Abstract
The regulation of cytokinin oxidase activity in callus tissues of Phaseolus vulgaris L. cv Great Northern has been examined using an assay based on the oxidation of N6-(Δ2-isopentenyl)adenine-8-14C (i6 Ade-8-14C) to adenine. Solutions of exogenous cytokinins applied directly to the surface of the callus tissues induced relatively rapid increases in cytokinin oxidase activity. The increase in activity was detectable after 1 hour and continued for about 8 hours, reaching values two- to three-fold higher than the controls. The cytokinin-induced increase in cytokinin oxidase activity was inhibited in tissues pretreated with cordycepin or cycloheximide, suggesting that RNA and protein synthesis may be required for the response. Rifampicin and chloramphenicol, at concentrations that inhibited the growth of Great Northern callus tissues, were ineffective in inhibiting the increase in activity. All cytokinin-active compounds tested, including both substrates and nonsubstrates of cytokinin oxidase, were effective in inducing elevated levels of the enzyme in Great Northern callus tissue. The cytokinin-active urea derivative, Thidiazuron, was as effective as any adenine derivative in inducing this response. The addition of Thidiazuron to the reaction volumes used to assay cytokinin oxidase activity resulted in a marked inhibition of the degradation of the labeled i6 Ade-8-14C substrate. On the basis of this result, it is possible that Thidiazuron may serve as a substrate for cytokinin oxidase, but other mechanisms of inhibition have not yet been excluded.
Full text
PDF

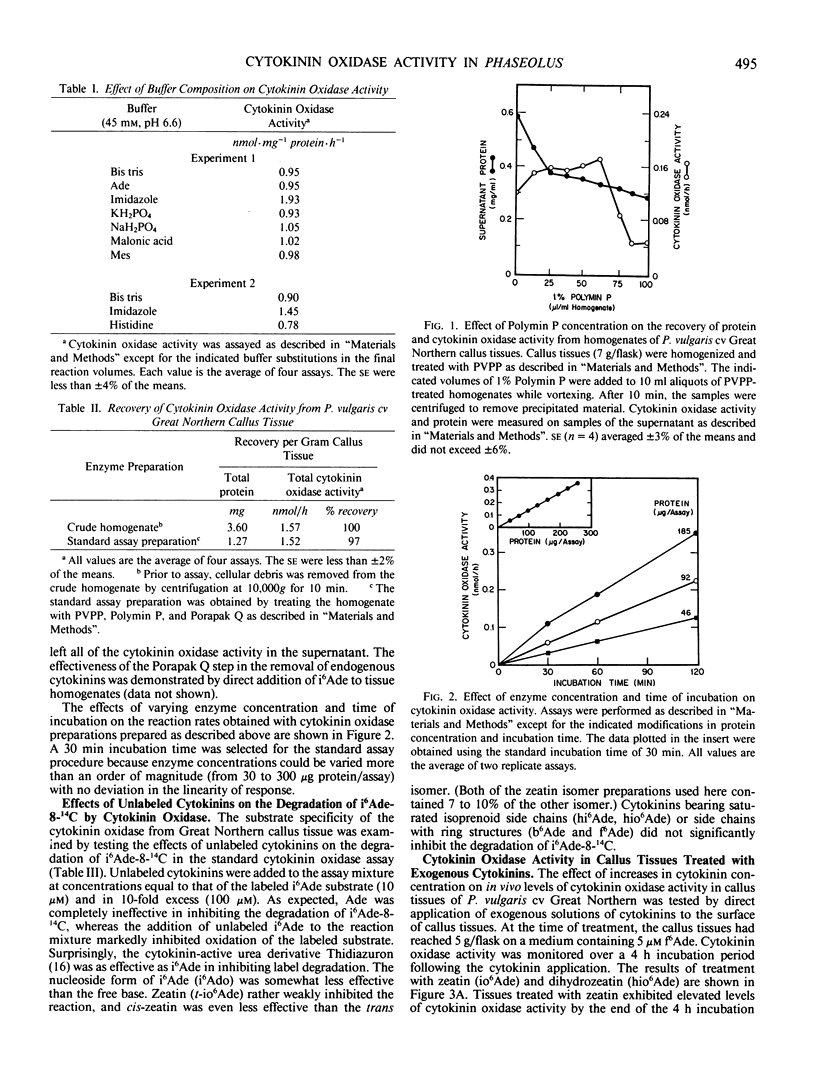
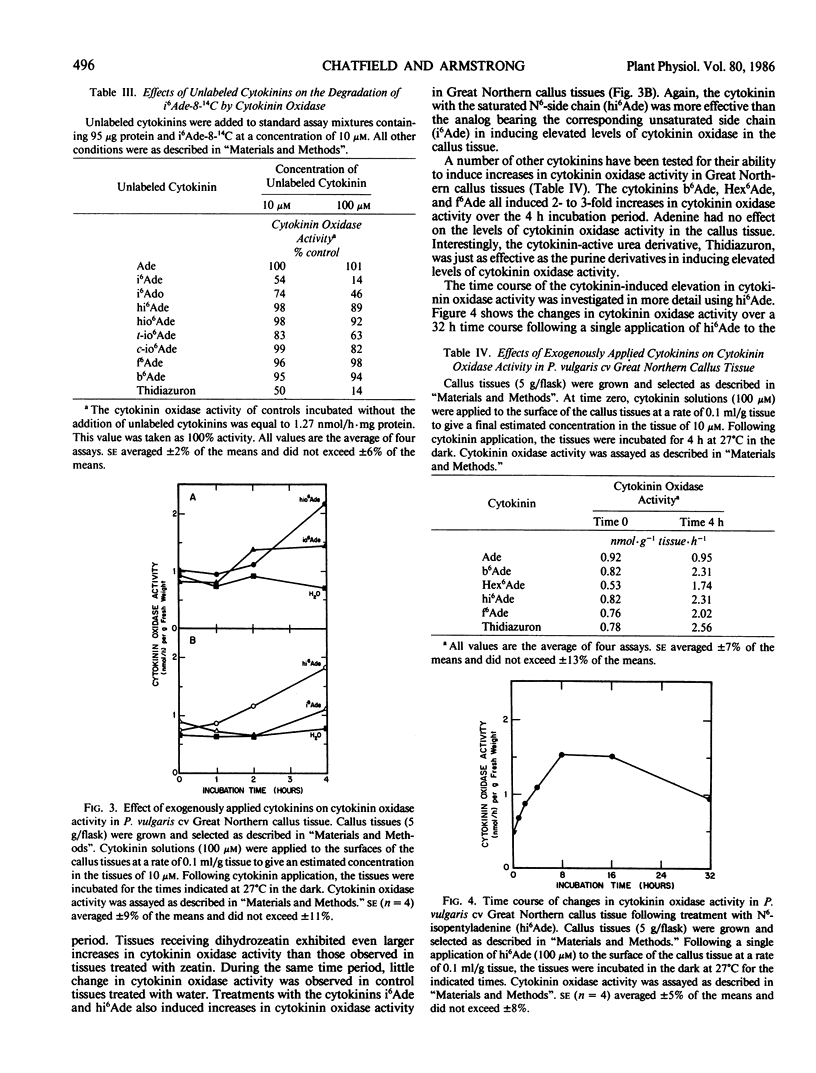
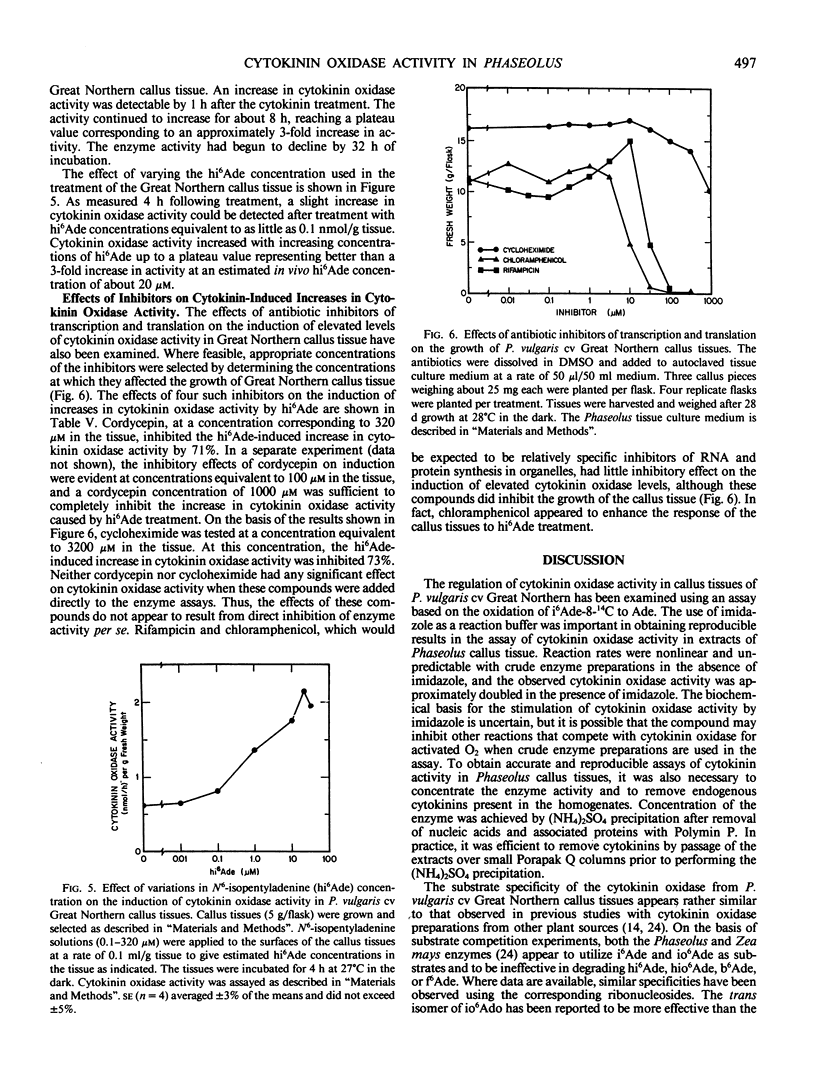
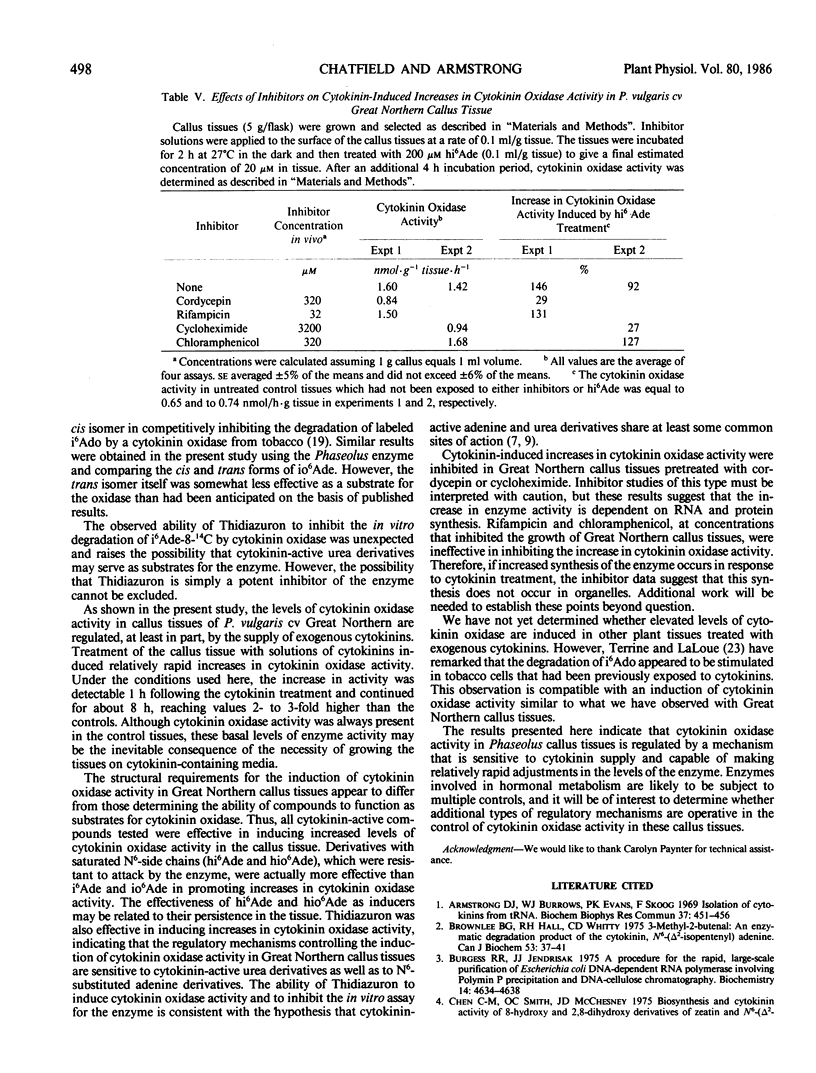

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong D. J., Burrows W. J., Evans P. K., Skoog F. Isolation of cytokinins from tRNA. Biochem Biophys Res Commun. 1969 Oct 22;37(3):451–456. doi: 10.1016/0006-291x(69)90936-x. [DOI] [PubMed] [Google Scholar]
- Brownlee B. G., Hall R. H., Whitty C. D. 3-Methyl-2-butenal: an enzymatic degradation product of the cytokinin, N-6-(delta-2 isopentenyl)adenine. Can J Biochem. 1975 Jan;53(1):37–41. doi: 10.1139/o75-006. [DOI] [PubMed] [Google Scholar]
- Burgess R. R., Jendrisak J. J. A procedure for the rapid, large-scall purification of Escherichia coli DNA-dependent RNA polymerase involving Polymin P precipitation and DNA-cellulose chromatography. Biochemistry. 1975 Oct 21;14(21):4634–4638. doi: 10.1021/bi00692a011. [DOI] [PubMed] [Google Scholar]
- Hall R. H., Alam S. N., McLennan B. D. N6-(delta 2-isopentenyl)adenosine: its conversion to inosine, catalyzed by adenosine aminohydrolases from chicken bone marrow and calf intestinal mucosa. Can J Biochem. 1971 Jun;49(6):623–630. doi: 10.1139/o71-089. [DOI] [PubMed] [Google Scholar]
- Mok M. C., Mok D. W., Dixon S. C., Armstrong D. J., Shaw G. Cytokinin structure-activity relationships and the metabolism of N-(delta-isopentenyl)adenosine-8-C in phaseolus callus tissues. Plant Physiol. 1982 Jul;70(1):173–178. doi: 10.1104/pp.70.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paces V., Kamínek M. Effect of ribosylzeatin isomers on the enzymatic degradation of N6-(delta2-isopentenyl) adenosine. Nucleic Acids Res. 1976 Sep;3(9):2309–2314. doi: 10.1093/nar/3.9.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paces V., Werstiuk E., Hall R. H. Conversion of N-(Delta-Isopentenyl)adenosine to Adenosine by Enzyme Activity in Tobacco Tissue. Plant Physiol. 1971 Dec;48(6):775–778. doi: 10.1104/pp.48.6.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson G. L. Determination of total protein. Methods Enzymol. 1983;91:95–119. doi: 10.1016/s0076-6879(83)91014-5. [DOI] [PubMed] [Google Scholar]
- Terrine C., Laloue M. Kinetics of N-(Delta-Isopentenyl)Adenosine Degradation in Tobacco Cells: EVIDENCE OF A REGULATORY MECHANISM UNDER THE CONTROL OF CYTOKININS. Plant Physiol. 1980 Jun;65(6):1090–1095. doi: 10.1104/pp.65.6.1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitty C. D., Hall R. H. A cytokinin oxidase in Zea mays. Can J Biochem. 1974 Sep;52(9):789–799. doi: 10.1139/o74-112. [DOI] [PubMed] [Google Scholar]


