Abstract
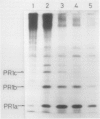
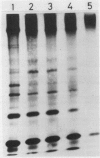
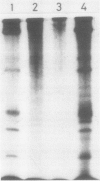
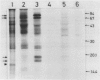
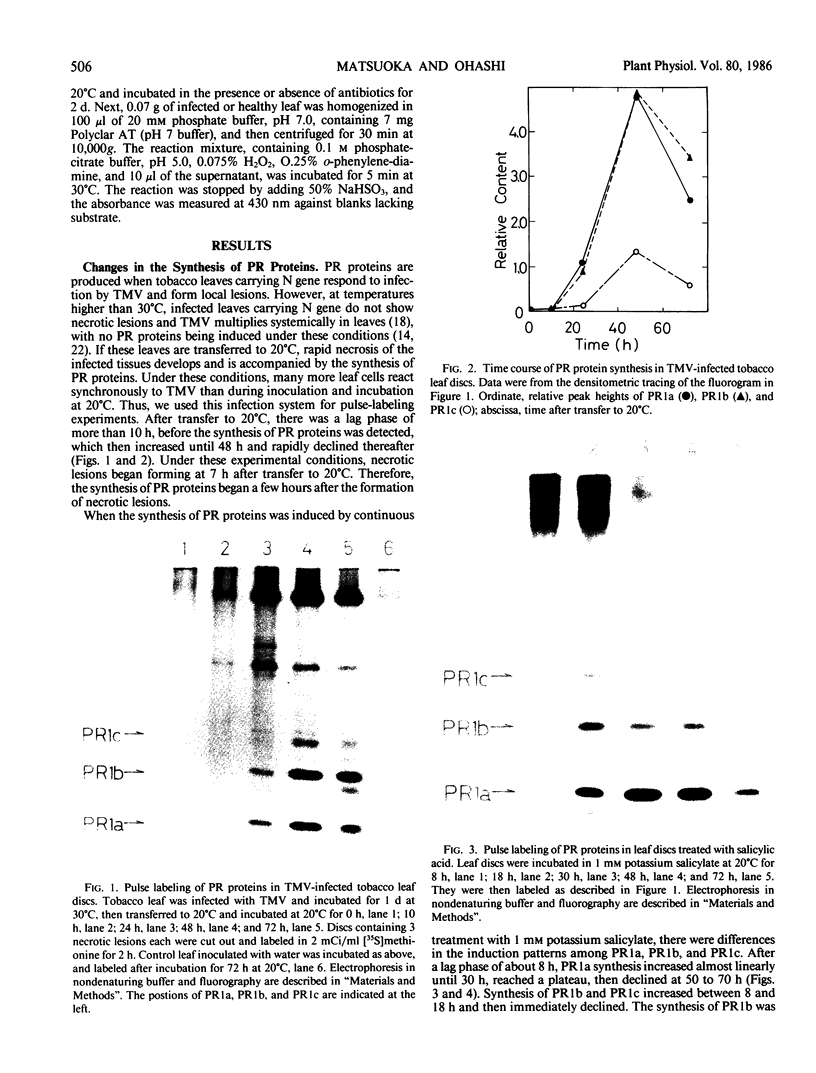
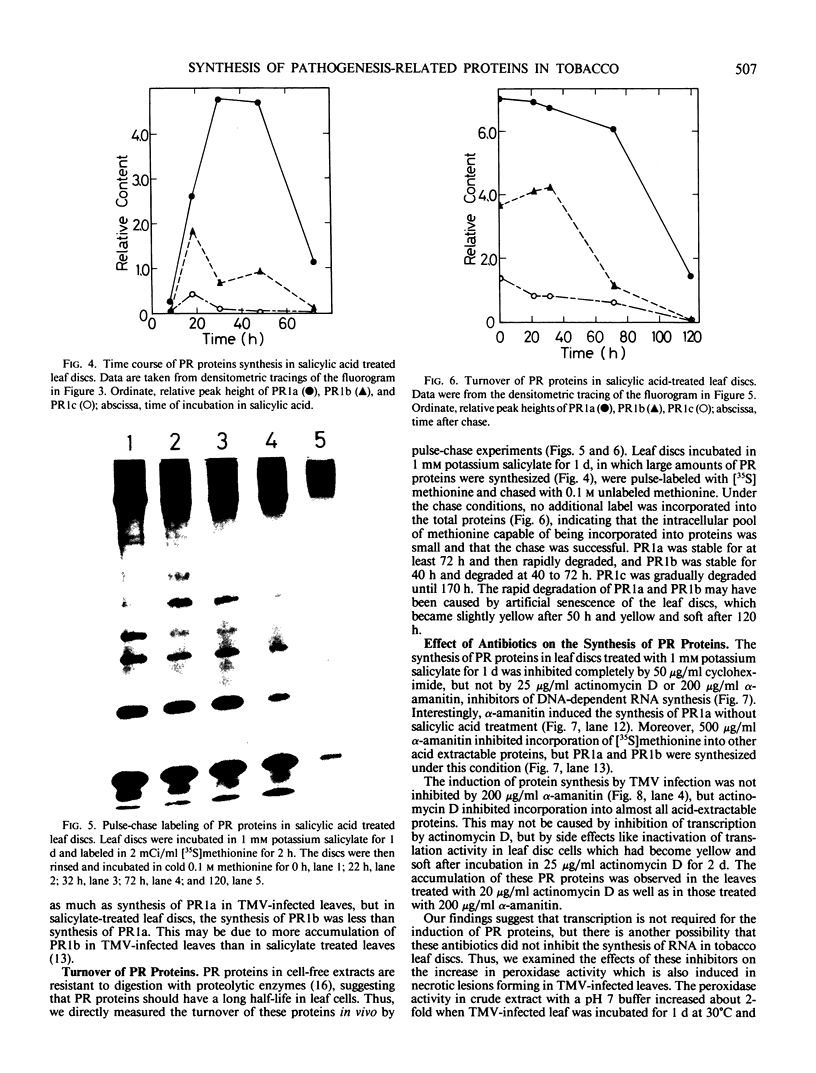
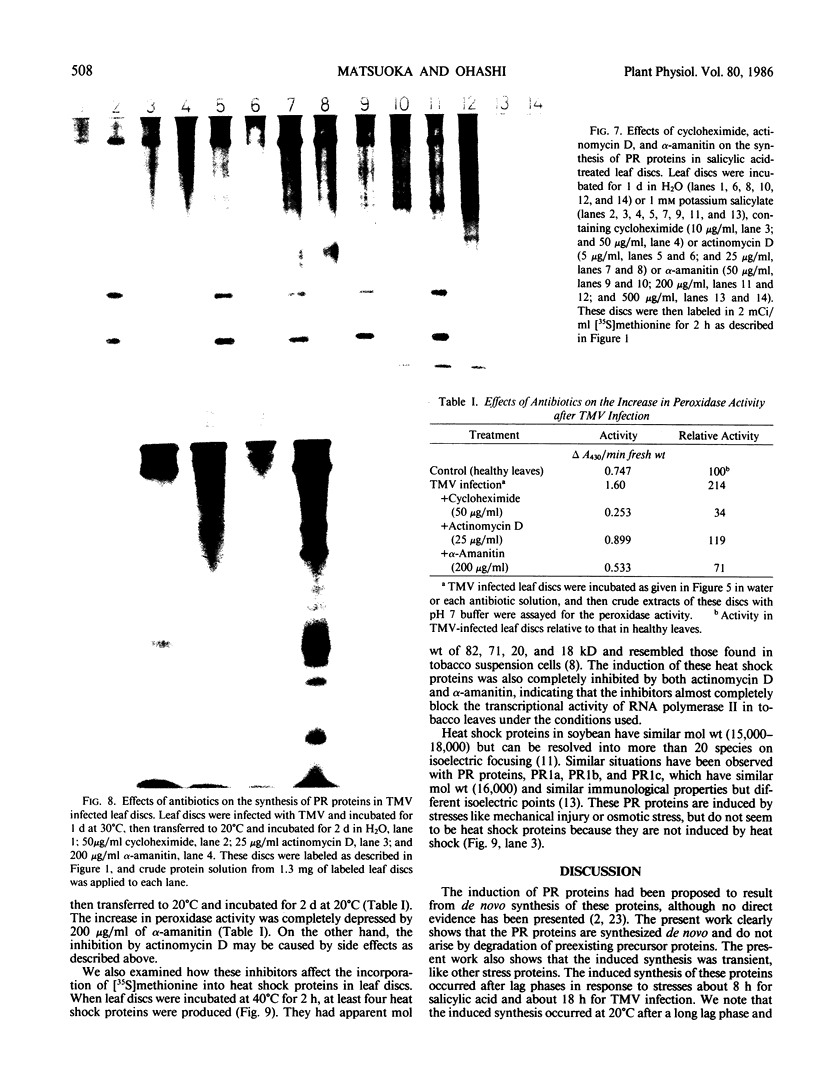
The synthesis of pathogenesis-related proteins (PR proteins), induced in tobacco leaves in response to infection with tobacco mosaic virus or treatment with salicylic acid, was studied with in vivo pulse-labeling experiments. PR proteins synthesis began after a lag phase of about 8 hours in leaf discs treated with salicylic acid and after more than 18 hours in those infected with tobacco mosaic virus. In both cases, the synthesis declined rapidly after 50 hours. The results show that the accumulation of PR proteins results from de novo synthesis and not from degradation of preexisting precursors and that the induced synthesis is transient like other stress-inducible proteins. The proteins have a half-life of at least 50 hours. The induction of these PR proteins was not inhibited by either 25 micrograms per milliliter of actinomycin D or 200 micrograms per milliliter of α-amanitin, which completely inhibited the increase of peroxidase activity in tobacco mosaic virus-infected leaf and the induction of heat shock proteins in tobacco leaf discs. These findings indicate that the induction of PR proteins is not regulated by a transcriptional step but by a translational step.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Hadwiger L. A., Jafri A., von Broembsen S., Eddy R. Mode of Pisatin Induction: Increased Template Activity and Dye-binding Capacity of Chromatin Isolated from Polypeptide-treated Pea Pods. Plant Physiol. 1974 Jan;53(1):52–63. doi: 10.1104/pp.53.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanabus J., Pikaard C. S., Cherry J. H. Heat Shock Proteins in Tobacco Cell Suspension during Growth Cycle. Plant Physiol. 1984 Jul;75(3):639–644. doi: 10.1104/pp.75.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lin C. Y., Roberts J. K., Key J. L. Acquisition of Thermotolerance in Soybean Seedlings : Synthesis and Accumulation of Heat Shock Proteins and their Cellular Localization. Plant Physiol. 1984 Jan;74(1):152–160. doi: 10.1104/pp.74.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White C. N., Hightower L. E. Stress mRNA metabolism in canavanine-treated chicken embryo cells. Mol Cell Biol. 1984 Aug;4(8):1534–1541. doi: 10.1128/mcb.4.8.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]