Abstract
Background
We investigated the prognostic significance of body mass index in small‐cell lung cancer and explored whether skeletal muscle status affects the body mass index–survival relationship.
Methods
This retrospective study evaluated data from patients who underwent platinum‐etoposide chemotherapy for small‐cell lung cancer between March 2010 and December 2021. Skeletal muscle status was assessed using non‐contrast computed tomography images of baseline positron‐emission tomography‐computed tomography, with the skeletal muscle index defined as the cross‐sectional area of skeletal muscle divided by height squared, and the average attenuation values of skeletal muscle. Cox proportional hazards regression analysis was used to determine the correlations of body mass index, skeletal muscle metrics, and overall survival.
Results
We analysed the data of 1146 Asian patients (1006 men and 140 women, with a median age of 67 years [interquartile range: 61–72 years]), including 507 and 639 patients with limited and extensive disease, respectively. Being underweight, defined as a body mass index <18.5 kg/m2, was associated with shorter overall survival, independent of clinical covariates in both the limited‐disease (hazard ratio, 1.77; 95% confidence interval, 1.01–3.09) and extensive‐disease (hazard ratio, 1.71; 95% confidence interval, 1.18–2.48) groups. The prognostic value of being underweight remained significant after additional adjustment for skeletal muscle index and attenuation in both limited‐disease (hazard ratio, 1.96; 95% confidence interval, 1.09–3.51) and extensive‐disease (hazard ratio, 1.75; 95% confidence interval, 1.17–2.61) groups.
Conclusions
Being underweight is an independent poor prognostic factor for shorter overall survival in Asian patients with small‐cell lung cancer, regardless of skeletal muscle status.
Keywords: Body mass index, Myopenia, Myosteatosis, Prognosis, Sarcopenia, Small‐cell lung cancer, Skeletal muscle
Introduction
Lung cancer is the leading cause of cancer‐related mortality and is responsible for an estimated 1.8 million deaths worldwide. 1 Small‐cell lung cancer (SCLC) accounts for 14% of lung cancer, which is an aggressive subtype known for early resistance to treatment and the tendency to present with metastasis at the time of diagnosis. 2 As a result, SCLC has a poor prognosis, with a median overall survival (OS) of 25–30 months in the limited‐disease (LD) stage, and 7–9.6 months in the extensive‐disease (ED) stage under standard care. 3 , 4 Despite efforts to treat SCLC, the prognosis of SCLC has not improved as much as that of non‐small cell lung cancer (NSCLC) in recent decades. 2 Therefore, identifying prognostic factors to improve the prognosis in SCLC patients is of utmost importance.
An association between body mass index (BMI) and OS in patients with lung cancer has been reported, with underweight patients showing worse OS, and with those with obesity demonstrating better outcomes. 5 However, the exact reason for this association remains uncertain, with possible explanations ranging from methodological limitations to potential biological mechanisms. 6 , 7 Because BMI cannot differentiate between skeletal muscle and fat, which have different biological functions, 8 and as its role in indicating body composition is limited, 9 various methods have been developed to assess body composition and overcome the limitations of BMI. One such method is computed tomography (CT), which enables the quantitative measurement of skeletal muscle status in terms of cross‐sectional area and attenuation value. Skeletal muscle mass and skeletal muscle attenuation (SMA) determined using CT have been suggested to be important prognostic factors for several types of cancer. 10 , 11
Previous studies have suggested the negative prognostic impact of low BMI 5 and skeletal muscle mass 12 , 13 in patients with SCLC. However, concurrent evaluation of BMI and skeletal muscle mass and their combined association with prognosis in this population have not been well‐studied. Considering the positive correlation between BMI and skeletal muscle mass, it is plausible that the prognostic significance of these factors is interrelated. Specifically, the effect of BMI on SCLC prognosis may be confounded by skeletal muscle status, which is an important factor in determining this relationship.
Smoking is a well‐known risk factor for chronic obstructive pulmonary disease (COPD), reduced BMI and skeletal muscle impairment. 14 , 15 Notably, almost all patients with SCLC are smokers. 16 Although associations of BMI and skeletal muscle mass with OS in patients with NSCLC have been suggested, 17 it remains unclear whether these associations hold true in patients with SCLC due to the distinct nature of this cancer. Therefore, the aim of this study was to investigate the prognostic significance of BMI in patients with SCLC and to evaluate the potential influence of skeletal muscle status on the BMI–survival relationship.
Materials and methods
This retrospective, single‐centre study was conducted at a tertiary referral medical centre. The Institutional Review Board of Samsung Medical Center (IRB file No. 2022‐04‐076) approved this study and waived the need for obtaining written informed consent. This study was conducted according to the principles of the Declaration of Helsinki.
Patients
From the Clinical Data Warehouse Darwin‐C (CDW) of Samsung Medical Center, we extracted data of 2547 Asian patients who received a first diagnosis of SCLC, based on histology, between March 2010 and December 2021. Among them, 1247 patients who underwent platinum‐etoposide chemotherapy within 90 days of pathological confirmation, as well as positron‐emission tomography‐CT (PET‐CT) within 60 days before starting chemotherapy, were enrolled. Thereafter, patients meeting the following criteria were excluded: (i) poor image quality on PET‐CT (e.g., metallic or streaking artefacts, inadequate position; n = 66), (ii) insufficient clinical data (n = 24), (iii) segmentation error during image analysis (n = 7), and (iv) chemotherapy in an adjuvant setting after surgery (n = 4) (Figure 1 ).
Figure 1.
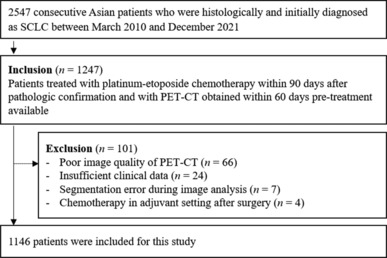
Flow chart illustrating selection of the study population. PET‐CT, positron‐emission tomography–computed tomography; SCLC, small‐cell lung cancer.
PET‐CT imaging
18F‐fluorodeoxyglucose (18F‐FDG) PET‐CT images were obtained using a Discovery STE PET‐CT scanner (GE Healthcare, Milwaukee, WI, USA). Sixty minutes after the intravenous injection of 18F‐FDG (5.0 MBq/kg), a non‐contrast CT scan of the whole body was performed using a 16‐slice helical CT scanner (acquisition parameters: 140 kVp, 30–170 mAs, pitch of 1.75, and section width of 3.75 mm). Subsequently, an emission scan was performed from the base of the skull to the thigh for 2.55 min/frame.
Image analysis
Non‐contrast baseline PET‐CT images were analysed using a commercially available deep learning‐based software (DeepCatch V.1.1.5; MedicalIP, Seoul, Korea). After automatic selection of the level of the third lumbar vertebrae, S1 skeletal muscles, including the internal and external obliques, transversus abdominus, rectus abdominus, psoas, quadratus lumborum and erector spinae muscles, were automatically segmented by using predetermined thresholds of −29 to 150 Hounsfield units (HU), while visceral fat and subcutaneous fat were automatically segmented based on predetermined thresholds of −190 to −30 HU (Figure 2 ). 17 A board‐certified radiologist with 7 years of experience in musculoskeletal imaging confirmed the appropriateness of the level selection and segmentation while blinded to patient information. The total cross‐sectional area (cm2) of these skeletal muscles and the visceral and subcutaneous fat for each patient were then divided by height‐squared to calculate the skeletal muscle index (SMI), visceral fat index (VFI), and subcutaneous fat index (SFI) (cm2/m2) of each patient. S2 SMA was measured, defined as the average CT attenuation value of the voxel within these skeletal muscles. Myopenia was defined as SMI of ≤52.4 cm2/m2 in men and ≤38.5 cm2/m2 in women. 8 SMA was dichotomized using sex‐specific medians as cutoff values, defining myosteatosis as the presence of an SMA lower than the relevant sex‐specific median.
Figure 2.
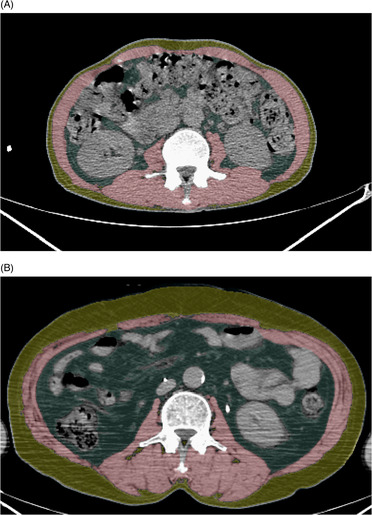
Examples of automated segmentation of skeletal muscles, visceral fat, and subcutaneous fat at the level of the third lumbar vertebrae. Skeletal muscles, visceral fat, and subcutaneous fat were visually highlighted by red, blue, and yellow overlays, respectively, in patients with LD‐ and ED‐SCLC, who had similar SMI, age, and sex, but different BMI. (A) A 56‐year‐old male patient with LD‐SCLC had a BMI of 18.1 kg/m2 and an SMI of 44.6 cm2/m2. He received CCRT and died 9.7 months after treatment. (B) A 56‐year‐old male patient with ED‐SCLC had a BMI of 26.8 kg/m2 and an SMI of 44.6 cm2/m2. He received palliative chemotherapy and died 20.8 months after treatment. BMI, body mass index; CCRT, concurrent chemoradiation therapy; ED, extensive disease; LD, limited disease; SMI, skeletal muscle index.
Data collection and endpoint
The following baseline characteristics were gathered by extraction using the CDW and electronic medical record reviews: age, sex, smoking status, height, body weight, blood neutrophil‐to‐lymphocyte ratio (NLR), Eastern Cooperative Oncology Group performance status (ECOG PS), Charlson co‐morbidity index, S3 and pulmonary function test results. BMI was calculated as body weight divided by height‐squared, and then categorized into underweight (<18.5 kg/m2), normal (18.5–22.9 kg/m2), overweight (23.0–24.9 kg/m2) or obese (≥25.0 kg/m2) according to the Asia‐Pacific classification. 18 We also recorded SCLC stage according to a modified version of the Veterans Administration Lung Cancer Study Group, 19 treatment agents used, and whether the patients underwent concurrent chemoradiation therapy. The diagnosis of COPD was made and the airflow limitation severity was classified according to the 2022 Global Initiative for Chronic Obstructive Lung Disease guidelines. 20
OS was the endpoint of this study, defined as the time from treatment initiation to death from any cause, and the survival data were collected from the electronic medical records or the database of the Ministry of the Interior and Safety of Korea.
Statistical analysis
Patient characteristics are presented as absolute numbers and percentages for categorical variables and as medians with interquartile ranges (IQR) for continuous variables. To compare the baseline characteristics of each BMI categories, the chi‐square test or Fisher's exact test was applied for categorical variables, as appropriate, according to the results of the normality test, and the Kruskal–Wallis test was applied for comparison of continuous variables.
Survival curves according to BMI categories, myopenia, and SMA for the LD and ED stages were estimated using the Kaplan–Meier method and were compared using the log‐rank test.
Cox proportional hazard regression analysis was performed to estimate the hazard ratios (HRs) and corresponding 95% confidence intervals (CIs) associated with BMI categories and body composition indices, including SMI, SMA, VFI and SFI, for the risk of mortality. SMI, SMA, VFI and SFI were treated as continuous variables (per sex‐specific standard deviation). Each variable was separately adjusted by age (per 1 year), sex (men/women), smoking status (never‐smoker/ex‐smoker/current‐smoker), ECOG PS (0/1/2 or more), Charlson co‐morbidity index (per 1 point), NLR (<3/≥3) and COPD status (normal/mild/moderate/severe) to construct Model I. Thereafter, Model II included BMI category, SMI and SMA and was adjusted using the same covariates to explore whether the prognostic impact of these variables was independent of each other. Analyses were performed separately for the LD and ED groups, which additionally included concurrent chemoradiation therapy status (no/yes) and combination immunotherapy (no/yes) as covariates. Interaction term analysis was also used to explore whether the prognostic values of BMI, SMI and SMA differed between the LD and ED stages.
Statistical analyses were performed using MedCalc Statistical Software version 20.118 (MedCalc Software Ltd., Ostend, Belgium) and Rex‐Pro (version 3.6.0; RexSoft, Co. Ltd., Seoul, Republic of Korea). Statistical significance was set at P < 0.05.
Results
A total of 1146 patients with a median age of 67 years (IQR 61–72 years; 1006 men and 140 women) were included in this study. The number of patients in the LD and ED stages was 507 (86 treated with etoposide + carboplatin and 421 with etoposide + cisplatin) and 639 (319 treated with etoposide + carboplatin and 320 treated with etoposide + cisplatin), respectively. In the ED group, 122 patients received immune checkpoint inhibitors as combination therapy (110 treated with atezolizumab, 9 with durvalumab and 3 with pembrolizumab). The median interval between the PET‐CT examination and treatment initiation was 7 days (IQR 3–14 days). Our cohort consisted of 58 underweight patients (5.1%), 405 patients (35.3%) with a normal BMI, 297 overweight patients (25.9%) and 386 obese patients (33.7%). Overall, 898 patients (78.4%) died during a median follow‐up of 13.4 months (IQR 7.7–22.6 months). A total of 110 patients (9.6%) had never smoked. Based on the cutoff values, 775 patients (67.6%) were categorized as having myopenia.
The baseline patient characteristics are summarized in Table 1 . Significant differences were observed among the BMI categories for age (P < 0.001), smoking status (P = 0.020), ECOG PS (P = 0.023), SCLC stage (P = 0.008) and NLR (P < 0.001). The underweight group was more likely to be older, currently smoking, and having an ECOG PS ≥ 2, ED stage, and higher NLR. No other significant differences were found among BMI categories.
Table 1.
Baseline patient characteristics.
| Characteristics | Total (n = 1146) | Underweight (n = 58) | Normal (n = 405) | Overweight (n = 297) | Obese (n = 386) | P‐value |
|---|---|---|---|---|---|---|
| Age (years) a | 67 (61, 72) | 68.5 (61, 75.5) | 67 (62, 73) | 67 (62, 72) | 65 (59, 70) | <0.001 b |
| Male sex | 1006 (87.8%) | 49 (84.5%) | 349 (86.2%) | 269 (90.6%) | 339 (87.8%) | 0.293 c |
| Smoking status | 0.020 c | |||||
| Never smoker | 110 (9.6%) | 3 (5.17%) | 42 (10.4%) | 19 (6.4%) | 46 (11.9%) | |
| Ex‐smoker | 450 (39.3%) | 18 (31.0%) | 144 (35.6%) | 126 (42.4%) | 162 (42.0%) | |
| Current smoker | 586 (51.1%) | 37 (63.8%) | 219 (54.1%) | 152 (51.2%) | 178 (46.1%) | |
| ECOG PS | 0.023 d | |||||
| 0–1 | 1065 (92.9%) | 52 (89.7%) | 368 (90.9%) | 275 (92.6%) | 370 (95.9%) | |
| ≥2 | 81 (7.1%) | 6 (10.3%) | 37 (9.1%) | 22 (7.4%) | 16 (4.2%) | |
| CCI | 0.464 c | |||||
| 0 | 423 (36.9%) | 19 (32.8%) | 164 (40.5%) | 100 (33.7%) | 140 (36.3%) | |
| 1 | 513 (44.8%) | 31 (53.5%) | 170 (42.0%) | 138 (46.5%) | 174 (45.1%) | |
| ≥2 | 210 (18.3%) | 8 (13.8%) | 71 (17.5%) | 59 (19.9%) | 72 (18.7%) | |
| Stage | 0.008 c | |||||
| LD | 507 (44.2%) | 23 (39.7%) | 166 (41.0%) | 120 (40.4%) | 198 (51.3%) | |
| ED | 639 (55.8%) | 35 (60.3%) | 239 (59.0%) | 177 (59.6%) | 188 (48.7%) | |
| NLR a | 2.43 (1.72, 3.55) | 3.46 (2.36, 5.18) | 2.58 (1.72, 3.82) | 2.49 (1.77, 3.55) | 2.15 (1.62, 3.02) | <0.001 b |
| COPD status | 0.206 c | |||||
| Yes | 392 (43.1%) | 16 (39.0%) | 124 (40.7%) | 95 (40.4%) | 157 (47.7%) | |
| No | 518 (56.9%) | 25 (61.0%) | 181 (59.3%) | 140 (59.6%) | 172 (52.3%) |
Unless otherwise indicated, the data are presented as the number of patients with percentages in parentheses.
CCI, Charlson co‐morbidity index; COPD, chronic obstructive pulmonary disease; ECOG PS, Eastern Cooperative Oncology Group performance status; ED, extensive disease; IQR, interquartile range; LD, limited disease; NLR, blood neutrophil‐to‐lymphocyte ratio.
Data are presented as medians with IQRs in parentheses.
Kruskal–Wallis test.
Chi‐squared test.
Fisher's exact test.
Survival analysis for LD stage
The median OS was 23.6 months (95% CI, 21.0–26.5 months). Kaplan–Meier curves and log‐rank tests showed that OS differed significantly among the different BMI categories (log‐rank P = 0.024), with reduced OS observed in underweight patients (median OS, 16.2 months; 95% CI, 9.6–33.8 months). Patients with myopenia (median OS, 20.5 months; 95% CI, 18.1–23.1 months) had shorter OS than those without myopenia (median OS, 27.5 months; 95% CI, 24.8–34.5 months) (log‐rank P = 0.003). OS was significantly shorter in patients with myosteatosis (median OS, 20.3 months; 95% CI, 17.1–23.1 months) than in patients without myosteatosis (median OS, 27.0 months; 95% CI, 23.7–32.4 months) (log‐rank P < 0.001) (Figure 3 ).
Figure 3.
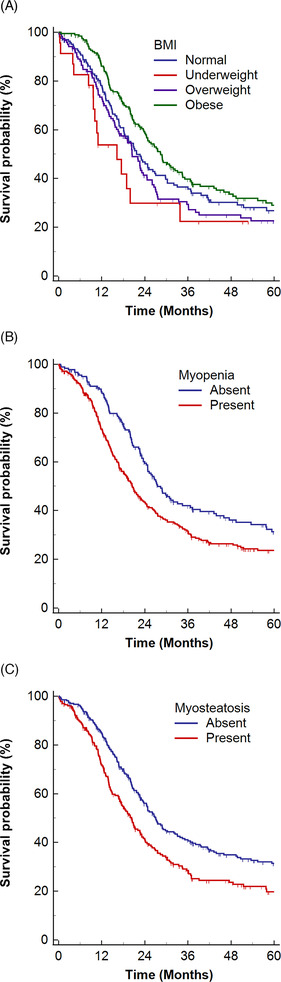
Kaplan–Meier estimates of overall survival in LD‐SCLC, with vertical dashes indicating censored data. The estimates are stratified according to (A) BMI categories, (B) myopenia, and (C) myosteatosis. BMI, body mass index; LD, limited disease; SCLC, small‐cell lung cancer.
In the Cox proportional hazard regression analysis adjusted for clinical covariates, underweight patients had a 77% increased risk of death as compared to patients with a normal BMI. A decrease of one sex‐specific standard deviation in SMA was associated with a 14% increased risk of death. However, overweight or obese BMI categories, SMI, VFI and SFI were not significantly associated with OS (Table 2 , Model I). The association observed between being underweight and SMA remained significant in Model II, in which BMI, SMI and SMA were entered together to adjust for each other (Table 2 ; Model II).
Table 2.
Association between body mass index, body composition indices and overall survival in limited disease group.
| Characteristics | Model I | Model II a | ||
|---|---|---|---|---|
| HR (95% CI) | P‐value | HR (95% CI) | P‐value | |
| BMI (kg/m2) | ||||
| Underweight (<18.5) | 1.77 (1.01–3.09) | 0.046 | 1.96 (1.09–3.51) | 0.024 |
| Normal (18.5–22.9) | 1 (reference) | 1 (reference) | ||
| Overweight (23.0–24.9) | 1.06 (0.79–1.42) | 0.721 | 0.94 (0.68–1.30) | 0.729 |
| Obese (≥25) | 1.01 (0.77–1.33) | 0.936 | 0.88 (0.62–1.26) | 0.490 |
| SMI (per 1 SD decreasing) | 1.02 (0.91–1.16) | 0.703 | 0.95 (0.81–1.12) | 0.519 |
| SMA (per 1 SD decreasing) | 1.14 (1.00–1.30) | 0.045 | 1.18 (1.03–1.36) | 0.020 |
| VFI (per 1 SD increasing) | 0.96 (0.86–1.07) | 0.454 | ‐ | ‐ |
| SFI (per 1 SD increasing) | 1.01 (0.90–1.13) | 0.928 | ‐ | ‐ |
All models were adjusted for the following covariates: age (per 1 year), sex (men/women), smoking status (never‐/ex‐/current‐smoker), ECOG PS (0/1/≥2), CCI (per 1 point), CCRT (yes/no), NLR (≥3/<3), COPD (normal/mild/moderate/severe).
BMI, body mass index; CCI, Charlson co‐morbidity index; CI, confidence interval; COPD, chronic obstructive pulmonary disease; ECOG PS, Eastern Cooperative Oncology Group performance status; HR, hazard ratio; NLR, blood neutrophil‐to‐lymphocyte ratio; SD, standard deviation; SFI, subcutaneous fat index; SMA, skeletal muscle attenuation; SMI, skeletal muscle index; VFI, visceral fat index.
BMI category, SMI, SMA were entered into model II with adjustment for covariates.
Survival analysis for ED stage
The median OS was 11.7 months (95% CI, 11.0–12.4 months). OS differed significantly according to BMI categories (log‐rank P = 0.014), where underweight patients had the shortest OS (median OS, 5.4 months; 95% CI, 3.3–10.1 months). Patients with myosteatosis (median OS, 10.4 months; 95% CI, 9.7–11.5 months) had significantly shorter OS than those without myosteatosis (median OS, 12.8 months; 95% CI, 11.9–14.0 months) (log‐rank P < 0.001). However, OS did not significantly differ between patients with (median OS, 11.3 months; 95% CI, 10.1–12.1 months) and without (median OS, 12.4 months; 95% CI, 11.3–13.2 months) myopenia (log‐rank P = 0.699) (Figure 4 ).
Figure 4.
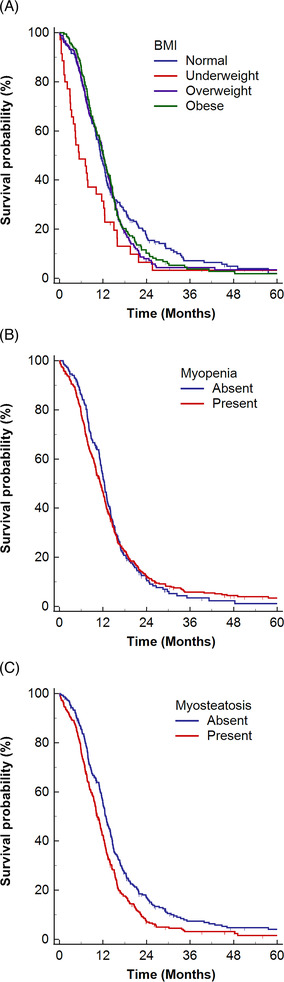
Kaplan–Meier estimates of overall survival in ED‐SCLC, with vertical dashes indicating censored data. The estimates are stratified according to (A) BMI categories, (B) myopenia, and (C) myosteatosis. BMI, body mass index; ED, extensive disease; SCLC, small‐cell lung cancer.
Being underweight was associated with poor OS and a 71% increased risk of death, independent of clinical covariates. However, neither being overweight or obese nor any of the body composition metrics were significantly associated with OS after adjustment for the same clinical covariates (Table 3 ; Model I). Nevertheless, the significant association observed in underweight patients persisted even after additional adjustments for SMI and SMA (Table 3 ; Model II).
Table 3.
Association between body mass index, body composition indices and overall survival in extensive disease group.
| Characteristics | Model I | Model II a | ||
|---|---|---|---|---|
| HR (95% CI) | P‐value | HR (95% CI) | P‐value | |
| BMI (kg/m2) | ||||
| Underweight (<18.5) | 1.71 (1.18–2.48) | 0.005 | 1.75 (1.17–2.61) | 0.007 |
| Normal (18.5–22.9) | 1 (reference) | 1 (reference) | ||
| Overweight (23.0–24.9) | 1.09 (0.88–1.36) | 0.407 | 1.07 (0.85–1.35) | 0.539 |
| Obese (≥25) | 1.13 (0.92–1.39) | 0.252 | 1.10 (0.83–1.45) | 0.523 |
| SMI (per 1 SD decreasing) | 1.01 (0.92–1.11) | 0.819 | 0.99 (0.87–1.12) | 0.835 |
| SMA (per 1 SD decreasing) | 1.03 (0.94–1.13) | 0.577 | 1.03 (0.93–1.14) | 0.530 |
| VFI (per 1 SD increasing) | 1.00 (0.91–1.09) | 0.941 | ‐ | ‐ |
| SFI (per 1 SD increasing) | 1.05 (0.96–1.15) | 0.305 | ‐ | ‐ |
All models were adjusted for the following covariates: age (per 1 year), sex (men/women), smoking status (never‐/ex‐/current‐smoker), ECOG PS (0/1/≥2), CCI (per 1 point), CCRT (yes/no), NLR (≥3/<3), COPD (normal/mild/moderate/severe).
BMI, body mass index; CCI, Charlson co‐morbidity index; CI, confidence interval; COPD, chronic obstructive pulmonary disease; ECOG PS, Eastern Cooperative Oncology Group performance status; HR, hazard ratio; NLR, blood neutrophil‐to‐lymphocyte ratio; SD, standard deviation; SFI, subcutaneous fat index; SMA, skeletal muscle attenuation; SMI, skeletal muscle index; VFI, visceral fat index.
BMI category, SMI, SMA were entered into the model II with adjustment for covariates.
Interaction of BMI, SMI and SMA with stage
The association between the BMI categories and OS was similar between patients in the LD and those in the ED stages (P for interaction = 0.616). Similarly, the association between SMI and OS was not significantly different between these two stages (P for interaction = 0.552). Although Cox proportional hazard regression analyses demonstrated that the prognostic effect of SMA on OS was more evident in the LD stage than in the ED stage (Tables 2 and 3 ), the association between SMA and OS did not reach statistical significance in terms of differences between the two stages (P for interaction = 0.063).
Discussion
In our study, being underweight was an independent poor prognostic factor in Asian patients with SCLC undergoing platinum‐etoposide chemotherapy, regardless of skeletal muscle status and clinical covariates. Furthermore, a decrease in SMA was another poor prognostic factor, and its impact was evident only in patients in the LD stage. Although myopenia has been reported to be an independent poor prognostic factor in previous studies, 12 , 13 its association with OS was not significant in multivariable analyses. In addition to the quantitative analysis of skeletal muscle status using CT images of a large number of patients, the strength of our study lies in the fact that we had separately analysed OS according to disease stage, considering their distinctively different prognosis, 21 and that we included various clinically relevant covariates.
Because BMI is an easily quantifiable biomarker and a key component in the diagnosis of cachexia, 22 many studies have investigated the association between BMI and prognosis in cancer patients. 5 , 23 , 24 Most studies have found that being underweight is a poor prognostic factor for several types of cancer, including lung cancer. 5 , 24 Although the exact mechanism has not been fully elucidated, cancer cachexia has been suggested as the underlying biological mechanism linking low BMI to poor prognosis in cancer patients. 12 , 25 Given that myopenia is widely regarded as a poor prognostic marker, 8 body composition has attracted attention as a potential explanation for the prognostic impact of BMI. 12 , 13 However, we found that being underweight was a poor prognostic factor for survival, regardless of the skeletal muscle status, refuting the previous notion that skeletal muscle may be a confounding factor. 7 Considering that our results also challenged the findings of previous studies that reported an association between fat depletion or ‘adipopenia’ and a poor prognosis in patients with cancer, 26 our study suggests that additional factors beyond body composition might contribute to the poor prognosis observed in underweight patients with SCLC. Given the correlation among underweight, treatment toxicity, and poor nutritional status, 27 it is plausible that these factors might serve as alternative explanations for the underweight‐survival relationship. This highlights the necessity for further investigations aimed at identifying the underlying mechanism, optimal treatment approaches, with the goal of improving outcomes in these patients.
Because L3 SMI has been introduced as a quantifiable metric that is linearly related to whole‐body muscle mass, 8 it has been widely used in numerous observational studies, defining an SMI below certain cutoffs as sarcopenia. 13 , 28 Although no standardized tool for defining sarcopenia exists, 29 a diagnosis of sarcopenia cannot be established solely based on decreased skeletal muscle quantity, known as myopenia, as low skeletal muscle strength and function are also essential components. 30 Thus, an additional surrogate marker is required to supplement the limitations of muscle quantity measurement. In this context, interest in muscle attenuation measured on CT has been growing, as it is considered an index that indirectly reflects muscle strength, 31 due to the observed association between decreased muscle attenuation caused by fat infiltration, referred to as myosteatosis, and poor prognosis in various types of cancer, including lung cancer. 10 , 11 Given the observed association between decreased SMA and poor OS in the LD group, regardless of BMI and SMI, our study results may imply that SMA has a higher prognostic value than SMI as a surrogate marker for sarcopenia.
Lung cancer is one of the malignancies in which the paradoxically beneficial impact of obesity on survival, termed the ‘obesity paradox’, appears in several meta‐analysis of different cohort. 32 , 33 Most of these studies involved patients with NSCLC and demontrated that obese patients, defined as having BMI ≥ 30 kg/m2, had improved survival after surgical resection, 34 chemotherapy 35 or immunotherapy. 36 In contrast, we observed that obesity, defined as having BMI ≥ 25 kg/m2, was not independently associated with improved OS in patients with SCLC, which was in line with previous studies that applied 25 kg/m2 37 or 30 kg/m2 38 as cutoffs to define obesity. While the prognostic impact of obesity seems to differ between NSCLC and SCLC, the cause of this phenomenon remains poorly understood, as does the obesity paradox. Given that smoking is suspected to be a strong confounding factor in the obesity paradox, 6 the disproportionally high smoker predominance in SCLC, 16 and the notion that smoking can affect weight and body compositions, 14 , 15 it might be a possible factor that should be investigated in this context in future studies.
This study had several limitations other than the intrinsic limitations of a retrospective study conducted in a single tertiary hospital. First, because our study only included Asians, selection bias may have been unavoidable. Moreover, the inclusion of only patients who underwent PET‐CT and the considerable male predominance in the patient cohort might have exacerbated the selection bias. Given the previous notion of heterogeneity in the association between BMI and SCLC prognosis, 5 care should be taken when generalizing the results of this study to other ethnicities. Second, while being a significant prognostic factor, the limited prevalence of underweight patients at 5.1% may constrain the clinical implications. Third, there might be an effect from residual or unmeasured confounding or reverse causation. 6 Although we adjusted for as many clinically relevant covariates as possible, the influence of these methodological problems may not have been eliminated. In particular, data regarding recent weight loss, a crucial factor known to have an unfavourable prognostic effect in patients with cancer, 39 was not available. The availability of disease burden information, including the number of metastases that impact patient prognosis, was also lacking. Finally, inferring a causal relationship between the variables and outcomes was challenging in this observational study and warrants future prospective studies.
In conclusion, being underweight is an independent poor prognostic factor in Asian patients with SCLC, irrespective of skeletal muscle status, for both patients in the LD and those in the ED stages, highlighting that BMI should be considered a prognostic biomarker, despite its limitations. SMA may have more prognostic value than cross‐sectional muscle area as a surrogate marker for sarcopenia, which indirectly reflects muscle quality. In addition to determining whether the associations observed in our study are similar in patients of other races, future studies should explore the underlying biology that links underweight and poor prognosis to find optimal treatment and improve clinical outcomes in patients with SCLC.
Conflict of interests
All the authors declare no potential conflicts of interests.
Funding
This work was supported by research funding from the Samsung Medical Center (No. SMO1220571) and the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Korean government (MSIT) (No. RS‐2023‐00222838).
Supporting information
Data S1. Supporting Information.
Acknowledgements
All the authors certify that they comply with the ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle. 40
Kwon YJ, Yoon YC, Kim HS, Cha MJ, Park S, Lee JH. 2023; Prognostic significance of body mass index in small‐cell lung cancer: Exploring the relationship with skeletal muscle status. Journal of Cachexia, Sarcopenia and Muscle, 14, 2939–2947, 10.1002/jcsm.13345
References
- 1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209–249. [DOI] [PubMed] [Google Scholar]
- 2. American Cancer Society . Cancer Facts & Figures 2023. Atlanta: American Cancer Society; 2023. https://www.cancer.org/research/cancer‐facts‐statistics/all‐cancer‐facts‐figures/2023‐cancer‐facts‐figures.html. Accessed 1 April 2023. [Google Scholar]
- 3. Faivre‐Finn C, Snee M, Ashcroft L, Appel W, Barlesi F, Bhatnagar A, et al. Concurrent once‐daily versus twice‐daily chemoradiotherapy in patients with limited‐stage small‐cell lung cancer (CONVERT): an open‐label, phase 3, randomised, superiority trial. Lancet Oncol 2017;18:1116–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Debieuvre D, Dayen C, Dixmier A, Pau D, Sibley‐Revelat A, Greenwood W, et al. FRESC: French Real world Extensive stage SCLC Cohorts: a retrospective study on patient characteristics and treatment strategy based on KBP‐2010. Lung Cancer 2022;164:1–7. [DOI] [PubMed] [Google Scholar]
- 5. Shepshelovich D, Xu W, Lu L, Fares A, Yang P, Christiani D, et al. Body mass index (BMI), BMI change, and overall survival in patients with SCLC and NSCLC: a pooled analysis of the International Lung Cancer Consortium. J Thorac Oncol 2019;14:1594–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Park Y, Peterson LL, Colditz GA. The plausibility of obesity paradox in cancer‐point. Cancer Res 2018;78:1898–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang XL, Liu YM, Shao H, Zheng X. Obesity paradox in lung cancer prognosis: evolving biological insights and clinical implications. J Thorac Oncol 2017;12:1478–1488. [DOI] [PubMed] [Google Scholar]
- 8. Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population‐based study. Lancet Oncol 2008;9:629–635. [DOI] [PubMed] [Google Scholar]
- 9. Nuttall FQ. Body mass index: obesity, BMI, and health: a critical review. Nutr Today 2015;50:117–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Caan BJ, Cespedes Feliciano EM, Prado CM, Alexeeff S, Kroenke CH, Bradshaw P, et al. Association of muscle and adiposity measured by computed tomography with survival in patients with nonmetastatic breast cancer. JAMA Oncol 2018;4:798–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sjøblom B, Grønberg BH, Wentzel‐Larsen T, Baracos VE, Hjermstad MJ, Aass N, et al. Skeletal muscle radiodensity is prognostic for survival in patients with advanced non‐small cell lung cancer. Clin Nutr 2016;35:1386–1393. [DOI] [PubMed] [Google Scholar]
- 12. Go SI, Park MJ, Song HN, Kang MH, Park HJ, Jeon KN, et al. Sarcopenia and inflammation are independent predictors of survival in male patients newly diagnosed with small cell lung cancer. Support Care Cancer 2016;24:2075–2084. [DOI] [PubMed] [Google Scholar]
- 13. Kim EY, Kim YS, Park I, Ahn HK, Cho EK, Jeong YM. Prognostic significance of CT‐determined sarcopenia in patients with small‐cell lung cancer. J Thorac Oncol 2015;10:1795–1799. [DOI] [PubMed] [Google Scholar]
- 14. Chiolero A, Faeh D, Paccaud F, Cornuz J. Consequences of smoking for body weight, body fat distribution, and insulin resistance. Am J Clin Nutr 2008;87:801–809. [DOI] [PubMed] [Google Scholar]
- 15. Park S, Kim SG, Lee S, Kim Y, Cho S, Kim K, et al. Causal linkage of tobacco smoking with ageing: Mendelian randomization analysis towards telomere attrition and sarcopenia. J Cachexia Sarcopenia Muscle 2023;14:955–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tseng JS, Chiang CJ, Chen KC, Zheng ZR, Yang TY, Lee WC, et al. Association of smoking with patient characteristics and outcomes in small cell lung carcinoma, 2011‐2018. JAMA Netw Open 2022;5:e224830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee JH, Yoon YC, Kim HS, Cha MJ, Kim JH, Kim K, et al. Obesity is associated with improved postoperative overall survival, independent of skeletal muscle mass in lung adenocarcinoma. J Cachexia Sarcopenia Muscle 2022;13:1076–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. World Health Organization . Regional Office for the Western Pacific. The Asia‐Pacific perspective: redefining obesity and its treatment. Sydney: Health Communications Australia; 2000. http://iris.wpro.who.int/handle/10665.1/5379. Accessed 1 April 2023. [Google Scholar]
- 19. Carter BW, Glisson BS, Truong MT, Erasmus JJ. Small cell lung carcinoma: staging, imaging, and treatment considerations. Radiographics 2014;34:1707–1721. [DOI] [PubMed] [Google Scholar]
- 20. Venkatesan P. GOLD report: 2022 update. Lancet Respir Med 2022;10:e20. [DOI] [PubMed] [Google Scholar]
- 21. Foster NR, Mandrekar SJ, Schild SE, Nelson GD, Rowland KM Jr, Deming RL, et al. Prognostic factors differ by tumor stage for small cell lung cancer: a pooled analysis of North Central Cancer Treatment Group trials. Cancer 2009;115:2721–2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011;12:489–495. [DOI] [PubMed] [Google Scholar]
- 23. Kroenke CH, Neugebauer R, Meyerhardt J, Prado CM, Weltzien E, Kwan ML, et al. Analysis of body mass index and mortality in patients with colorectal cancer using causal diagrams. JAMA Oncol 2016;2:1137–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Parr CL, Batty GD, Lam TH, Barzi F, Fang X, Ho SC, et al. Body‐mass index and cancer mortality in the Asia‐Pacific Cohort Studies Collaboration: pooled analyses of 424,519 participants. Lancet Oncol 2010;11:741–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fearon KC, Glass DJ, Guttridge DC. Cancer cachexia: mediators, signaling, and metabolic pathways. Cell Metab 2012;16:153–166. [DOI] [PubMed] [Google Scholar]
- 26. Choi H, Park YS, Na KJ, Park S, Park IK, Kang CH, et al. Association of adipopenia at preoperative PET/CT with mortality in stage i non‐small cell lung cancer. Radiology 2021;301:645–653. [DOI] [PubMed] [Google Scholar]
- 27. Muscaritoli M, Arends J, Bachmann P, Baracos V, Barthelemy N, Bertz H, et al. ESPEN practical guideline: clinical nutrition in cancer. Clin Nutr 2021;40:2898–2913. [DOI] [PubMed] [Google Scholar]
- 28. Nakamura R, Inage Y, Tobita R, Yoneyama S, Numata T, Ota K, et al. Sarcopenia in resected NSCLC: effect on postoperative outcomes. J Thorac Oncol 2018;13:895–903. [DOI] [PubMed] [Google Scholar]
- 29. Carvalho do Nascimento PR, Bilodeau M, Poitras S. How do we define and measure sarcopenia? A meta‐analysis of observational studies. Age Ageing 2021;50:1906–1913. [DOI] [PubMed] [Google Scholar]
- 30. Cruz‐Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019;48:16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Correa‐de‐Araujo R, Addison O, Miljkovic I, Goodpaster BH, Bergman BC, Clark RV, et al. Myosteatosis in the context of skeletal muscle function deficit: an interdisciplinary workshop at the National Institute on Aging. Front Physiol 2020;11:963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yu D, Zheng W, Johansson M, Lan Q, Park Y, White E, et al. Overall and central obesity and risk of lung cancer: a pooled analysis. J Natl Cancer Inst 2018;110:831–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shen N, Fu P, Cui B, Bu CY, Bi JW. Associations between body mass index and the risk of mortality from lung cancer: a dose‐response PRISMA‐compliant meta‐analysis of prospective cohort studies. Medicine (Baltimore) 2017;96:e7721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li S, Wang Z, Huang J, Fan J, Du H, Liu L, et al. Systematic review of prognostic roles of body mass index for patients undergoing lung cancer surgery: does the ‘obesity paradox’ really exist? Eur J Cardiothorac Surg 2017;51:817–828. [DOI] [PubMed] [Google Scholar]
- 35. Dahlberg SE, Schiller JH, Bonomi PB, Sandler AB, Brahmer JR, Ramalingam SS, et al. Body mass index and its association with clinical outcomes for advanced non‐small‐cell lung cancer patients enrolled on Eastern Cooperative Oncology Group clinical trials. J Thorac Oncol 2013;8:1121–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kichenadasse G, Miners JO, Mangoni AA, Rowland A, Hopkins AM, Sorich MJ. Association between body mass index and overall survival with immune checkpoint inhibitor therapy for advanced non‐small cell lung cancer. JAMA Oncol 2020;6:512–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Abdel‐Rahman O. Impact of baseline characteristics on extensive‐stage SCLC patients treated with etoposide/carboplatin: A secondary analysis of a phase III study. Clin Respir J 2018;12:2519–2524. [DOI] [PubMed] [Google Scholar]
- 38. Evcil FY, Önal Ö, Özkan EE. The Effect of Body Mass Index on Survival in Lung Cancer. Nutr Cancer 2023;75:857–866. [DOI] [PubMed] [Google Scholar]
- 39. Martin L, Senesse P, Gioulbasanis I, Antoun S, Bozzetti F, Deans C, et al. Diagnostic criteria for the classification of cancer‐associated weight loss. J Clin Oncol 2015;33:90–99. [DOI] [PubMed] [Google Scholar]
- 40. von Haehling S, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2021. J Cachexia Sarcopenia Muscle 2021;12:2259–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting Information.


