact that we used CT‐derived body composition measurements to explore whether body composition provides a link between obese BMI and improved survival. However, several limitations should be acknowledged. First, it was conducted retrospectively at a single tertiary center. Second, the fact that we included only patients who underwent abdominal CT may have led to selection bias, considering controversies regarding the added benefit of this imaging modality in patients with lung cancer. 39 Third, the study included only Asians with a lower prevalence of morbidly obese BMI compared to Western populations, as described above, which may have led to further selection bias. Fourth, data regarding treatment duration and toxicities, including immune‐related adverse events, which are important clinical factors and outcomes, were unavailable. Fifth, the possibility of unmeasured or residual confounding and reverse causation cannot be excluded, even after adjusting for clinically relevant covariates, which has been discussed earlier.
In conclusion, obese BMI was associated with improved survival in Asian patients who received ICI therapy for advanced NSCLC, independent of body composition status. Excessive skeletal muscle mass did not provide additional protective effect on survival, and neither subcutaneous nor visceral fat could explain the improved survival of patients with obese BMI. Our results showed that BMI should be considered a prognostic marker that can be easily assessed, despite its limitations. Further studies are needed to investigate its true prognostic value and underlying biology to account for the obesity paradox.
Conflict of interest
The authors declare no potential conflicts of interest.
Acknowledgements
This work was supported by research funding of Samsung Medical Center (No. SMO1220571), Institute of Information & Communications Technology Planning & Evaluation (IITP) grant funded by the Korea government (MSIT) (No. 2021‐0‐02068, Artificial Intelligence Innovation Hub) and the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. NRF‐2022R1A2C1003999). All the authors certify that they comply with the ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2019. 40
Lee JH, Kang D, Ahn JS, Guallar E, Cho J, Lee HY. 2023; Obesity paradox in patients with non‐small cell lung cancer undergoing immune checkpoint inhibitor therapy. Journal of Cachexia, Sarcopenia and Muscle, 14, 2898–2907, 10.1002/jcsm.13367
Ji Hyun Lee and Danbee Kang contributed equally to this work.
Contributor Information
Juhee Cho, Email: alfadur2j@gmail.com.
Ho Yun Lee, Email: hoyunlee96@gmail.com.
References
- 1. Park Y, Peterson LL, Colditz GA. The plausibility of obesity paradox in cancer‐point. Cancer Res 2018;78:1898–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lee DH, Giovannucci EL. The obesity paradox in cancer: epidemiologic insights and perspectives. Curr Nutr Rep 2019;8:175–181. [DOI] [PubMed] [Google Scholar]
- 3. Li S, Wang Z, Huang J, Fan J, Du H, Liu L, et al. Systematic review of prognostic roles of body mass index for patients undergoing lung cancer surgery: does the ‘obesity paradox’ really exist? Eur J Cardiothorac Surg 2017;51:817–828. [DOI] [PubMed] [Google Scholar]
- 4. Kichenadasse G, Miners JO, Mangoni AA, Rowland A, Hopkins AM, Sorich MJ. Association between body mass index and overall survival with immune checkpoint inhibitor therapy for advanced non‐small cell lung cancer. JAMA Oncol 2020;6:512–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liu X, Hogg GD, DeNardo DG. Rethinking immune checkpoint blockade: ‘beyond the T cell’. J Immunother Cancer 2021;9:e001460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gunjur A, Manrique‐Rincon AJ, Klein O, Behren A, Lawley TD, Welsh SJ, et al. ‘Know thyself’ ‐ host factors influencing cancer response to immune checkpoint inhibitors. J Pathol 2022;257:513–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lutz CT, Quinn LS. Sarcopenia, obesity, and natural killer cell immune senescence in aging: altered cytokine levels as a common mechanism. Aging (Albany NY) 2012;4:535–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cespedes Feliciano EM, Kroenke CH, Caan BJ. The obesity paradox in cancer: how important is muscle? Annu Rev Nutr 2018;38:357–379. [DOI] [PubMed] [Google Scholar]
- 9. Caan BJ, Meyerhardt JA, Kroenke CH, Alexeeff S, Xiao J, Weltzien E, et al. Explaining the obesity paradox: the association between body composition and colorectal cancer survival (C‐SCANS study). Cancer Epidemiol Biomarkers Prev 2017;26:1008–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Martin L, Birdsell L, Macdonald N, Reiman T, Clandinin MT, McCargar LJ, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol 2013;31:1539–1547. [DOI] [PubMed] [Google Scholar]
- 11. Kang SH, Jeong WK, Baik SK, Cha SH, Kim MY. Impact of sarcopenia on prognostic value of cirrhosis: going beyond the hepatic venous pressure gradient and MELD score. J Cachexia Sarcopenia Muscle 2018;9:860–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Choi H, Park YS, Na KJ, Park S, Park IK, Kang CH, et al. Association of adipopenia at preoperative PET/CT with mortality in stage i non‐small cell lung cancer. Radiology 2021;30:210576. [DOI] [PubMed] [Google Scholar]
- 13. Lee JH, Hyung S, Lee J, Choi SH. Visceral adiposity and systemic inflammation in the obesity paradox in patients with unresectable or metastatic melanoma undergoing immune checkpoint inhibitor therapy: a retrospective cohort study. J Immunother Cancer 2022;10:e005226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ha J, Park T, Kim HK, Shin Y, Ko Y, Kim DW, et al. Development of a fully automatic deep learning system for L3 selection and body composition assessment on computed tomography. Sci Rep 2021;11:21656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Park HJ, Shin Y, Park J, Kim H, Lee IS, Seo DW, et al. Development and validation of a deep learning system for segmentation of abdominal muscle and fat on computed tomography. Korean J Radiol 2020;21:88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 17. World Health Organization . Regional Office for the Western Pacific. The Asia‐Pacific perspective: redefining obesity and its treatment. Sydney: Health Communications Australia; 2000. https://apps.who.int/iris/handle/10665/206936 [Google Scholar]
- 18. Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al. Pembrolizumab for the treatment of non‐small‐cell lung cancer. N Engl J Med 2015;372:2018–2028. [DOI] [PubMed] [Google Scholar]
- 19. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–247. [DOI] [PubMed] [Google Scholar]
- 20. Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 1996;15:361–387. [DOI] [PubMed] [Google Scholar]
- 21. O'brien RM. A caution regarding rules of thumb for variance inflation factors. Qual Quant 2007;41:673–690. [Google Scholar]
- 22. Gonzalez MC, Pastore CA, Orlandi SP, Heymsfield SB. Obesity paradox in cancer: new insights provided by body composition. Am J Clin Nutr 2014;99:999–1005. [DOI] [PubMed] [Google Scholar]
- 23. Lee JH, Yoon YC, Kim HS, Cha MJ, Kim JH, Kim K, et al. Obesity is associated with improved postoperative overall survival, independent of skeletal muscle mass in lung adenocarcinoma. J Cachexia Sarcopenia Muscle 2022;13:1076–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer 2007;7:763–777. [DOI] [PubMed] [Google Scholar]
- 25. Migita T, Ruiz S, Fornari A, Fiorentino M, Priolo C, Zadra G, et al. Fatty acid synthase: a metabolic enzyme and candidate oncogene in prostate cancer. J Natl Cancer Inst 2009;101:519–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Albiges L, Hakimi AA, Xie W, McKay RR, Simantov R, Lin X, et al. Body mass index and metastatic renal cell carcinoma: clinical and biological correlations. J Clin Oncol 2016;34:3655–3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sanchez A, Furberg H, Kuo F, Vuong L, Ged Y, Patil S, et al. Transcriptomic signatures related to the obesity paradox in patients with clear cell renal cell carcinoma: a cohort study. Lancet Oncol 2020;21:283–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Barbi J, Patnaik SK, Pabla S, Zollo R, Smith RJ Jr, Sass SN, et al. Visceral obesity promotes lung cancer progression‐toward resolution of the obesity paradox in lung cancer. J Thorac Oncol 2021;16:1333–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang Z, Aguilar EG, Luna JI, Dunai C, Khuat LT, Le CT, et al. Paradoxical effects of obesity on T cell function during tumor progression and PD‐1 checkpoint blockade. Nat Med 2019;25:141–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shimizu H, Shimomura Y, Hayashi R, Ohtani K, Sato N, Futawatari T, et al. Serum leptin concentration is associated with total body fat mass, but not abdominal fat distribution. Int J Obes Relat Metab Disord 1997;21:536–541. [DOI] [PubMed] [Google Scholar]
- 31. Esposito A, Marra A, Bagnardi V, Frassoni S, Morganti S, Viale G, et al. Body mass index, adiposity and tumour infiltrating lymphocytes as prognostic biomarkers in patients treated with immunotherapy: a multi‐parametric analysis. Eur J Cancer 2021;145:197–209. [DOI] [PubMed] [Google Scholar]
- 32. Hotamisligil GS. Inflammation and metabolic disorders. Nature 2006;444:860–867. [DOI] [PubMed] [Google Scholar]
- 33. Cederholm T, Jensen GL, Correia M, Gonzalez MC, Fukushima R, Higashiguchi T, et al. GLIM criteria for the diagnosis of malnutrition ‐ a consensus report from the global clinical nutrition community. J Cachexia Sarcopenia Muscle 2019;10:207–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Antoun S, Lanoy E, Ammari S, Farhane S, Martin L, Robert C, et al. Protective effect of obesity on survival in cancers treated with immunotherapy vanishes when controlling for type of cancer, weight loss and reduced skeletal muscle. Eur J Cancer 2023;178:49–59. [DOI] [PubMed] [Google Scholar]
- 35. Cortellini A, Bersanelli M, Buti S, Cannita K, Santini D, Perrone F, et al. A multicenter study of body mass index in cancer patients treated with anti‐PD‐1/PD‐L1 immune checkpoint inhibitors: when overweight becomes favorable. J Immunother Cancer 2019;7:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dixon JB, Egger GJ. A narrow view of optimal weight for health generates the obesity paradox. Am J Clin Nutr 2014;99:969–970. [DOI] [PubMed] [Google Scholar]
- 37. Laird BJA, Skipworth RJE. The obesity paradox in cancer: is bigger better? J Cachexia Sarcopenia Muscle 2022;13:1440–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lennon H, Sperrin M, Badrick E, Renehan AG. The obesity paradox in cancer: a review. Curr Oncol Rep 2016;18:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schneider BJ, Ismaila N, Aerts J, Chiles C, Daly ME, Detterbeck FC, et al. Lung cancer surveillance after definitive curative‐intent therapy: ASCO guideline. J Clin Oncol 2020;38:753–766. [DOI] [PubMed] [Google Scholar]
- 40. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2021. J Cachexia Sarcopenia Muscle 2021;12:2259–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]


