Cardiac metastasis in patients with head and neck cancer is rare.1-3 Considering the cases reported from 1985 to 2020, oral cancer was the most common head and neck cancers leading to cardiac metastasis, accounting for 62% of all cases. Conversely, only 12% of oropharyngeal carcinoma cases resulted in metastatic cardiac tumors.3 Oropharyngeal carcinoma has a low incidence of distant metastases, with the lungs and liver being the most common sites and cardiac metastases being rare.4,5 A 72-year-old woman was diagnosed with left oropharyngeal carcinoma at the age of 67 years. Subsequently, the patient underwent tumor resection along with left cervical lymph node dissection. The oncological diagnosis was highly differentiated, p16 negative, pT2N2bM0, and stage IVa squamous carcinoma. Four years later, local recurrence was noted, with poorly differentiated carcinoma. The patient underwent another tumor resection, right neck lymph node dissection, and tongue reconstruction using a rectus flap. After six months, follow-up contrast-enhanced computed tomography revealed a mass in the right atrial appendage (RAA) extending into the right ventricle (Fig. 1A). Transthoracic echocardiography revealed a mobile mass between the right atrium and right ventricle (Fig. 1B). Magnetic resonance imaging revealed a connection between the tumor in the RAA and the mobile mass (Fig. 1C). Whole-body positron emission tomography-computed tomography revealed high tumor accumulation in the RAA (Fig. 1D). Since the obtained images did not reveal any other tumors, distant metastasis from the oropharyngeal carcinoma was suspected. The mass was large and mobile enough to cause severe tricuspid stenosis or pulmonary embolism, increasing the likelihood of sudden death. Treatment options included immediate surgical resection or implementation of palliative care. Surgical resection could help prevent sudden death. Palliative care would help the patient avoid physical exhaustion due to surgery and allow her to spend time at home. Therefore, the patient preferred surgical resection to palliative care. Subsequently, the surgical procedure was performed immediately. The main tumor occupied the RAA and slightly invaded the right ventricle (Fig. 2A). The mobile mass was saccular and connected to the tumor in the RAA (Fig. 2B). We performed en bloc resection of the tumor. Furthermore, the right atrial wall defect was repaired using a bovine pericardial patch (Fig. 2C). The tumor had invaded the right coronary artery. Thus, tumor resection resulted in a 4 × 2 mm oval defect on the surface of the right coronary artery. The injured area was repaired using an elliptical autologous pericardial patch. Complete tumor resection would have required removal of a part of the right ventricle. Hence, complete tumor resection was not performed to preserve cardiac function. The excision margin of the tumor was likely positive. Therefore, recurrence might be unavoidable. Postoperative computed tomography confirmed that almost all portions of the tumor had been resected. The repaired right coronary artery was patent, with no stenosis or aneurysm (Fig. 2D). The postoperative course was uneventful. Four weeks after surgery, the patient underwent radiation therapy and was subsequently discharged home on postoperative day 39, with unchanged activities of daily living. Pathological examination showed that both solid tumors (Fig. 2E) in the RAA and mobile tumors were densely infiltrated by tumor cells containing distinct nucleoli and large atypical nuclei. Both cardiac tumors resembled a previously recurrent oropharyngeal carcinoma (Fig. 2F) and were found to be cardiac metastases of the carcinoma, with supporting evidence of positive immunostaining for the epithelial markers: AE1/AE3, CAM5.2, and CK-MNF116. Histologically, the surgical margins were positive. However, the patient was readmitted for recurrent carcinoma of the right ventricular stump and metastasis to the pleura and retroperitoneum on postoperative day 89. The patient eventually expired on postoperative day 98. Recently, it has been reported that late gadolinium enhancement cardiovascular magnetic resonance could be used as a prognostic marker for cardiac metastases.6 Bussani et al reported that among 662 patients with autopsy-evidenced cardiac metastases, lesions were present in the pericardium (69%), epicardium (34%), myocardium (32%), and endocardium (5%).1 Pun et al also reported that the location of cardiac metastasis varied. Moreover, 44% of cases with cardiac metastasis involved the right ventricle, 19% involved the right atrium, 28% involved the left ventricle, 9% involved the left atrium, and 25% involved the pericardium, while multi-chamber involvement was identified in 22% of cases.7 Intraoperative pathological examination may be used to determine the need for complete resection.8 Cryocoagulation could also prevent local recurrence of cancer.8,9 Between 2017 and 2021, out of nine reported surgical cases of metastatic cardiac tumors, six had positive resection margins, one had negative intraoperative findings, and two were unknown.8-11 Generally, patients with distant metastases are considered inoperable and only palliative treatments, such as chemotherapy or tumor irradiation, are indicated.12 In this case, cardiac surgery was performed to avoid an acute cardiac event. However, the effect of palliative cardiac surgery on prognosis remains unclear.
Fig. 1.
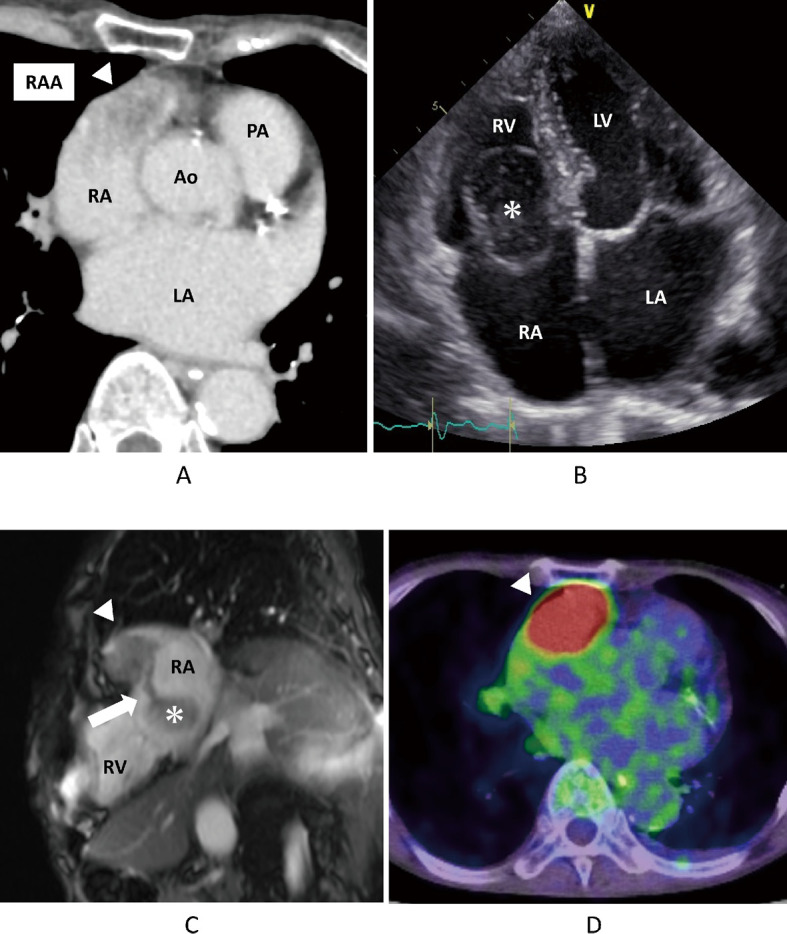
Preoperative findings
Fig. 1A: Preoperative enhanced-CT.
Fig. 1B: Preoperative transthoracic echocardiography.
Fig. 1C: Preoperative magnetic resonance image.
Fig. 1D: Preoperative positron emission tomography-CT.
The arrowheads indicate the tumor in the RAA. The asterisks indicate a mobile mass. The arrow indicates the stem between the two tumors.
CT: computed tomography
RAA: right atrial appendage
RA: right atrium
RV: right ventricle
LA: left atrium
LV: left ventricle
Ao: aorta
PA: pulmonary artery
Fig. 2.
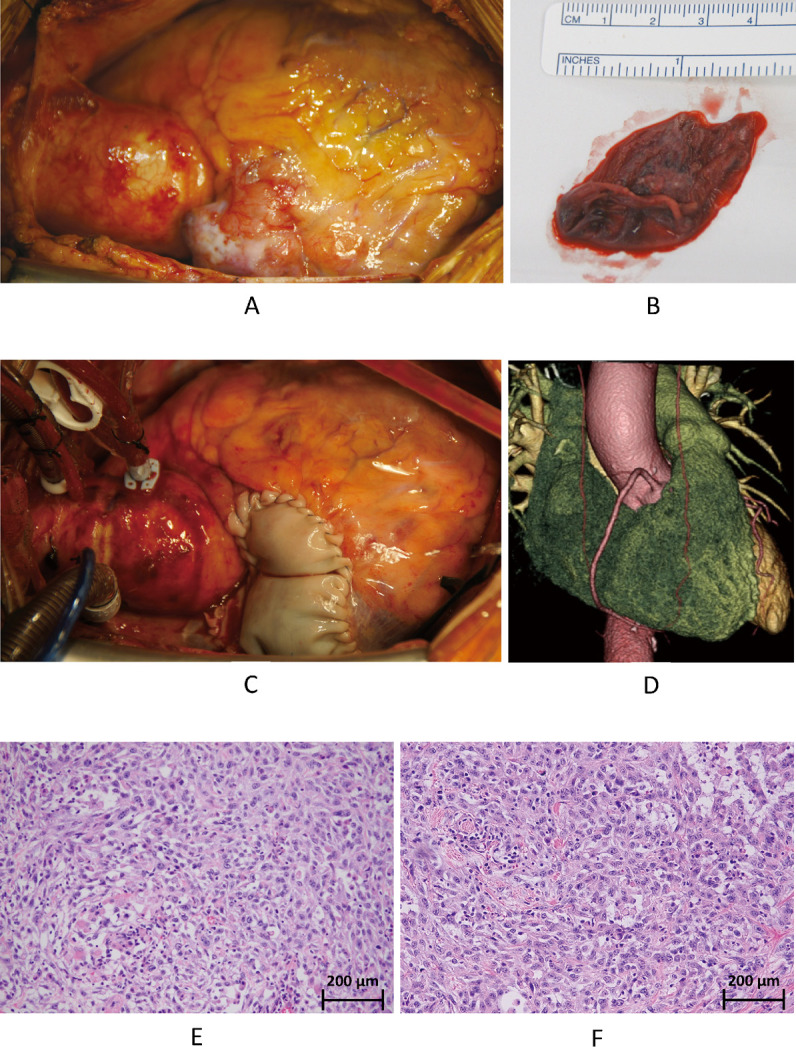
Intraoperative and postoperative findings and histopathological examination
Fig. 2A: Before tumor removal.
Fig. 2B: The mobile tumor.
Fig. 2C: Repaired right atrium.
Fig. 2D: Postoperative 3-dementional CT.
Fig. 2E: Tumor in the RAA (hematoxylin and eosin staining, ×200).
Fig. 2F: Local recurrence (hematoxylin and eosin staining, ×200).
CT: computed tomography
RAA: right atrial appendage
ACKNOWLEDGEMENTS
We thank the patient and her family for approving of the submission.
We would like to thank Editage (www.editage.com) for English language editing.
CONFLICTS OF INTEREST
Nothing to declare.
Abbreviations
- RAA
right atrial appendage
REFERENCES
- 1.Bussani R, De-Giorgio F, Abbate A, Silvestri F. Cardiac metastases. J Clin Pathol. 2007;60(1):27–34. doi: 10.1136/jcp.2005.035105. [DOI] [PMC free article] [PubMed]
- 2.Mukai K, Shinkai T, Tominaga K, Shimosato Y. The incidence of secondary tumors of the heart and pericardium: a 10-year study. Jpn J Clin Oncol. 1988;18(3):195–201. doi: 10.1093/oxfordjournals.jjco.a039238. [DOI] [PubMed]
- 3.Dewan K, Klug T, Ardestani A, Shires CB. Heart breaking diagnosis: cardiac metastases from mucosal head and neck squamous cell carcinoma. Case report and review of current literature. Otolaryngol Case Rep. 2020;17:100248. doi: 10.1016/j.xocr.2020.100248. [DOI]
- 4.Bathia R, Bahadur S. Distant metastasis in malignancies of the head and neck. J Laryngol Otol. 1987;101(9):925–928. doi: 10.1017/S0022215100102993. [DOI] [PubMed]
- 5.Pattni N, Rennie A, Hall T, Norman A. Cardiac metastasis of oral squamous cell carcinoma. BMJCase Rep. 2015;2015:bcr2015211275. doi: 10.1136/bcr-2015-211275. [DOI] [PMC free article] [PubMed]
- 6.Chan AT, Dinsfriend W, Kim J, et al. Risk stratification of cardiac metastases using late gadolinium enhancement cardiovascular magnetic resonance: prognostic impact of hypo-enhancement evidenced tumor avascularity. J Cardiovasc Magn Reson. 2021;23(1):42. doi: 10.1186/s12968-021-00727-2. [DOI] [PMC free article] [PubMed]
- 7.Pun SC, Plodkowski A, Matasar MJ, et al. Pattern and prognostic implications of cardiac metastases among patients with advanced systemic cancer assessed with cardiac magnetic resonance imaging. J Am Heart Assoc. 2016;5(5):e003368. doi: 10.1161/JAHA.116.003368. [DOI] [PMC free article] [PubMed]
- 8.Kiryu K, Kadohama T, Tanaka F, Takagi D, Yamamoto H. Significance of surgical therapy for right heart malignant tumors: a 5-case report [in Japanese]. Jpn J Phlebol. 2020;31(2):57–63. doi: 10.7134/phlebol.20-8. [DOI]
- 9.Yamaguchi R, Watanabe M, Ito F, Kataoka G. Metastatic cardiac tumor in the left atrium from esophageal cancer [in Japanese]. Kyobu Geka. 2021;74(13):1078–1083. doi: 10.15106/j_kyobu74_1078. [DOI] [PubMed]
- 10.Burazor I, Aviel-Ronen S, Imazio M, et al. Metastatic cardiac tumors: from clinical presentation through diagnosis to treatment. BMC Cancer. 2018;18(1):202. doi: 10.1186/s12885-018-4070-x. [DOI] [PMC free article] [PubMed]
- 11.Suenaga T, Kawamura N, Miyata K, Mohri M, Yamamoto H. Cardiac metastasis of rectal cancer diagnosed by intracardiac echocardiography-guided tumor biopsy and treated by surgical resection [in Japanese]. Sinzo. 2019;51(11):1175–1181. doi: 10.11281/shinzo.51.1175. [DOI]
- 12.Reynen K, Köckeritz U, Strasser RH. Metastases to the heart. Ann Oncol. 2004;15(3):375–381. doi: 10.1093/annonc/mdh086. [DOI] [PubMed]


