Abstract
Introduction
Mutations in the 79 exons of the dystrophin gene result in muscle wasting and weakness of varying clinical severity, ranging from severe/typical Duchenne muscular dystrophy (DMD) to intermediate DMD and mild Becker muscular dystrophy (BMD), depending on the frameshift of the mutation. We previously reported that males with DMD have progressively declining appendicular lean mass (ALM) and ALM index (ALMI) with age and worsening functional motor ability compared with healthy controls. These indices have not been studied in patients with intermediate DMD and BMD phenotypes and across DMD genotypes. In this study, we compared age‐related trajectories of ALM and ALMI of patients who had (1) BMD without functional mobility deficits with patients who had DMD at different stages of disease and healthy controls; (2) a DMD intermediate phenotype with patients who had a typical DMD phenotype; and (3) DMD categorized by genotype.
Methods
We conducted a retrospective review of ALM and ALMI data from 499 patients (ages 5–23 years) with DMD (466 typical and 33 intermediate) and 46 patients (ages 5–21 years) with BMD (without functional mobility deficits and functional mobility score of 1). Patients were grouped according to age reflecting disease stage (ages 5 to <7, 7 to <10, 10 to <14, and 14 to <20 years) and genotype (mutations in exons 1–30, 31–44, 45–62, and 63–79).
Results
ALM and ALMI trajectories of patients with BMD paralleled those of healthy controls until adolescence, in contrast to patients with DMD. ALMI Z‐scores of patients with BMD remained within ±2 SD without decline while those of patients with DMD fell below −2 SD around age 12 years. Patients with BMD had increasing ALM and ALMI with age, with peak accrual between ages 10 to <14 years. ALMI declined after age 14 years for those with intermediate DMD compared with 10 years for patients with typical DMD. Patients with mutations in exons 63–79 had a greater decline in ALMI as compared with those with other genotypes after age 10 years.
Conclusions
Age‐related changes in ALMI in patients with BMD and intermediate DMD differ from those with typical DMD, reflecting their clinical phenotypes. ALM and ALMI should be further studied in patients with BMD and DMD subtypes for their potential value as surrogate markers to characterize the severity of BMD and DMD and inform clinical care decisions and clinical trial designs.
Keywords: Appendicular lean mass (ALM), Appendicular lean mass index (ALMI), Becker muscular dystrophy (BMD), Dual energy‐X‐ray absorptiometry (DXA), Duchenne muscular dystrophy (DMD), Genotype
Introduction
Duchenne muscular dystrophy (DMD) and Becker muscular dystrophy (BMD) are X‐linked recessive disorders associated with mutations in the dystrophin gene that codes for the sarcolemmal muscle protein, dystrophin. Depending on the type of mutation—frameshift versus in‐frame—the severity of the phenotype ranges from that of severe DMD, through intermediate DMD to that of BMD. 1 , 2 Frameshift mutations of the dystrophin gene resulting in a non‐functional dystrophin protein or absence of dystrophin predict the DMD phenotype while in‐frame mutations predict the milder BMD phenotype. Exceptions to this frame rule occur when alternate splicing occurs around the deleted exons. 1 , 3
The clinical manifestations of the skeletal muscle involvement of patients with BMD ranges from that of muscle cramps with no detectable muscle weakness, to the ‘typical BMD phenotype’ with later onset of muscle weakness with mild clinical progression to loss of ambulation in the fifth decade of life and beyond; and the severe BMD phenotype with wheel‐chair dependence around age 17 years. 1 In contrast, patients with typical DMD present with varying degrees of pelvic girdle weakness by age 3–5 years, manifested by a Gower's manoeuvre to hand rising from the floor, difficulty with running and climbing steps, and inability to jump. Without glucocorticoid treatment, patients with typical DMD progress to loss of independent ambulation by age 12 (patients with intermediate DMD by age 16), cardiopulmonary dysfunction in the teen years and death in their early twenties. With current standard of care glucocorticoid therapy, DMD patients progress gradually from an early‐ambulatory phase characterized by stable or improving motor function to a mid‐ambulatory phase characterized by declining ambulation and increasing difficulty in rising from the floor and climbing steps; this is followed by a late‐ambulatory phase that is associated with loss of the ability to rise from the floor or climb steps. Patients in the early non‐ambulatory phase have good upper extremity function enabling independent feeding, which is lost with progression to the late non‐ambulatory phase characterized by total dependence for feeding and all activities of daily living.
The pathological correlation of the clinical phenotype of DMD is progressive fibro‐fatty replacement of muscle tissue. 4 This progressive muscle wasting in patients with DMD has been found to be associated with altered body composition with a decrease in lean body mass and elevated body fat mass. 5 , 6 , 7 As such, non‐invasive assessment of lean mass, particularly appendicular lean mass (ALM), may prove valuable for measuring disease severity, which may in turn inform clinical care decisions and clinical trial designs. We previously reported that males with DMD have progressive decline in ALM and ALM index (ALMI) with age and worsening functional motor ability compared with healthy controls. 8 ALM and ALMI changes in patients with the milder intermediate DMD phenotype and BMD have not been reported. No genotype–phenotype studies of ALM and ALMI in patients with DMD genetic subtypes have been reported to date.
We hypothesized that patients with typical DMD and intermediate DMD, who have earlier onset of progressive loss of muscle strength and motor function, have lower muscle mass as compared with paediatric patients with BMD, who have no functional mobility deficits as defined by functional mobility criteria. In this study, we aimed to compare age‐related trajectories of ALM and ALMI of patients who had (1) BMD without functional mobility deficits with patients who had DMD at different stages of disease and healthy controls; (2) intermediate DMD phenotype with patients who had a typical DMD phenotype and BMD; and (3) DMD categorized by four genotype groups—mutations in exons 1–30, 31–44, 45–62, and 63–79.
Methods
We performed a retrospective study of longitudinal ALM measured by dual energy X‐ray absorptiometry (DXA) (Hologic, Marlborough, MA) of patients with DMD and BMD (ages 5–23 years) seen at Cincinnati Children's Comprehensive Neuromuscular Care Center between 1 January 2009 and 31 March 2017. DXA scans are performed annually as part of the centre's standard‐of‐care protocol for patients with DMD and BMD to monitor bone health 9 and body composition. The Institutional Review Board at Cincinnati Children's Hospital Medical Center approved this study (IRB # 2017‐2480). The Functional Mobility Status (FMS) score is obtained at each clinic visit and is an 8‐grade scale categorizing ambulation, mobility status and amount of support needed for activities of daily living, with lower scores indicating greater functional mobility. A FMS score of 1 (FMS 1) is defined by mild abnormalities in gait and the ability to climb stairs without assistance.
Inclusion criteria for the present study were male sex; clinical presentation consistent with DMD (signs and symptoms of pelvic girdle weakness by ages 3–5 years) or paediatric patients with BMD who had an FMS of 1 and no signs of pelvic girdle weakness, and a dystrophin gene mutation and/or muscle biopsy dystrophin findings confirming a diagnosis of DMD or BMD; age at DXA whole body composition assessment between 5.0 and 22.9 years; and evaluation in the clinic within 90 days of the DXA scan. Exclusion criteria were artefact presence in the DXA scan affecting scan results; patients with DMD receiving investigational muscle anabolic therapeutic agents, patients with other coexistent primary diagnoses affecting ambulation and/or muscle mass or function; and >1 year of daily glucocorticoid treatment in patients with BMD.
DXA lean mass data for healthy male controls ages (5–23 years) were obtained from the National Institute of Child Health and Human Development Data and Specimen Hub database that stored the data for the National Institute of Child Health and Human Development funded multi‐site ‘Bone Mineral Density in Childhood Study (BMDCS)’. The BMDCS was a 7‐year longitudinal study (conducted between July 2002 and November 2010) of healthy children aged 5 to 19 years at study entry, who had no medical conditions known to adversely affect bone health or growth, and a height and body mass index (BMI) between the 3rd and 97th percentiles for age and sex, who underwent annual measurements of standing height and body composition. 10 , 11 Body composition for the healthy controls and our patients with DMD and BMD was measured by whole body DXA scans acquired with Hologic densitometers (Marlborough, MA).
Standing heights were obtained for ambulatory DMD patients (73% of the DMD cohort). Heights for non‐ambulatory patients with DMD were derived from the following methods: arm span for those with no upper extremity contractures and, for those with upper extremity contractures, segmental arm span, ulna length and estimations derived by extrapolation from their growth curve trajectories. Data from scans with missing heights were excluded from the analyses involving a height index (ALMI). Standing heights were obtained for all patients with BMD.
All whole body DXA scans were analysed using the National Health and Nutrition Examination Survey body composition analysis adjustment 12 to yield measures of bone, lean soft tissue and fat mass. We used lean soft tissue mass (lean mass—bone mass) as our measure of lean body mass (LBM). ALM was calculated as the sum of LBM in the arms and legs. LBM index (LBMI) and ALMI were calculated as LBM or ALM divided by the square of the height in meters (kg/m2), respectively.
Statistical analyses
All analyses were conducted with the use of R 3.5.0 software (The R Foundation for Statistical Computing) and SAS version 9.4 software (SAS Institute Inc., Cary, NC). A piece‐wise linear, or segmental, regression was decided upon for the ease of interpretability at the expense of incurring extra mathematical complexity.
As described previously, we developed ALM‐ and ALMI‐for‐age reference curves using data from the BMDCS healthy controls with the use of Generalized Additive Models for Location, Scale, and Shape (GAMLSS library) in R. 8 Specifically, the LMS method was used to estimate smoothed growth curves that could be summarized by the Box–Cox power transformation (L), the median (M), and the generalized coefficient of variation (S). 13 , 14 Several statistical and visual diagnostic tools were used to guide the choice of our final model. These tools included worm plots, analysis of residuals, Owen D‐trend plots, as well as an examination of the percentile of smoothed curves that were superimposed on the empirical data. This modelling approach does not account for the fact that data for healthy controls were longitudinal.
Z‐scores for ALM and ALMI were calculated for patients with DMD (typical and intermediate phenotypes) and BMD using the parameters generated from the LMS model. We assessed age‐related trajectory Z‐scores using mixed models to account for the multiple observations per person.
A repeated measures model was fit where the response was modelled as a piece‐wise linear function of age in years. The cut‐point ages where the line segments changed the slope (knots) were based on expert clinical opinion regarding the stages of ambulation for glucocorticoid treated patients with DMD. Specifically, these were early‐ambulatory (<7 years), mid‐ambulatory (7 to <10 years), late‐ambulatory (10 to <14 years), and non‐ambulatory (14 to 23 years). The curves shown in the figures gave the fitted line segments for the cohorts based on this longitudinal model, and the points corresponded to the raw data values, ignoring the subject effect.
Results
Characteristics of patients with Duchenne muscular dystrophy and Becker muscular dystrophy
Our sample included 466 patients with typical DMD and 33 with intermediate DMD who underwent 2460 and 171 DXA scans respectively; and 46 patients with BMD who underwent 137 scans (Table 1A, Table S1). The median age at DXA scan was 11.0 years (range 5.0–22.8) for patients with DMD, 11.6 years (5.1–21.1) for patients with BMD and 13.9 years (5.0–23.3) for healthy controls (N = 693). Patients with DMD were treated with glucocorticoids (mean age of initiation was 5.7 years [2.5–12.8 years]; most (80.6%) were on daily deflazacort and the remainder on daily prednisone or with a history of having transitioned between the two glucocorticoid types (n = 10 patients). Nineteen per cent of patients with DMD had a history of growth hormone treatment, and 14% had testosterone treatment. (Of note, a sensitivity analysis revealed no material differences in ALM or ALMI between those with and without these treatments; data not shown.) Patients with BMD were not treated with glucocorticoids. In accordance with our clinic's cardiac care practice, patients with DMD are prescribed angiotensin converting ezyme inhibitors or angiotensin receptor blockers after age 10 years or with cardiac imaging findings of decreased left ventricular systolic function (left ventricular ejection fraction <55%) whichever is earlier. Antifibrotics (spironolactone or eplerenone) are added upon the finding of myocardial fibrosis on contrast cardiac MRI studies with gadolinium. Addition of beta‐blockers follows with further changes in cardiac function.
Table 1.
(A) The distribution of patients with DMD, DMD intermediate and BMD by four age groups and number of DEXA scans [*5 to <7 years; 7 to <10 years; 10 to <14 years; 14 to <20 years and >20 years]. (B) The distribution of patients with DMD by four genotypes.
| A. | ||||
|---|---|---|---|---|
| Number of DXA scans | ||||
| DMD | DMD intermediate | BMD | ||
| Age group | N = 466 | N = 33 | N = 46 | |
| 5 to <7 years | 254 | 22 | 16 | |
| 7 to < 10 years | 611 | 46 | 34 | |
| 10 to < 14 years | 765 | 54 | 48 | |
| 14 to < 20 years | 785 | 46 | 33 | |
| ≥20 years | 45 | 3 | 6 | |
| B. | ||||
|---|---|---|---|---|
| DMD | ||||
| Mutation site | Number | No. of DXA scans | ||
| Exon 1–30 | 159 | 772 | ||
| Exon 31–44 | 73 | 373 | ||
| Exon 45–62 | 253 | 1,132 | ||
| Exon 63–79 | 12 | 52 | ||
Mutations at exons 1–30, 31–44, 45–62 and 63–79.
BMD, Becker muscular dystrophy; DMD, Duchenne muscular dystrophy; DXA, dual‐energy X‐ray absorptiometry.
Median BMI Z‐score was 1.1 (−0.2 to 3.5) for patients with DMD, 0.5 (−2.7 to 2.7) for patients with BMD and 0.3 (−2.9 to 2.6) for healthy controls. Median height‐for‐age Z‐score was −2.1 (−7.4 to 2.2), −0.2 (−2.4 to 2.8) and 0.1 (−2.5 to 3.0) for the same groups, respectively. Median percentage of whole body fat was 37.9% (14.0 to 67.5), 29.1% (16.4 to 49.4) and 23.1% (11.6 to 47.2), respectively. Standing height was used for ambulatory patients with DMD (73% of the DMD cohort) and BMD (100% of the BMD cohort). Other descriptive characteristics of the study population are detailed in Table 1.
Age‐related trajectories of appendicular lean mass and appendicular lean mass index in patients with Becker muscular dystrophy compared with patients with Duchenne muscular dystrophy and healthy controls
Figure 1 shows the fitted line segments for the three cohorts of healthy controls, BMD patients and DMD patients (Table 2). The reference curves show consistent gains in ALM and ALMI with age for healthy controls, including a noticeable increase around the time of the pubertal growth spurt (10 to <14 years). Unlike patients with DMD, the ALM and ALMI trajectories of patients with BMD paralleled those of healthy controls until adolescence. Likewise, ALM and ALMI Z‐scores of patients with BMD remained within ±2 SD without decline (Figure 2). In contrast, in patients with DMD, mean ALM Z‐scores declined steadily from around age 7 years, falling below −2.0 by age 10 years, while height‐adjusted ALMI Z‐scores fell below −2.0 after age 13 years.
Figure 1.
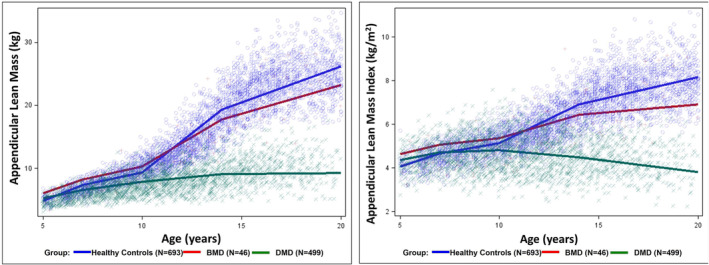
Appendicular lean mass (ALM) and appendicular lean mass index (ALMI) by age of patient with DMD (N = 499), BMD (N = 46) and healthy controls (N = 693).
Table 2.
Rate of change of appendicular lean mass (kg) and appendicular lean mass index (kg/m2) in four age groups of mutations health controls (HC) and patients with BMD and DMD.
| Healthy controls | BMD | DMD | P‐values | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N = 693 | N = 46 | N = 499 | |||||||
| Age (years) | Estimate | SE | Estimate | SE | Estimate | SE | HC vs. BMD | HC vs. DMD | BMD vs. DMD |
| ALM | |||||||||
| 5 to <7 | 1.25 | 0.14 | 1.08 | 0.46 | 0.67 | 0.06 | 0.7149 | 0.0002 | 0.3820 |
| 7 to <10 | 0.69 | 0.05 | 0.70 | 0.19 | 0.43 | 0.03 | 0.9380 | <0.00001 | 0.1446 |
| 10 to <14 | 2.48 | 0.03 | 1.86 | 0.13 | 0.31 | 0.02 | <0.00001 | <0.00001 | <0.00001 |
| 14 to <20 | 1.15 | 0.02 | 0.91 | 0.12 | 0.04 | 0.02 | 0.0620 | <0.00001 | <0.00001 |
| ≥20 | −0.37 | 0.09 | −1.32 | 0.47 | −0.39 | 0.10 | 0.0443 | 0.8962 | 0.0493 |
| ALMI | |||||||||
| 5 to <7 | 0.30 | 0.03 | 0.21 | 0.13 | 0.17 | 0.03 | 0.4918 | 0.0033 | 0.7532 |
| 7 to <10 | 0.16 | 0.01 | 0.10 | 0.05 | 0.03 | 0.01 | 0.2331 | <0.00001 | 0.1942 |
| 10 to <14 | 0.44 | 0.01 | 0.27 | 0.04 | −0.08 | 0.01 | <0.00001 | <0.00001 | <0.00001 |
| 14 to <20 | 0.21 | 0.01 | 0.08 | 0.03 | −0.12 | 0.01 | 0.0002 | <0.00001 | <0.00001 |
| ≥20 | 0.00 | 0.02 | −0.22 | 0.13 | −0.10 | 0.05 | 0.1017 | 0.0650 | 0.3774 |
BMD, Becker muscular dystrophy; DMD, Duchenne muscular dystrophy; HC, healthy controls; SE, standard error.
Figure 2.
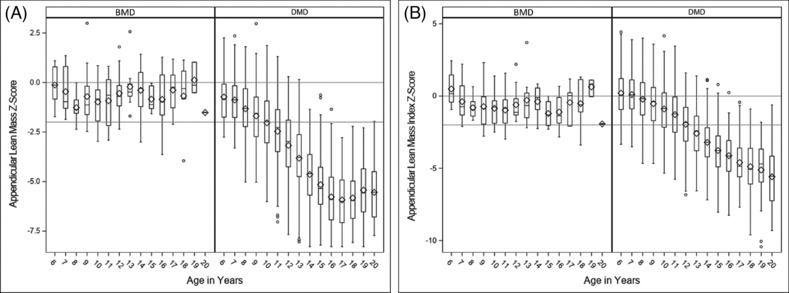
Boxplots of ALM (A) and ALMI (B) for patients with DMD and BMD by age. Boxes represent interquartile ranges; vertical bars indicate ranges.
Age‐related trajectories of appendicular lean mass and appendicular lean mass index in patients with intermediate Duchenne muscular dystrophy compared with patients with typical Duchenne muscular dystrophy and Becker muscular dystrophy
The age‐related trajectories of ALM and ALMI for the different age groups of patients with milder DMD intermediate compared with patients with typical DMD and BMD (who had normal motor function) are shown in Figure 3 and Table 3. Patients with BMD had the highest rate of change for ALMI during the 10 to <14 years (pubertal) age range, while patients with typical DMD and intermediate DMD had their highest rate of change before 7 years of age, followed by declining rates of change thereafter, consistent with progressive loss of muscle accrual over the life span of patients with DMD.
Figure 3.
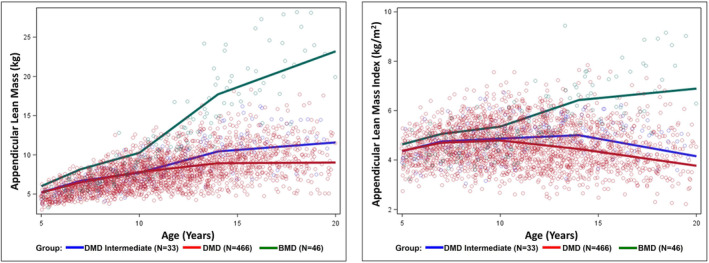
ALM and ALMI by age of patient with DMD (N = 499), DMD intermediate (N = 33), and BMD (N = 46).
Table 3.
Rate of change of appendicular lean mass (kg) and appendicular lean mass index (kg/m2) in four age groups of patients. With DMD (N = 466), DMD intermediate (N = 33), and BMD (N = 46).
| DMD intermediate (DMDI) | DMD | BMD | P‐values | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N = 33 | N = 466 | N = 46 | |||||||
| Age (years) | Estimate | SE | Estimate | SE | Estimate | SE | DMDI vs. DMD | DMDI vs. BMD | DMD vs. BMD |
| ALM | |||||||||
| 5 to <7 | 0.74 | 0.23 | 0.66 | 0.07 | 1.08 | 0.46 | 0.7388 | 0.5081 | 0.3643 |
| 7 to <10 | 0.35 | 0.11 | 0.44 | 0.03 | 0.70 | 0.19 | 0.4379 | 0.1037 | 0.1594 |
| 10 to <14 | 0.68 | 0.08 | 0.28 | 0.02 | 1.86 | 0.13 | <0.0001 | <0.0001 | <0.0001 |
| 14 to <20 | 0.19 | 0.07 | 0.01 | 0.02 | 0.91 | 0.12 | 0.0204 | <0.0001 | <0.0001 |
| ≥20 | −2.33 | 0.56 | −0.28 | 0.10 | −1.32 | 0.47 | 0.0003 | 0.1671 | 0.0289 |
| ALMI | |||||||||
| 5 to <7 | 0.20 | 0.08 | 0.16 | 0.03 | 0.21 | 0.13 | 0.6844 | 0.9316 | 0.7086 |
| 7 to <10 | 0.04 | 0.04 | 0.03 | 0.01 | 0.10 | 0.05 | 0.8534 | 0.3557 | 0.1994 |
| 10 to <14 | 0.03 | 0.03 | −0.09 | 0.01 | 0.27 | 0.04 | 0.0001 | <0.0001 | <0.0001 |
| 14 to <20 | −0.14 | 0.03 | −0.11 | 0.01 | 0.08 | 0.03 | 0.3012 | <0.0001 | <0.0001 |
| ≥20 | −0.72 | 0.20 | −0.07 | 0.05 | −0.22 | 0.13 | 0.0017 | 0.0361 | 0.2785 |
BMD, Becker muscular dystrophy; DMD, Duchenne muscular dystrophy; DMDI, Duchenne muscular dystrophy intermediate; SE, standard error.
Age‐related trajectories of appendicular lean mass and appendicular lean mass index in patients with Duchenne muscular dystrophy by genotype group
Patients with DMD who had mutations in exons 63–79 showed greater declines in ALMI after age 10 years as compared with patients with the other three DMD genotype groups (Figure 4 and Table 4).
Figure 4.
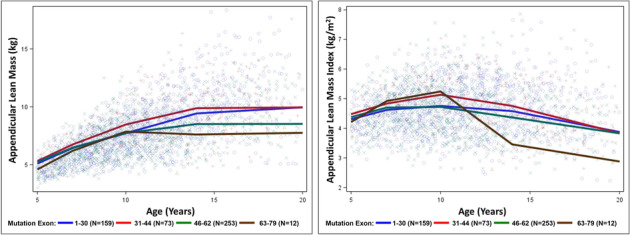
ALM and ALMI of patients with DMD by four genotypes [1–30 (N = 159); 31–44 (N = 73); 46–62 (N = 253); and 63–79 (N = 12)].
Table 4.
Rate of change of appendicular lean mass (kg) and appendicular lean mass index (kg/m2) in DMD patients per 4 genotypes of DMD from ages 5 to 23 years.
| 1: Exon 1–30 | 2: Exon 31–44 | 3: Exon 45–62 | 4: Exon 63–79 | P‐values | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | Estimate | SE | Estimate | SE | Estimate | SE | Estimate | SE | 1 v 2 | 1 vs. 3 | 1 vs. 4 | 2 vs. 3 | 2 vs. 4 | 3 vs. 4 |
| ALM | ||||||||||||||
| 5 to <7 | 0.68 | 0.12 | 0.72 | 0.22 | 0.62 | 0.07 | 0.80 | 0.28 | 0.8616 | 0.7200 | 0.6734 | 0.6842 | 0.8103 | 0.5378 |
| 7 to <10 | 0.42 | 0.05 | 0.57 | 0.09 | 0.42 | 0.03 | 0.55 | 0.10 | 0.1288 | 0.9143 | 0.2391 | 0.0868 | 0.8721 | 0.1886 |
| 10 to <14 | 0.43 | 0.03 | 0.35 | 0.05 | 0.19 | 0.02 | −0.06 | 0.09 | 0.1802 | <0.0001 | <0.0001 | 0.0017 | <0.0001 | 0.0070 |
| 14 to <20 | 0.09 | 0.04 | 0.01 | 0.04 | 0.00 | 0.03 | 0.02 | 0.08 | 0.1836 | 0.0481 | 0.4520 | 0.8490 | 0.8794 | 0.7716 |
| ≥20 | 0.66 | 0.20 | −0.73 | 0.21 | −0.78 | 0.12 | −0.72 | 1.67 | <0.0001 | <0.0001 | 0.4108 | 0.8261 | 0.9966 | 0.9712 |
| ALMI | ||||||||||||||
| 5 to <7 | 0.16 | 0.05 | 0.18 | 0.09 | 0.16 | 0.04 | 0.36 | 0.15 | 0.8404 | 0.9659 | 0.2253 | 0.8514 | 0.3252 | 0.2196 |
| 7 to <10 | 0.04 | 0.02 | 0.10 | 0.04 | 0.01 | 0.01 | 0.11 | 0.06 | 0.1854 | 0.1423 | 0.3056 | 0.0173 | 0.9027 | 0.0933 |
| 10 to <14 | −0.04 | 0.01 | −0.09 | 0.02 | −0.09 | 0.01 | −0.45 | 0.05 | 0.0313 | 0.0115 | <0.0001 | 0.7975 | <0.0001 | <0.0001 |
| 14 to <20 | −0.12 | 0.02 | −0.15 | 0.02 | −0.09 | 0.01 | −0.10 | 0.04 | 0.1410 | 0.1665 | 0.6245 | 0.0050 | 0.1996 | 0.8730 |
| ≥20 | 0.37 | 0.09 | −0.21 | 0.09 | −0.32 | 0.07 | −0.14 | 0.89 | <0.0001 | <0.0001 | 0.5664 | 0.3351 | 0.9398 | 0.8466 |
ALM, appendicular lean mass; ALMI, appendicular lean mass index; BMD, Becker muscular dystrophy; DMD, Duchenne muscular dystrophy; SE, standard error.
Discussion
We characterized age‐related trajectories of ALM and ALMI of patients (aged 5–23 years) with DMD, including differentiating those with the intermediate DMD phenotype, and BMD. As long‐term treatment with systemic daily glucocorticoids has been the standard of care for DMD, 15 ALMI of this glucocorticoid treated DMD cohort is a more accurate representation of appendicular lean muscle mass changes than ALM due to growth failure resulting from long‐term glucocorticoid treatment. In our earlier analysis of ALMI of glucocorticoid‐treated patients with DMD, 8 we found that, by age 14 years, the mean age of loss of ambulation, 16 , 17 the median ALMI Z‐score was below −2, suggesting a ALMI threshold for loss of ambulatory function. Patients with DMD have a declining rate of accrual of lean mass (adjusted for height), with accrual rate of about 57% that of healthy controls before age 7 years and about 20% that of healthy 7‐ to 10‐year‐olds, with progressive loss of lean mass over the second decade of life. In contrast, healthy males and paediatric patients with BMD with no functional mobility deficits (FMS 1) accrue lean mass steadily with age, with the peak accrual rate occurring during the early part of the second decade, coinciding with puberty.
When compared with healthy controls, the mean ALM and ALMI of patients with BMD without functional mobility deficits (FMS 1) were within 2 SD of healthy controls, albeit mainly between 0 and −2 SD. Notably, the peak rate of change of ALMI between ages 10 to 14 years (during puberty) for patients with BMD was only 60% that of healthy controls, raising the need for longitudinal surveillance of ALMI changes with disease progression with increasing age.
Of note, the major clinical milestones for changes in motor function in patients with DMD mirror the trajectories of ALMI changes. At about age 7 years, patients with DMD transition from a stable ‘plateau’ phase to onset of decline in motor function. 18 At about age 10 years, the 6‐min‐walk test distance changes from an improvement of 20 m/year to a decline of 85 m/year. 19 The median age of loss of ambulation of DMD patients treated with daily glucocorticoids is 14 years. 16 , 17
The rapid gain in appendicular lean muscle mass in healthy controls, and to a lesser magnitude in paediatric patients with BMD, coincides with puberty onset after the age of 10 years. Reference curves available for lean BMI (LBMI) for males and females by Weber et al. 20 showed a similar rapid accrual of lean body mass during puberty (11 to 16 years for males and 8 to 12 years for females) that was steeper for males, with LBMI values that were consistently greater in males than females. This is likely related to the increase in serum testosterone concentrations in males during puberty. In addition to the pathological decline in muscle mass due to muscle degeneration in patients with DMD, coexistent pubertal delay and hypogonadism in glucocorticoid‐treated patients may also contribute to decreased accrual of muscle mass, thereby amplifying the differences in lean mass findings in boys with DMD compared with healthy controls during adolescence. In contrast, non‐glucocorticoid‐treated paediatric patients with BMD who had normal puberty had ALMI values within 2 SD of healthy controls, with discrepancies between BMD and healthy controls after age 10 years reflecting underlying muscle pathology but not hypogonadism. These observations highlight the clinical need to treat glucocorticoid‐induced testosterone deficiency in patients with DMD for the potential benefit on muscle mass and motor function.
Compared with healthy controls and paediatric patients with BMD, our patients with DMD are not obese with a median BMI Z‐score of 1.1. However, with their reduced lean mass, patients with DMD with a higher percentage of body fat puts them at risk for sarcopenic obesity despite a normal BMI, as seen in the normal aging population with sarcopenia. 21 With improving survival of DMD patients with disease modifying and transformative therapies, there is a need to monitor the older glucocorticoid‐treated patients for cardiometabolic syndrome from sarcopenic obesity. 22 , 23
Patients with deletions of exons 3–7 or 45 of the dystrophin gene present clinically with the milder intermediate DMD phenotype. 24 , 25 , 26 We compared ALMI of these patients (N = 33) with the remaining patients who had a typical DMD phenotype and noted a trend towards greater ALMI for patients with DMD intermediate as compared with typical DMD, consistent with their milder clinical motor phenotype (some of whom were ambulating independently into their late teens and early twenties).
The dystrophin gene of 79 exons encodes for the three full‐length dystrophin isoforms of Dp 427 (cortical, muscle and purkinje cell) and four other shorter isoforms for dystrophin (Dp260, Dp140, Dp116 and Dp71) that are known to localize in the brain, peripheral nerve, and retina. Mutations occurring upstream of exon 30 are predicted to affect only the expression of the full‐length isoforms while preserving the expression of the shorter isoforms. Mutations between exons 31 and 44 affect the expression of Dp260 in addition to affecting the expression of full‐length isoforms, while mutations between exons 45 and 62 will additionally affect the expression of Dp140 and Dp116. Mutations downstream of exon 63 affect the expression of all dystrophin protein isoforms, including Dp71. Dp71 is known to be ubiquitous and expressed in all tissues except for skeletal muscle, where its expression is confined exclusively to myoblasts, while Dp427 is not expressed until after the cells begin myogenic differentiation and is the major isoform expressed in mature fibres. 27 While a genotype–phenotype correlation between mutations affecting Dp140 and Dp71 and neuropsychological functioning have been reported, 28 , 29 , 30 , 31 there are no known genotype–phenotype correlations for motor function with regards to mutations affecting the shorter isoforms of dystrophin, as predicted by the localization of these shorter isoforms to the central nervous system and not to skeletal muscle. Our finding of 12 patients with mutations downstream of exon 63 with a significant trend towards lower ALM as compared with those with mutations upstream from exon 63 (N = 485) needs further clinical validation with larger samples. As genomic deletions downstream of exon 55 are rare (the hot spots are in exons 2–20 [19% of total deletions] and 45–55 [73% of total deletions] 32 ) collaborative studies with larger patient registries may be needed to investigate if patients with mutations affecting all dystrophin isoforms have a faster rate of muscle loss despite the localization of the shorter isoforms in non‐skeletal muscle.
While we report clinical data that has been systematically collected on a large and generalizable cohort of 499 patients with DMD and 46 patients with BMD, who have been treated according to a standard care protocol in a single center, we note limitations in our study inherent to its retrospective nature. Our retrospective data are limited in providing information about the factors influencing pathophysiology of ALM/ALMI in DMD/BMD patients. Although the sample sizes of patients with intermediate DMD and BMD are smaller than for typical DMD, they were still sufficient and robust to provide meaningful and plausible information, with differences between groups identified by the regression models.
In conclusion, this study of ALMI in patients aged 5–23 years with DMD and BMD extends current knowledge and highlights the different rates of ALM changes in patients with typical and intermediate DMD, and paediatric patients with BMD without functional mobility deficits. These ALMI findings reflect the clinical features of earlier onset of muscle weakness in patients with DMD who exhibit progression of motor function loss from the first decade of life, in contrast to patients with BMD. Sherlock et al had recently showed that whole body DXA lean body mass measures can be used as biomarkers for disease progression and treatment effects in DMD. 33 Our findings further support the potential use of ALM and ALMI as surrogate biomarkers to characterize the severity of BMD and DMD, to inform genotype–phenotype correlations, clinical care decisions and clinical trial designs and outcomes.
Conflict of interest
None of the authors has any conflict of interest to disclose.
Supporting information
Table S1. Supporting Information
Acknowledgements
We wish to thank Jean Bange and Ray Hu for their assistance with data collection and Sarah Figueira for her technical assistance with preparation for this manuscript. All authors hereby confirm that we are in compliance with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle.
Wong B. L., Summer S., Horn P. S., Rutter M. M., Rybalsky I., Tian C., et al (2023) Appendicular lean mass index changes in patients with Duchenne muscular dystrophy and Becker muscular dystrophy, Journal of Cachexia, Sarcopenia and Muscle, 14, 2804–2812, doi: 10.1002/jcsm.13357
References
- 1. Bushby KM, Gardner‐Medwin D. The clinical, genetic and dystrophin characteristics of Becker muscular dystrophy. I Natural history. J Neurol 1993;240:98–104. [DOI] [PubMed] [Google Scholar]
- 2. Worton RG, Thompson MW. Genetics of Duchenne muscular dystrophy. Annu Rev Genet 1988;22:601–629. [DOI] [PubMed] [Google Scholar]
- 3. Koenig M, Beggs AH, Moyer M, Scherpf S, Heindrich K, Bettecken T, et al. The molecular basis for Duchenne versus Becker muscular dystrophy: correlation of severity with type of deletion. Am J Hum Genet 1989;45:498–506. [PMC free article] [PubMed] [Google Scholar]
- 4. Flanigan KM. The muscular dystrophies. Semin Neurol 2012;32:255–263. [DOI] [PubMed] [Google Scholar]
- 5. Kanda F, Fujii Y, Takahashi K, Fujita T. Dual‐energy X‐ray absorptiometry in neuromuscular diseases. Muscle Nerve 1994;17:431–435. [DOI] [PubMed] [Google Scholar]
- 6. Palmieri GM, Bertorini TE, Griffin JW, Igarashi M, Karas JG. Assessment of whole body composition with dual energy X‐ray absorptiometry in Duchenne muscular dystrophy: correlation of lean body mass with muscle function. Muscle Nerve 1996;19:777–779. [DOI] [PubMed] [Google Scholar]
- 7. Skalsky AJ, Han JJ, Abresch RT, Shin CS, McDonald CM. Assessment of regional body composition with dual‐energy X‐ray absorptiometry in Duchenne muscular dystrophy: correlation of regional lean mass and quantitative strength. Muscle Nerve 2009;39:647–651. [DOI] [PubMed] [Google Scholar]
- 8. Summer SS, Wong BL, Rutter MM, Horn PS, Tian C, Rybalsky I, et al. Age‐related changes in appendicular lean mass in males with Duchenne muscular dystrophy: a retrospective review. Muscle Nerve 2021;63:231–238. [DOI] [PubMed] [Google Scholar]
- 9. Wong BL, Rybalsky I, Shellenbarger KC, Tian C, McMahon MA, Rutter MM, et al. Long‐term outcome of interdisciplinary management of patients with Duchenne muscular dystrophy receiving daily glucocorticoid treatment. J Pediatr 2017;296–303. [DOI] [PubMed] [Google Scholar]
- 10. Zemel BS, Kalkwarf HJ, Gilsanz V, Lappe JM, Oberfield S, Shepherd JA, et al. Revised reference curves for bone mineral content and areal bone mineral density according to age and sex for black and non‐black children: results of the bone mineral density in childhood study. J Clin Endocrinol Metab 2011;96:3160–3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kalkwarf HJ, Zemel BS, Gilsanz V, Lappe JM, Horlick M, Oberfield S, et al. The bone mineral density in childhood study: bone mineral content and density according to age, sex, and race. J Clin Endocrinol Metab 2007;92:2087–2099. [DOI] [PubMed] [Google Scholar]
- 12. Schoeller DA, Tylavsky FA, Baer DJ, Chumlea WC, Earthman CP, Fuerst T, et al. QDR 4500A dual‐energy X‐ray absorptiometer underestimates fat mass in comparison with criterion methods in adults. Am J Clin Nutr 2005;81:1018–1025. [DOI] [PubMed] [Google Scholar]
- 13. Box GEP, Cox DR. An analysis of transformations. Journal of the Royal Statistical Society: Series B (Methodological) 1964;26:211–252. [Google Scholar]
- 14. Cole TJ, Green PJ. Smoothing reference centile curves: the LMS method and penalized likelihood. Stat Med 1992;11:1305–1319. [DOI] [PubMed] [Google Scholar]
- 15. Birnkrant DJ, Bushby K, Bann CM, Apkon SD, Blackwell A, Brumbaugh D, et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and neuromuscular, rehabilitation, endocrine, and gastrointestinal and nutritional management. Lancet Neurol 2018;17:251–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bello L, Gordish‐Dressman H, Morgenroth LP, Henricson EK, Duong T, Hoffman EP, et al. Prednisone/prednisolone and deflazacort regimens in the CINRG Duchenne Natural History Study. Neurology 2015;85:1048–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ricotti V, Ridout DA, Scott E, Quinlivan R, Robb SA, Manzur AY, et al. Long‐term benefits and adverse effects of intermittent versus daily glucocorticoids in boys with Duchenne muscular dystrophy. J Neurol Neurosurg Psychiatry 2013;84:698–705. [DOI] [PubMed] [Google Scholar]
- 18. Mazzone E, Vasco G, Sormani MP, Torrente Y, Berardinelli A, Messina S, et al. Functional changes in Duchenne muscular dystrophy: a 12‐month longitudinal cohort study. Neurology 2011;77:250–256. [DOI] [PubMed] [Google Scholar]
- 19. Hamuro L, Chan P, Tirucherai G, AbuTarif M. Developing a natural history progression model for Duchenne muscular dystrophy using the six‐minute walk test. CPT Pharmacometrics Syst Pharmacol 2017;6:596–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Weber DR, Moore RH, Leonard MB, Zemel BS. Fat and lean BMI reference curves in children and adolescents and their utility in identifying excess adiposity compared with BMI and percentage body fat. Am J Clin Nutr 2013;98:49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cruz‐Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European Consensus on Definition and Diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010;39:412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stenholm S, Harris TB, Rantanen T, Visser M, Kritchevsky SB, Ferrucci L. Sarcopenic obesity: definition, cause and consequences. Curr Opin Clin Nutr Metab Care 2008;11:693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dominguez LJ, Barbagallo M. The cardiometabolic syndrome and sarcopenic obesity in older persons. J Cardiometab Syndr 2007;2:183–189. [DOI] [PubMed] [Google Scholar]
- 24. Winnard AV, Mendell JR, Prior TW, Florence J, Burghes AH. Frameshift deletions of exons 3‐7 and revertant fibers in Duchenne muscular dystrophy: mechanisms of dystrophin production. Am J Hum Genet 1995;56:158–166. [PMC free article] [PubMed] [Google Scholar]
- 25. Carsana A, Frisso G, Tremolaterra MR, Lanzillo R, Vitale DF, Santoro L, et al. Analysis of dystrophin gene deletions indicates that the hinge III region of the protein correlates with disease severity. Annals of Hum Genet 2005;69:253–259. [DOI] [PubMed] [Google Scholar]
- 26. Winnard AV, Klein CJ, Coovert DD, Prior T, Papp A, Snyder P, et al. Characterization of translational frame exception patients in Duchenne/Becker muscular dystrophy. Hum Mol Genet 1993;2:737–744. [DOI] [PubMed] [Google Scholar]
- 27. Bermúdez de León M, Montañez C, Gómez P, Luz Morales‐Lázaro S, Tapia‐Ramírez V, Valadez‐Graham V, et al. Dystrophin Dp71 expression is down‐regulated during myogenesis: role of Sp1 and Sp3 on the Dp71 promoter activity. J Biol Chem 2005;280:5290–5299. [DOI] [PubMed] [Google Scholar]
- 28. Fatma D, Angeard N, Demerre B, Martie I, Benyaou R, Leturcq F, et al. 2009. Analysis of Dp71 contribution in the severity of mental retardation through comparison of Duchenne and Becker patients differing by mutation consequences on Dp71 expression. Hum Mol Genet 2009;18:3779–3394. [DOI] [PubMed] [Google Scholar]
- 29. Darmahkasih A, Rybalsky R, Tian C, Shellenbarger KC, Horn PS, Lambert JT, et al. Neurodevelopmental, behavioral, and emotional symptoms common in Duchenne muscular dystrophy. Muscle Nerve 2020;61:466–474. [DOI] [PubMed] [Google Scholar]
- 30. Felisari GF, Boneschi M, Bardoni A, Sironi M, Comi GP, Robotti M, et al. Loss of Dp140 dystrophin isoform and intellectual impairment in Duchenne dystrophy. Neurology 2000;55:559–564. [DOI] [PubMed] [Google Scholar]
- 31. Pane M, Lombardo ME, Alfieri P, D'Amico A, Bianco F, Vasco G, et al. Attention deficit hyperactivity disorder and cognitive function in Duchenne muscular dystrophy: phenotype‐genotype correlation. J Pediatr 2012;161:705–709. [DOI] [PubMed] [Google Scholar]
- 32. Li D, Adams AM, Johnsen RD, Fletcher S, Wilton SD. Morpholino oligomer‐induced dystrophin isoforms to map the functional domains in the dystrophin protein. Molecular Therapy ‐ Nucleic Acids 2020;22:263–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sherlock SP, Palmer J, Wagner KR, Abdel‐Hamid HZ, Tian C, Mah JK, et al. Dual‐energy X‐ray absorptiometry measures of lean body mass as a biomarker for progression in boys with Duchenne muscular dystrophy. Sci Rep 2022;12:18762. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Supporting Information


