Abstract
Background
Sex disparities constitute a significant issue in hepatocellular carcinoma (HCC). However, the mechanism of gender dimorphism in HCC is still not completely understood.
Methods
5‐Hydroxymethylcytosine (5hmC)‐Seal technology was utilised to detect the global 5hmC levels from four female and four male HCC samples. Methylation of XIST was detected by Sequenom MassARRAY methylation profiling between HCC tissues (T) and adjacent normal liver tissues (L). The role of Tet methylcytosine dioxygenase 2 (TET2) was investigated using diethylnitrosamine (DEN)‐administered Tet2 −/− female mice, which regulated XIST in hepatocarcinogenesis. All statistical analyses were carried out by GraphPad Prism 9.0 and SPSS version 19.0 software.
Results
The results demonstrated that the numbers of 5hmC reads in the first exon of XIST from female HCC tissues (T) were remarkably lower than that in female adjacent normal liver tissues (L). Correspondingly, DNA methylation level of XIST first exon region was significantly increased in female T than in L. By contrast, no significant change was observed in male HCC patients. Compared to L, the expression of XIST in T was also significantly downregulated. Female patients with higher XIST in HCC had a higher overall survival (OS) and more extended recurrence‐free survival (RFS). Moreover, TET2 can interact with YY1 binding to the promoter region of XIST and maintain the hypomethylation state of XIST. In addition, DEN‐administered Tet2 −/− mice developed more tumours than controls in female mice.
Conclusions
Our study provided that YY1 and TET2 could interact to form protein complexes binding to the promoter region of XIST, regulating the methylation level of XIST and then affecting the expression of XIST. This research will provide a new clue for studying sex disparities in hepatocarcinogenesis.
Highlights
XIST was significantly downregulated in HCC tissues and had gender disparity.
Methylation levels in the XIST first exon were higher in female HCC tissues, but no significant change in male HCC patients.
The TET2–YY1 complex regulate XIST expression in female hepatocytes.
Other ways regulate XIST expression in male hepatocytes.
Keywords: DNA methylation, hepatocellular carcinoma, TET2, XIST
1. XIST was significantly downregulated in HCC tissues and had gender disparity.
2. Methylation levels in the XIST first exon were higher in female HCC tissues, but no significant change in male HCC patients.
3. The TET2–YY1 complex regulate XIST expression in female hepatocytes.
4. Other ways regulate XIST expression in male hepatocytes.

1. INTRODUCTION
Primary liver cancer is prevalent and has one of the highest fatality rates, which poses a massive threat to human health. 1 , 2 It is more common in men than in women. The incidence rate and mortality of liver cancer in men are 14.1 and 12.9 per 100 000, respectively, while in women, the incidence rate and mortality are 5.2 and 4.8 per 100 000, respectively. 1 Over the past few decades, researchers believed that sex hormone was the key to the gender differences in hepatocellular carcinoma (HCC). Estrogen inhibits HCC development and procession via miRNAs, DNA repair and obesity‐associated pathways. 3 , 4 , 5 , 6 Instead, androgen and androgen receptors serve a promoting role. 7 , 8 Moreover, the autosome gene CYP39A1 with female‐preferential expression is a potent inhibitor of HCC development, which provides a possible mechanism for gender differences in HCC. 9 Meanwhile, some transcripts that escape X chromosome inactivation (XCI) also lead to gender disparity of HCC, such as FTX, JPX and XIST. In HCC, previous studies found that long non‐coding RNA (lncRNA) FTX was differentially expressed between sexes, and inhibited HCC proliferation and metastasis. 10 JPX is another non‐coding gene that can activate XIST, which balances the activator and inhibitor to control XCI. 11 Studies also confirmed that JPX was decreased in HCC, resulting in inferior overall survival (OS) of HCC. 12 , 13 XIST is a lncRNA located in the XCI and an essential mediator of X inactivation. 14 Evidence accumulated suggested that XIST played critical regulatory roles in sex disparities diseases, such as autoimmune diseases, sex disparities cancers, neurological disorders and so on. 15 However, molecular mechanisms still need to be explored in depth.
Earlier studies had found an overall tendency to DNA hypomethylation in many cancers. 16 Sex‐specific methylation patterns were also extensive in the liver. 17 , 18 DNA methylation is a dynamic process accompanied by DNA demethylation. 5‐Methylcytosine (5mC) can be gradually oxidised by Tet methylcytosine dioxygenase (TET) enzymes (TET1, TET2 and TET3) to 5‐hydroxymethylcytosine (5hmC). 19 , 20 Due to the wide distribution of 5mC and 5hmC in the human genome, high chemical stability and close correlation with gene expression, some scholars have proposed that 5mC and 5hmC can be applied as an ideal biomarker for cancer diagnosis. 21
Li et al. collected samples from colorectal cancer (CC), gastric cancer (GC), HCC, thyroid, pancreatic cancer and healthy individuals, analysed 5hmC levels of circulating cell‐free DNA (cfDNA) in plasma and determined 5hmC levels of genomic DNA (gDNA) in tumour tissues and the adjacent healthy tissues. They found that the global 5hmC level of tumour gDNA was significantly downregulated. Compared with control plasma cfDNA, 5hmC levels of cancer patient's cfDNA also had lower levels. 21 Cai et al. applied 5hmC‐Seal technology to capture 5hmC sequences in cfDNA samples from 2554 Chinese subjects and found that 5hmC can be a marker for HCC staging and prognosis. 22 Interestingly, the incidence of CC, GC, HCC, thyroid and pancreatic cancer all exist in sex dimorphism. 23 , 24 Unfortunately, these studies did not distinguish the gender of tissue samples.
In this study, we first use 5hmC‐Seal technology to detect whether there are gender differences in 5hmC distribution from four female and four male HCC patients. Results demonstrated that 5hmC distribution was significantly different between female and male HCC patients. The number of 5hmC reads in the first exon of XIST from female HCC tissues was remarkably lower than in adjacent normal liver tissues. Then, we clarify the regulatory mechanism of XIST’s tumour suppressive function on female HCC. Studies may offer a novel understanding of the mechanisms of HCC gender disparity.
2. MATERIALS AND METHODS
2.1. Patient samples and clinical data
We randomly obtained 112 pairs (cohorts 1, 2 and 3) of HCC and adjacent normal liver tissues (at least 3 cm away from the tumour border and with no microscopic tumour cells) from Eastern Hepatobiliary Hospital (Shanghai, China) operated between 2009 and 2016 (including complete clinical and follow‐up data). The samples were stored at −80°C until experimental treatment performed. The clinical characteristics of patients are listed in Tables S1 and S2. This research was approved by the ethics committee of the Naval Medical University. All patients signed the informed consent.
2.2. 5hmC‐Seal sequencing
5hmC‐Seal sequencing was performed as described previously. 25 DNA extraction, DNA quantification and qualification, library preparation and quantification, sequencing and data analysis were all performed by the Yunbios platform (Yunbios).
2.3. Cell lines and cell culture
HCC‐1016 and HCC‐3527 (female primary HCC cell lines) were obtained from the tumour tissues of HCC patients. 26 Cells were cultured in a high‐glucose Dulbecco's Modified Eagle's Medium (DMEM) with 10% foetal bovine serum at 37°C, 5% CO2, and kept the humidity at a certain level.
2.4. Sequenom MassARRAY methylation
Quantitative methylation analysis of the XIST first exon region of HCC patients and the XIST exon region of mice was performed by using Sequenom MassARRAY methylation spectroscopy (CapitalBio). The primers used to amplify target regions are listed in Table S3.
2.5. hMeDIP‐qPCR and MeDIP‐qPCR assay
We performed immunoprecipitation using the Hydroxymethylated DNA Immunoprecipitation (hMeDIP) kit (ab117134, Abcam) and quantified the enrichment of 5hmC DNA at the target loci by quantitative real‐time polymerase chain reaction (qPCR). Methylated DNA Immunoprecipitation (MeDIP) kit (ab117135, Abcam) was used to quantified the enrichment of 5mC DNA at the target loci according the user manual. The primers are shown in Table S3.
2.6. TaqMan copy number assay
We determined the TET2 gene copy number by TaqMan gene copy number assay in female samples using the RNaseP gene as a standard reference gene according to the instructions. Cycling conditions were: 95°C for 10 min and 40 cycles of 95°C for 15 s and 60°C for 1 min. The copy number of target gene was analysed and calculated by CopyCaller software 2.1 (Applied Biosystems).
2.7. Chromatin immunoprecipitation
The SimpleChIP Plus Enzyme Chromatin IP Kit (#9005, CST) was used for chromatin immunoprecipitation (ChIP) assays. We incubated for 20 min at room temperature for cross‐linking of the liver tissue mixture, made from mix of finely minced human liver tissue (10 mg) and 42.5 μL of 37% formaldehyde. We homogenise suspended tissue, washed twice with ice‐cold phosphate‐buffered saline (PBS) using a B‐type Dounce homogeniser, suspended by centrifugation after adding glycine to stop the cross‐linking reaction. Then, resuspended in Kit Buffer A and incubated with micrococcal nuclease for 20 min at 37°C. We disrupted nuclei by sonication, removed debris by centrifugation and treated clarified nuclear extracts with TET2 antibody (ab94580, Abcam, 1:50) or YY1 antibody (ab109228, Abcam, 1:100). Immunoprecipitation with protein G magnetic beads was performed after incubation at 4°C overnight. ChIP‐enriched DNA was analysed by qPCR using specific primers, as described in Table S3.
2.8. Co‐immunoprecipitation
We performed co‐immunoprecipitation (Co‐IP) using the Thermo Scientific Pierce Co‐IP kit. First, immobilised TET2 antibody (ab94580, Abcam, 1:50) with AminoLink Plus conjugated resin for 2 h, rinsed the resin and incubated it with tissue lysates overnight. Then, re‐washed the resin and eluted the protein using elution buffer using a primary YY1 antibody (ab109228, Abcam, 1:1000) and 800‐CW goat anti‐rabbit immunoglobulin G (IgG) as a secondary antibody (1:5000; LI‐COR Biosciences Inc.) Finally, membranes were analysed using the Odyssey infrared scanner (LI‐COR Biosciences Inc.).
2.9. Immunofluorescence staining
We washed HCC‐1016 and HCC‐3527 cells (cultured on 13 mm round glass coverslips) three times with cold PBS. Coverslips with samples were fixed in 3% paraformaldehyde for 30 min and then permeabilised with permeabilisation solution for an additional 30 min. TET antibody (ab94580, Abcam, 1:200) and YY1 antibody (ab199815, Abcam, 1:100) were incubated with cells at room temperature for 1 h. Subsequently, cells were washed three times and incubated with goat anti‐rabbit IgG H&L (ab150077, Abcam) for 1 h. Coverslips were washed five times and then stained with Hoechst 33342 (1 mg/mL; Sigma) for 10 min. Zeiss laser confocal scanning microscope (Carl Zeiss) used to analyse cell samples.
2.10. Dual‐luciferase reporter assay
The dual‐luciferase reporter plasmid was purchased from Obio Technology (Shanghai) Corp., Ltd. YY1 binding sites on the XIST promoter was analysed by JASPAR database. The luciferase reporter plasmid construct contained the XIST promoter containing YY1 binding sites (wild type, WT) or lacking YY1 binding sites (mutant type, MUT). Dual‐luciferase reporter assay was performed using Dual‐Luciferase Reporter Assay Kit (Hanbio) accordance with the manufacturer's instructions. The sequence of XIST promoter is listed in Supporting Information.
2.11. Animal models
Shanghai Biomodel Organism Science & Technology Development Co., Ltd. was entrusted to knock out the Tet2 gene of C57BL/6J mice (Tet2 −/−). Untreated age and sex‐matched littermate C57BL/6J mice were used as corresponding WT mice. All mice were fed freely standard diet and water. Maintain a 12‐h on and 12‐h off light cycle at 24°C ± 2°C and 65% ± 5% humidity. Animal studies were approved by the Institutional Animal Care and Use Committee of Naval Medical University (Shanghai, China) and performed under the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Mouse models of HCC (WT and Tet2 −/− mice) were established by intraperitoneally injecting 30 mg/kg body weight of diethylnitrosamine (DEN; Sigma) at 15 days of age. Mice were humanely sacrificed 40 weeks after sample injection. Additional materials and methods are listed in Supporting Information.
2.12. Statistical analysis
Student's t‐test was used to analysis the difference between two sets of data. A chi‐squared test was applied to verify the relationship between XIST and clinicopathological features. For assessing the difference in patient survival, Kaplan–Meier analysis and log‐rank test were used. To identify independent influencing factors based on variables selected by Cox univariate analysis, the Cox proportional hazards model was used. Pearson or Spearman correlation coefficient was used to verify statistical correlation. According to different usage scenarios, the data are statistically significant in the following p‐values: * p < .05, ** p < .01, *** p < .001 and **** p < .0001. All statistical analyses were carried out by GraphPad Prism 9.0 and SPSS version 19.0 software.
3. RESULTS
3.1. 5hmC profiles differ between female and male HCC patients
5hmC‐Seal sequencing was utilised to generate genome‐wide 5hmC profiles from four female and four male HCC patients. Principal component analysis verified that there was a markedly difference between HCC tissues (T) and adjacent normal liver tissues (L) whether in female or male patients (Figure 1A). In addition, a striking separation can be seen between female and male patients (Figure 1A). Then, we compared the distribution of differential peaks across chromosomes. Overall, the significant difference can be observed across the X chromosome was that there were almost all 5hmC downregulated peaks in female HCC tissues (F‐T) compared to adjacent normal liver tissues (F‐L) (Figure 1B). However, there were almost all upregulated peaks located on the X chromosome in male HCC tissues (M‐T) compared to adjacent normal liver tissues (M‐L) (Figure 1C). Next, we counted the number of different peaks located on human chromosomes. A total of 533 5hmC downregulated peaks and one upregulated peak were located on the X chromosome in females (Figure 1D), but only six downregulated peaks and 33 upregulated peaks were located on the X chromosome in males (Figure 1E). So, there was also a marked difference in the number of different peaks located on the X chromosome between female and male patients.
FIGURE 1.

Characteristics of 5‐hydroxymethylcytosine (5hmC) distribution in four female and four male hepatocellular carcinoma (HCC) patients. (A) Principal component analysis (PCA) of genome‐wide hydroxymethylation level in HCC patients. Each data point represents an individual sample. The distribution of the differentia peaks between tumour tissues and adjacent normal liver tissues in female patients (B) and male patients (C) is shown across the human chromosomes. Peaks coloured in red represent significant upregulated hydroxymethylation levels in tumour tissues compared to adjacent normal liver tissues. Peaks coloured in green represent downregulated hydroxymethylation levels in tumour tissues compared to adjacent normal liver tissues. The number of differential peaks between tumour tissues and adjacent normal liver tissues in female patients (D) and male patients (E) located on human chromosomes. (F) Heatmap of 168 genes escaping X chromosome inactivation (XCI) between female and male tissue samples. (G) Integrative Genomics Viewer (IGV) snapshot of XIST shown the 5hmC signal peaks in female and male samples. (H) Normalised counts of 5hmC reads in the first exon of XIST. (I) The 5hmC level in the XIST first exon from three HCC female patients by hMeDIP assay and qPCR. (J) XIST was quantified using DNA agarose gel electrophoresis.
Given that XCI produce dosage compensation by randomly inactivating one of the X chromosomes in females. 27 Some genes can escape XCI to protect females from complete functional loss. 28 The 5hmC reads number of genes (Table S4) escaping XCI on the basis of previous research 29 was analysed. The heatmap result demonstrated that 5hmC modification signal of the XCI escape gene coding region in M‐T and M‐L was very low, and there was no difference. In contrast, the 5hmC modification signal of the XCI escape gene coding region in F‐T was lower than that in F‐L (Figure 1F).
In addition, XIST is located in the XCI and plays a vital role in random XCI. 15 , 30 The 5hmC signal peaks in XIST were markedly increased in female samples than in male samples (Figure 1G). Further analysis showed that the number of 5hmC reads in the first exon of XIST from F‐T were remarkably lower than that in F‐L. However, there was no difference between M‐T and M‐L (Figure 1H). Then, hMeDIP was used to detect 5hmC (hydroxymethylation marker) levels in the first exon of XIST in the female HCC patients. The results revealed that the 5hmC level of the XIST first exon in F‐T was lower compared to that in F‐L (Figure 1I,J).
3.2. Methylation levels in the XIST first exon were higher in female HCC tissues
Subsequently, we examined the correlation between the level of DNA methylation and XIST using the Shiny Methylation Analysis Resource Tool (SMART, http://www.bioinfo‐zs.com/smartapp/) in HCC samples. We select the probe (cg:05533223, cg:03554089 and cg:12653510) located in XIST first exon region for data analysis. An extremely negative correlation was seen between XIST expression and methylation in female HCC tissues (Figures 2A and S1A,B). In male HCC tissues, a significantly negative correlation was observed between XIST expression and methylation at the probe cg:12653510 (Figure S1C). However, there was no correlation at the other two probes (Figures 3A and S1D) in male HCC tissues.
FIGURE 2.

Methylation levels in the XIST first exon were higher in female hepatocellular carcinoma (HCC) tissues. (A) Correlation between the level of methylation and XIST in 118 female HCC tissues. Pearson correlation analysis was used to measure the correlation of female data. (B) Methylation mass spectrum primers and sequence of XIST first exon region (yellow indicates the detection sites, a total of 12). (C) Methylation levels of CpG sites in XIST first exon region from nine female patients. Quantitative methylation analysis results are shown in a colour scale: light green (0% methylation), green (50% methylation) and dark red (100% methylation). The grey circles represent the missing data at a given CpG site. (D) Mean methylation levels of CpG sites in XIST first exon region from nine pairs of female tumour tissues and adjacent normal liver tissues. (E) Methylation level of each CpG site between the two groups in XIST first exon.
FIGURE 3.
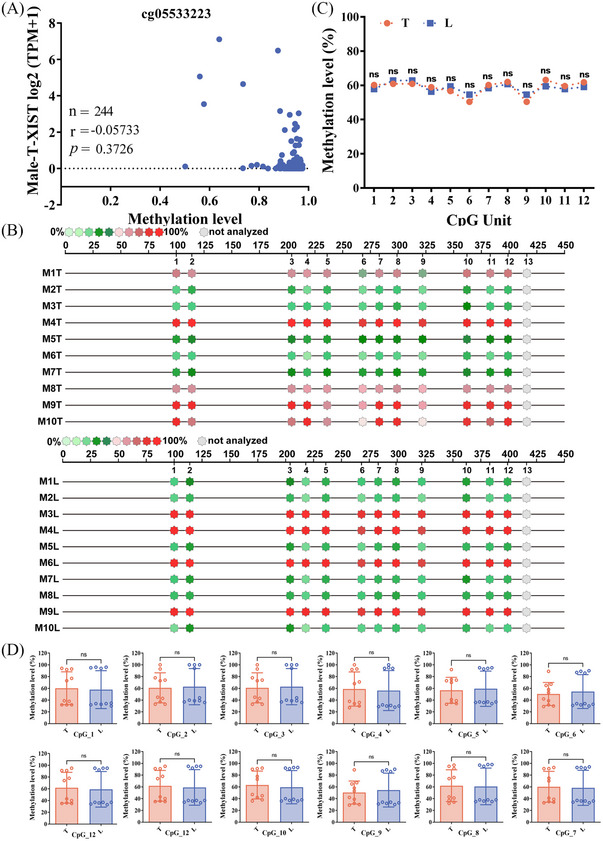
Methylation levels in the XIST first exon were no significant change in male hepatocellular carcinoma (HCC) tissues. (A) Correlation between the level of methylation and XIST in 244 male HCC tissues. Spearman correlation analysis was used to measure the correlation of female data. (B) Methylation levels of CpG sites in XIST first exon region from 10 male patients. Quantitative methylation analysis results are shown in a colour scale: light green (0% methylation), green (50% methylation) and dark red (100% methylation). The grey circles represent the missing data at a given CpG site. (C) Mean methylation levels of CpG sites in XIST first exon region in 10 pairs of male tumour tissues and adjacent normal liver tissues. (D) Methylation level of each CpG site between the two groups in the XIST first exon.
Then, the methylation level of XIST first exon region was detected by Sequenom MassARRAY methylation profiling. The detection primers (the probe cg05533223 was also located in the target sequence, Figure 2B) for XIST first exon region were designed to detect nine female HCC patients and 10 male HCC patients. Twelve Cytosine Guanine dinucleotide (CpG) sites were analysed in this region. We quantified the level of CpG site methylation in XIST first exon region and compared between T and L. For female patients, methylation levels at all CpG sites in T were conspicuously higher than in L (Figure 2C–E). Conversely, no significant change was observed in male HCC patients (Figure 3B–D).
3.3. XIST was significantly downregulated in HCC tissues and predicted a poor prognosis
Subsequently, XIST expression level was firstly detected in 30 female HCC patients (cohort 1) and 30 male HCC patients (cohort 2). The clinical information samples of cohorts 1 and 2 are obtained in Tables S1 and S2. XIST was significantly downregulated in T compared with L in both HCC female and male patients (Figure 4A). Moreover, XIST was higher in females than males, whether in T or in L (Figure 4A). Considering the effect of XIST on XCI and the high expression abundance in female liver tissue, we further detected XIST in 82 HCC female patients (cohort 3, including cohort 1). XIST was significantly downregulated in F‐T compared with F‐L (Figure 4B).
FIGURE 4.

Differential expression of XIST in hepatocellular carcinoma (HCC) patients. (A) Expression of XIST in tumour tissues (T) and adjacent normal liver tissues (L) between genders (cohorts 1 and 2). (B) Expression of XIST in T and L (cohort 3). (C and D) Kaplan–Meier survival curves depicting overall survival (OS) (C) and recurrence‐free survival (RFS) (D) of HCC female patients with diverse XIST expression. (E) Cox univariate analysis of the factors related to OS of 82 female patients. (F) Cox univariate analysis of the factors related to RFS of 82 female patients. (G) Cox multivariate analysis of the factors associated with OS of 82 female patients. (H) Cox multivariate analysis of the factors associated with RFS of 82 female patients.
Then, we tried to determine whether XIST in female HCC was associated with specific clinicopathological characteristics. Based on the median XIST expression in HCC tissues, the 82 female HCC patients were assigned to the high‐XIST expression group (n = 41) and low‐XIST expression group (n = 41). Although XIST did not have significant correlations with age, liver cirrhosis, Edmondson's grade, tumour‐node metastasis stage, tumour number, pathological satellite, microvascular invasion (MVI), HBsAg, plasma alpha‐fetoprotein level, albumin/globulin and γ‐glutamyl transpeptidase, but lower levels of XIST were associated with larger tumour diameters and poorer encapsulation status, and were also associated with more advanced Barcelona Clinic liver cancer stage and more portal vein tumour thrombosis (Table 1).
TABLE 1.
Clinical characteristics of 82 hepatocellular carcinoma patients according to XIST expression levels.
| Lnc‐XIST | ||||
|---|---|---|---|---|
| Feature | High | Low | χ 2 | p‐Value |
| All cases | 41 | 41 | ||
| Age (years) | .0499 | .8233 | ||
| ≤50 | 18 | 17 | ||
| >50 | 23 | 24 | ||
| Liver cirrhosis | .4802 | .4884 | ||
| Without | 28 | 25 | ||
| With | 13 | 16 | ||
| Edmondson's grade | 2.216 | .1366 | ||
| I/II | 6 | 2 | ||
| III/IV | 35 | 39 | ||
| TNM | .0489 | .825 | ||
| I | 20 | 19 | ||
| II/III | 21 | 22 | ||
| Tumour number | .7343 | .3915 | ||
| 1 | 35 | 32 | ||
| >1 | 6 | 9 | ||
| Tumour diameter (cm) | 4.556 | .0328 * | ||
| ≤3 | 13 | 5 | ||
| >3 | 28 | 36 | ||
| Encapsulation | 8.613 | .0033 ** | ||
| None or incomplete | 29 | 39 | ||
| Complete | 12 | 2 | ||
| Pathological satellite | .4564 | .4993 | ||
| Absent | 23 | 26 | ||
| Present | 18 | 15 | ||
| MVI | .497 | .4808 | ||
| Without | 29 | 26 | ||
| With | 12 | 15 | ||
| HBsAg | .213 | .6444 | ||
| Negative | 3 | 2 | ||
| Positive | 38 | 39 | ||
| BCLC | 4.473 | .0344 * | ||
| A | 32 | 23 | ||
| B/C | 9 | 18 | ||
| Portal vein tumour thrombus | 3.905 | .0481 * | ||
| Without | 40 | 35 | ||
| With | 1 | 6 | ||
| AFP (ug/L) | .0604 | .8058 | ||
| ≤20 | 11 | 12 | ||
| >20 | 30 | 29 | ||
| Albumin/globulin | 1.268 | .2602 | ||
| ≤1.5 | 27 | 22 | ||
| >1.5 | 14 | 19 | ||
| γ‐GT (U/L) | .2253 | .635 | ||
| ≤50 | 29 | 27 | ||
| >50 | 12 | 14 | ||
Note: The median expression level was used as the cutoff. For analysis of correlation between XIST levels and clinical features, Pearson's chi‐squared tests were used. Results were considered statistically significant at p < .05.
Abbreviations: AFP, alpha‐fetoprotein; BCLC, Barcelona Clinic liver cancer staging system; Lnc‐XIST, long non‐coding RNA XIST; MVI, microvascular invasion; TNM, tumour‐node metastasis; γ‐GT, γ‐glutamyl transpeptidase.
Bold values represent significant differences.
p < .05.
p < .01.
Furthermore, as a result of Kaplan–Meier survival analysis, patients with higher XIST in HCC had a conspicuously better prognosis than those with lower XIST, with a higher OS (p = .0015) (Figure 4C) and more extended recurrence‐free survival (RFS) (p = .0006) (Figure 4D). A consistent conclusion was reached with our clinical results that female patients with higher XIST had better OS and RFS by using the Kaplan–Meier plotter database (https://kmplot.com/analysis/index.php?p = service&cancer = liver_rnaseq) (Figure S2).
Cox univariate analysis showed that tumour diameter, pathological satellite and XIST expression levels in HCC patients were conspicuously associated with OS (Figure 4E). Only the XIST expression level was conspicuously associated with RFS in HCC patients (Figure 4F). Cox multivariate analysis showed that XIST expression levels and pathological satellites were risk factors for OS and independent (Figure 4G). XIST expression levels also play the same role for HCC patients' RFS (Figure 4H).
Subsequently, we established stable XIST‐silenced HCC‐1016 cells by infecting XIST‐silenced lentivirus. XIST was validated by qPCR (Figure S3A). Results showed that XIST knockdown considerably promoted cell proliferation by Cell Counting Kit‐8 (CCK8) assay and EdU (5‐ethynyl‐2'‐deoxyuridine) immunofluorescence staining (Figure S3B–D). Previous study also found that targeted deletion of Xist in the blood compartment of mice can induce an aggressive, lethal blood cancer. 31 Therefore, we believe that XIST may play a cancer‐suppressing role in HCC and contribute partially to the sex difference of HCC due to different liver expressions in different sexes.
3.4. TET2 is significantly downregulated in HCC tissues
Methyltransferases and demethylases (TETs) maintain the dynamic balance of DNA. TETs catalyse the oxidation of 5mC to 5hmC, which begins the demethylation of active DNA. Considering the decreased levels of 5hmC modification and the higher methylation levels in the first exon of XIST in female HCC tissues (Figures 1I,J and 2C–E), the expression of TET1, TET2 and TET3 were further examined in cohort 1. Only TET2 was significantly downregulated in T compared with L (Figure 5A). The immunohistochemistry results also confirmed that TET2 was downregulated in HCC tissues (Figure 5B).
FIGURE 5.

Tet methylcytosine dioxygenase 2 (TET2) is significantly downregulated in hepatocellular carcinoma (HCC) tissues. (A) Expression of TET1, TET2 and TET3 of tumour tissues (T) and adjacent normal liver tissues (L) in 30 females (cohort 1). (B) Histochemistry score of TET2 in 30 pairs females T and L (right) and representative samples (left). (C) The deletion of TET2 sCNA in various cancer types was obtained from the TIMER2.0 database. (D) The data were analysed from GSE38323. (E) The copy number of TET2 in cohort 1 tumour tissues and adjacent normal liver tissues. (F) Correlation between the TET2 expression level and copy number in cohort 1 tumour tissues. Correlation between the expression level of TET2 and XIST in cohort 1 tumour tissues (G) and adjacent normal liver tissues. (H) Pearson correlation analysis was used to measure the correlation.
In recent years, some articles have reported that copy number variation can activate oncogenes and inactivate tumour suppressor genes, which are considered a key factor in many types of cancer, including liver cancer. 32 , 33 , 34 , 35 We found that the copy number of TET2 was deficient in liver hepatocellular carcinoma (Figure 5C) by the TIMER2.0 database (http://timer.comp‐genomics.org/timer/). To explore the alteration of TET2 genomic copy number in HCC, we used published data (GSE38323) to perform a data mining process. 36 The results showed that deletion of the TET2 genome was present in 50% of female HCC tissues (p = 4.40E‐10, n = 52), and deletion of the TET2 genome occurred in 40.60% of male HCC tissues (p = 2.85E‐34, n = 234) (Figure 5D). We then assayed the copy number change of TET2 in cohort 1 HCC samples (females) using TaqMan copy number. These results showed that the copy number of TET2 genome is lacking in HCC tissues (Figure 5E), and its copy number was positively correlated with TET2 mRNA expression level in HCC tissues (r = .4835, p = .0068, Figure 5F). In addition, the mRNA expression level of TET2 and XIST in T and L from cohort 1 was detected. The result demonstrated that XIST expression level was positively correlated with TET2 mRNA expression, whether in T (r = .4474, p = .0132, Figure 5G) or in L (r = .8946, p < .0001, Figure 5H). Therefore, we speculated that the downregulated expression of TET2 was caused by the deletion of copy number, which might increase the methylation level of XIST first exon and lead to the downregulated expression of XIST in female HCC.
3.5. TET2 form complexes with YY1 binding to the promoter region of XIST and regulating the methylation level of XIST
However, how does TET2 regulate the methylation level of XIST? A published study showed that loss of transcription factor YY1 could prevent XIST expression, and YY1 was bound solely to the unmethylated XIST allele. 37 Consequently, we suspected whether TET2 could form complexes with YY1 binding to the promoter region of XIST and regulating the methylation level of XIST. Firstly, to validate whether YY1 directly binds to the XIST promoter, the JASPAR database was analysed for YY1 binding sites within the XIST gene promoter sequences (Figure 6A). Then, the dual‐luciferase reporter plasmid system was constructed which contained the full length of XIST promoter region containing YY1 binding sites (WT) or lacking YY1 binding sites (MUT). The results showed that the plasmids co‐transfected with YY1 and XIST promoter (MUT) could significantly decrease the expression abundance of luciferase in HCC‐1016 cell line (Figure 6B). It suggested that YY1 could bind to the promoter of XIST and promote transcription. Moreover, we performed a ChIP‐qPCR assay in female adjacent normal liver tissues. The results demonstrated that TET2 and YY1 could specifically bind to the XIST promoter (Figure 6C,D). Furthermore, physical contact between TET2 and YY1 was further suggested by Co‐IP experiments performed using female adjacent normal liver tissues. It was shown that these two proteins could be co‐precipitated (Figure 6E). In addition, the spatial distribution of TET2 and YY1 coincides and interacts with each other by immunofluorescence assays in HCC‐1016 and HCC‐3527 cells (Figure 6F). To demonstrate whether the TET2–YY1 complex can regulate the expression of XIST, we knocked down YY1 in HCC‐1016 cells by transfecting siRNA (Figure 6G,H). The result showed that knockdown YY1 significantly downregulated the expression level of XIST (Figure 6I), and the methylation level of its promoter region was increased by MeDIP analysis (Figure 6J,K). Taken together, TET2 could form complexes with YY1 binding to the promoter region of XIST in female cells and regulating the methylation level of XIST, thereby affecting the expression of XIST.
FIGURE 6.

Tet methylcytosine dioxygenase 2 (TET2) forms complexes with YY1 binding to the promoter region of XIST. (A) Analysis the binding sites of YY1 in human XIST promoter region based on the JASPAR database (left) and the binding sites in the XIST promoter region (right, yellow indicates the detection sites, red font indicates the primers of XIST promoter region). (B) Fluorescence intensity expressed by dual luciferin of plasmid containing either the wild type (WT) or MUT. (C) Bar plot representing qPCR values of the chromatin immunoprecipitation (ChIP) results in female adjacent normal liver tissues. (D) XIST was quantified using DNA agarose gel electrophoresis. (E) Co‐immunoprecipitation (Co‐IP) of YY1 by TET2 was determined. The female adjacent normal liver tissues were immunoprecipitated with TET2 antibody, followed by western blotting assays for YY1 antibody. (F) Micrographs of representative immunofluorescence staining for TET2 (green) and YY1 expression (red) in hepatocellular carcinoma (HCC)‐1016 (up) and HCC‐3527 (down) cells. The arrow indicates colocalisation of TET2 and YY1. Scale bar, 50 μm. (G and H) The expression of YY1 in HCC‐1016 cells (knocked down YY1 in HCC‐1016 cells by transfecting siRNA) by western blot analysis. (I) Expression of YY1 and XIST in HCC‐1016 cells (knocked down YY1 in HCC‐1016 cells by transfecting siRNA) by qPCR. (J) XIST was quantified using DNA agarose gel electrophoresis. (K) The 5‐methylcytosine (5mC) level in the XIST promoter region by MeDIP assay and qPCR.
3.6. TET2 functions as a tumour suppressor in hepatocarcinogenesis
To explore the role of TET2 in hepatocarcinogenesis, Tet2 −/− mice were constructed. The expression level of Tet2 and Xist in the liver tissue of female mice was detected. Figure 7A,B indicates that Tet2 and Xist in Tet2 −/− mice were conspicuously lower than in WT mice. Moreover, we established a chronic DEN model by injecting liver carcinoma pro‐DEN into WT and Tet2 −/− female and male mice and sacrificing them 40 weeks after DEN administration. The number of tumours in each female mouse was measured. Macroscopic liver analysis revealed that DEN‐administered Tet2 −/− female mice had more tumours than in controls (Figure 7C,D), and tumour incidence in male DEN‐induced mice was remarkably higher than in female mice (Figure S4 and Table S5). Next, the protein expression of cell proliferation (Ki67) and vessel density (CD31) in the liver cancer tissues from DEN‐administered female Tet2 −/− mice and WT mice were examined. It exhibited a significantly increased proliferation rate and tumour vessel density in the liver cancer tissues compared with the DEN‐administered WT mice (Figure 7E,F). Methylation of CpG sites of the Xist first exon region in liver tissues between 10 WT and 10 Tet2 −/− female mice was quantified by Sequenom MassARRAY methylation profiling. All 13 CpG sites in the first exon region were divided into 11 CpG site units (Figure 7G). As shown in Figure 7H,I, methylation levels in Tet2 −/− mice were conspicuously increased over the corresponding methylation levels in WT mice. All results suggested that TET2 in hepatocytes could inhibit DNA methylation modifications in the first exon region of XIST and maintain high levels of XIST expression in female liver tissues, thereby inhibiting tumourigenesis.
FIGURE 7.

Tet methylcytosine dioxygenase 2 (TET2) plays a protective role in hepatocellular carcinoma (HCC). Expression of Tet2 (A) and Xist (B) in female wild‐type (WT) mice (n = 9) and Tet2 −/− mice (n = 7). (C) The tumour number per mice in diethylnitrosamine (DEN)‐administered female mice. (D) Representative pictures of the livers (arrows depict tumours). Immunohistochemistry (IHC) detection and quantification of Ki67 (E) and CD31 (F) protein expression in tumours from DEN‐administered female mice. (G) Methylation mass spectrometry detection product sequence of Xist first exon region (yellow indicates the detection sites, a total of 13). (H) Mean methylation levels of CpG sites in Xist first exon region in liver tissues between 10 WT mice and 10 TET2 −/− mice of females. (I) Methylation level of each CpG site between WT mice and 10 TET2 −/− mice of female in Xist first exon.
4. DISCUSSION
As our study confirmed, the 5hmC distribution was significantly different between female and male HCC patients. The number of 5hmC reads in the first exon of XIST from female tumour tissues were remarkably lower than in female adjacent liver tissues. On further analysis, XIST was downregulated in tumour tissues, which in females was higher than in males. XIST was positively correlated with OS and RFS in female HCC tissues. We found that TET2‐mediated DNA demethylation is vital in the molecular mechanism of XIST expression. TET2 achieves XIST’s DNA hypomethylation state by binding to the YY1 transcription factor in female normal liver tissue. Furthermore, the decrease of TET2 in female HCC tissue was owing to the loss of TET2 copy number.
Epidemiological data suggest that males had a greater number of liver cancer than females. Generally, the androgen axis promotes hepatocarcinogenesis, while the estrogen axis acts as a tumour suppressor. However, clinical trials of sex hormone‐specific therapies for HCC have not yielded satisfactory results. 38 , 39 There are even some conflicting findings. Ligand‐activated androgen receptors may inhibit HCC metastasis by activating the p38 pathway to induce apoptosis. 40 It has also been shown that androgen receptors could improve cell adhesion by activating the AKT signalling pathway, thereby reducing cell migration. 41 It can be seen that there may be deeper reasons behind the gender differences in HCC.
In addition to sex hormones, there is a very different event between the two sexes—XCI. lncRNA XIST regulates the formation of XCI in female mammals to balance the X‐linked gene expression between sexes. 27 , 42 However, XCI is incomplete, with approximately 23% of genes escaping from XCI, causing higher expression levels in XX female XCI escapers than in XY males. 29 Our results indicate that the sex dimorphism associated with XIST in hepatocarcinogenesis is mainly reflected in the presence of two X chromosomes in female hepatocytes, one of which achieves XCI under DNA methylation regulation. 43 XIST is an XCI escape gene that needs to be highly expressed in hepatocytes to help achieve XCI in female cells, and its promoter region (including the first exon region) has a lower level of DNA methylation. The DNA methylation level of the XIST promoter region (including the first exon region) is significantly increased in female HCCs (Figure 2C–E), leading to a decrease in its expression level (Figure 4B). In male hepatocytes, there is only one X chromosome, which does not require XCI. The DNA methylation level of the XIST promoter region (including the first exon region) is already relatively high (Figure 3B–D) in male hepatocytes. No significant changes were found in the comparison between male hepatocytes and HCC cells (Figure 3B–D). These escapes from XCI may contribute to gender‐biased disease. 10 , 44 Perhaps, genes that escape from XCI not only contribute to achieving XCI, 45 but also have other functions, such as inhibiting tumour development. 46 Compared to males, female hepatocytes need to reduce the high expression of XCI escape genes (such as XIST), which is actually equivalent to a higher HCC threshold for females than males. Our results suggest that the low expression of TET2 caused by genome copy number deletion in female HCC cells may downregulate the expression of XIST through DNA demethylation.
Why this regulatory relationship does not hold true in male hepatocytes? Our identified transcription factor YY1, which interacts with TET2, may play a crucial role in this process. Published study has shown that YY1 only binds to unmethylated XIST alleles. 37 Our results indicate that high methylation levels in the XIST promoter region (Figure 3B–D) may hinder the binding of YY1, and the binding of TET2 in this region naturally decreases in male hepatocytes (Figure S5), and the expression level of TET2 naturally does not regulate the expression of XIST. The decrease in XIST expression in male HCC tissues (Figure 4A) may be caused by other reasons, such as RNA post‐transcriptional modifications, etc. 47 , 48 In female hepatocytes, the XIST promoter region is in a low methylation state (Figure 2C–E), and YY1–TET2 complex may bind in this region, maintaining the low methylation state. In female HCC cells, low expression of TET2 is caused by copy number deletion (Figure 5), resulting in a decrease in 5hmC levels and an increase in 5mC levels in this region, thereby reducing the expression of XIST. It can be seen that the 5hmC and 5mC states of the XCI escape gene XIST promoter region are inconsistent, which may be the reason for the different susceptibility of males and females to HCC.
The role of XIST on tumourigenesis and progression is complicated and indistinguishable. Yildirim et al. have demonstrated that deleting XIST is sufficient to induce an aggressive, lethal blood cancer in mice. 31 A recent study showed that loss of XIST hampers mammary stem cell differentiation and promotes tumourigenesis. 46 Some researchers have explicitly proposed that XIST is involved in carcinogenesis and tumour suppressor pathways in tumour pathology. 49 There were also many studies indicating that XIST had the role of the oncogene. An analysis of published sequencing data on cancer tissues revealed that XIST was activated and expressed in various male tumours characteristics of XCI. 30 Much evidence shows that XIST plays a vital role in the proliferation, invasion, migration, apoptosis and chemosensitivity of non‐small‐cell lung cancer (NSCLC) cells. 50 , 51 XIST’s role in HCC is even more chaotic. XIST was less expressed in HCC tissues and inhibited HCC cell proliferation and metastasis by specifically regulating miR‐92b. 12 , 52 , 53 It has also reported that XIST was upregulated in HCC tissues. 54 , 55 , 56 The reason may be that some studies did not accurately distinguish the gender of tissue sample sources (some studies put together the data of male and female patients), or the sample size was too small. In our research, detection data from male and female HCC tissues were processed and analysed separately (Figure 4A). Results confirmed that the expression of XIST in females was much higher than in males. In a large sample size (n = 82) cohort of female patients with HCC, we further clarified that XIST was downregulated in HCC tissues (Figure 4B). Female patients with lower XIST expression had significantly poorer prognoses than those patients with higher XIST expression (Figure 4C–H). Downregulating XIST expression considerably promoted cell proliferation (Figure S3B–D). From the above results, it can be speculated that XIST may work like a tumour suppressor in female HCC.
How to regulate the transcriptional activation of XIST is also an interesting issue for researchers. With the deepening of XCI research, lncRNA regulators targeting XIST have been discovered, such as JPX and TSIX, which can promote or inhibit, expanding the scope of XCI research. 57 , 58 However, people do not know much about the regulatory mechanism of XIST expression under pathological conditions. Thanks to the SMART database, we established the relationship between the level of DNA methylation and XIST in female HCC tissues but not in males.
In summary, this study shows that the downregulation of XIST expression can lead to poor prognosis in female HCC, and TET2 can form a complex with YY1 and bind to the XIST promoter region, maintain the hypomethylation of this region, as well as promote XIST expression. All the above results suggest that XIST may act as tumour suppressor in female hepatocarcinogenesis. Of course, our study also has study limitations, such as the clinical samples only derived from the same hospital. Moreover, it is unknown whether the decreased expression of XIST induces HCC in vivo. We cannot definitively state that XIST down‐expression in female HCC cases is causal for HCC. The questions will be explored in the following study.
5. CONCLUSIONS
This study found that the expression level of XIST regulated by the YY1–TET2 complex is associated with the prognosis of female HCC patients. These results showed that XIST may act as a tumour suppressor gene in female hepatocarcinogenesis. A better understanding of the XCI regulatory network may help to develop relevant targeted therapy for clinical treatment HCC in females.
AUTHOR CONTRIBUTIONS
Fu Yang, Hui Liu and Shuhan Sun designed the study. Zhihui Dai and Sijie Wang performed data analysis and prepared the manuscript. Xinggang Guo collected and analysed clinical data. Yuefang Wang, Haozan Yin, Chenyang Mu and Jan Tan performed the experiments. All authors approved the submitted version.
CONFLICT OF INTEREST STATEMENT
The authors declare they have no conflicts of interest.
ETHICS STATEMENT
This research was approved by the ethics committee of the Naval Medical University. All patients were consented by an informed consent process.
Supporting information
Figure S1 The correlation between the methylation level and the relative expression of XIST in hepatocellular carcinoma (HCC) tissues using the Shiny Methylation Analysis Resource Tool (SMART) date. The correlation between the methylation level and the relative expression of XIST in 118 female (A) and in 244 male (C) HCC tissues (probe:cg12653510). The correlation between the methylation level and the relative expression of XIST in 113 female (B) and in 235 male (D) HCC tissues (probe:cg03554089). The correlation of female date was measured by Pearson correlation analysis. The correlation of male date was measured by Spearman correlation analysis.
Figure S2 The prognosis of patients with higher expression of XIST are better from the database of Kaplan–Meier plotter. (A) The overall survival of 118 female liver cancer patients. (B) The recurrence‐free survival of 106 female liver cancer patients. (C) The overall survival of 246 male liver cancer patients. (D) The recurrence‐free survival of 210 male liver cancer patients.
Figure S3 XIST suppresses hepatocellular carcinoma (HCC)‐1016 cell proliferation. (A) Relative expression level of XIST in XIST‐silenced HCC‐1016 cells compared with control cells determined by real‐time polymerase chain reaction (PCR). (B) Proliferation of HCC‐1016 cells assessed by CCK8 assay. XIST silencing promote cell proliferation. (C) EdU immunofluorescence staining of HCC‐1016 cells. (D) The percentage of EdU‐positive nuclei.
Figure S4 Representative pictures of the livers (arrows depict tumours) from diethylnitrosamine (DEN)‐administered male mice.
Figure S5 TET2 and YY1 could not binding to the promoter region of XIST in male adjacent normal liver tissues by chromatin immunoprecipitation (ChIP)‐qPCR. (A) Bar plot representing qPCR values of the ChIP results in male adjacent normal liver tissues. (B) XIST was quantified using DNA agarose gel electrophoresis.
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
ACKNOWLEDGEMENTS
The research was supported by the National Key Research and Development Program of China (2023YFC2505900, 2016YFC1302303), the National Natural Science Foundation of China (nos. 81972657, 81830085 and 81972575).
Dai Z, Wang S, Guo X, et al. Gender dimorphism in hepatocarcinogenesis—DNA methylation modification regulated X‐chromosome inactivation escape molecule XIST . Clin Transl Med. 2023;13:e1518. 10.1002/ctm2.1518
Zhihui Dai, Sijie Wang and Xinggang Guo contributed equally to this study.
Contributor Information
Hui Liu, Email: liuhuigg@hotmail.com.
Fu Yang, Email: yangfusq1997@smmu.edu.cn.
DATA AVAILABILITY STATEMENT
Data supporting this study will be made available upon reasonable request.
REFERENCES
- 1. Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209‐249. [DOI] [PubMed] [Google Scholar]
- 2. Zheng B, Zhu Y‐J, Wang H‐Y, Chen L. Gender disparity in hepatocellular carcinoma (HCC): multiple underlying mechanisms. Sci China Life Sci. 2017;60:575‐584. [DOI] [PubMed] [Google Scholar]
- 3. Jiang R, Deng L, Zhao L, et al. miR‐22 promotes HBV‐related hepatocellular carcinoma development in males. Clin Cancer Res. 2011;17(17):5593‐5603. [DOI] [PubMed] [Google Scholar]
- 4. Liu W‐H, Yeh S‐H, Lu C‐C, et al. MicroRNA‐18a prevents estrogen receptor‐alpha expression, promoting proliferation of hepatocellular carcinoma cells. Gastroenterology. 2009;136(2):683‐693. [DOI] [PubMed] [Google Scholar]
- 5. Tummala KS, Gomes AL, Yilmaz M, et al. Inhibition of de novo NAD(+) synthesis by oncogenic URI causes liver tumorigenesis through DNA damage. Cancer Cell. 2014;26(6):826‐839. [DOI] [PubMed] [Google Scholar]
- 6. Shen M, Shi H. Estradiol and estrogen receptor agonists oppose oncogenic actions of leptin in HepG2 cells. PLoS One. 2016;11(3):e0151455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wu M‐H, Ma W‐L, Hsu C‐L, et al. Androgen receptor promotes hepatitis B virus‐induced hepatocarcinogenesis through modulation of hepatitis B virus RNA transcription. Sci Transl Med. 2010;2(32):32ra35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang L, Wu J, Wu Q, et al. Sex steroid axes in determining male predominance in hepatocellular carcinoma. Cancer Lett. 2023;555:216037. [DOI] [PubMed] [Google Scholar]
- 9. Ji F, Zhang J, Liu N, et al. Blocking hepatocarcinogenesis by a cytochrome P450 family member with female‐preferential expression. Gut. 2022;71(11):2313‐2324. [DOI] [PubMed] [Google Scholar]
- 10. Liu F, Yuan J‐H, Huang J‐F, et al. Long noncoding RNA FTX inhibits hepatocellular carcinoma proliferation and metastasis by binding MCM2 and miR‐374a. Oncogene. 2016;35(41):5422‐5434. [DOI] [PubMed] [Google Scholar]
- 11. Tian D, Sun S, Lee JT. The long noncoding RNA, Jpx, is a molecular switch for X chromosome inactivation. Cell. 2010;143(3):390‐403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ma W, Wang H, Jing W, et al. Downregulation of long non‐coding RNAs JPX and XIST is associated with the prognosis of hepatocellular carcinoma. Clin Res Hepatol Gastroenterol. 2017;41(2):163‐170. [DOI] [PubMed] [Google Scholar]
- 13. Lin X‐Q, Huang Z‐M, Chen X, Wu F, Wu W. XIST induced by JPX suppresses hepatocellular carcinoma by sponging miR‐155‐5p. Yonsei Med J. 2018;59(7):816‐826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Żylicz JJ, Bousard A, Žumer K, et al. The implication of early chromatin changes in X chromosome inactivation. Cell. 2019;176(1‐2):182‐197.e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li J, Ming Z, Yang L, Wang T, Liu G, Ma Q. Long noncoding RNA XIST: mechanisms for X chromosome inactivation, roles in sex‐biased diseases, and therapeutic opportunities. Genes Dis. 2022;9(6):1478‐1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nishiyama A, Nakanishi M. Navigating the DNA methylation landscape of cancer. Trends Genet. 2021;37(11):1012‐1027. [DOI] [PubMed] [Google Scholar]
- 17. Reizel Y, Spiro A, Sabag O, et al. Gender‐specific postnatal demethylation and establishment of epigenetic memory. Genes Dev. 2015;29(9):923‐933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. García‐Calzón S, Perfilyev A, De Mello VD, Pihlajamäki J, Ling C. Sex differences in the methylome and transcriptome of the human liver and circulating HDL‐cholesterol levels. J Clin Endocrinol Metab. 2018;103(12):4395‐4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tahiliani M, Koh KP, Shen Y, et al. Conversion of 5‐methylcytosine to 5‐hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science. 2009;324(5929):930‐935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ito S, Shen L, Dai Q, et al. Tet proteins can convert 5‐methylcytosine to 5‐formylcytosine and 5‐carboxylcytosine. Science. 2011;333(6047):1300‐1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li W, Zhang X, Lu X, et al. 5‐Hydroxymethylcytosine signatures in circulating cell‐free DNA as diagnostic biomarkers for human cancers. Cell Res. 2017;27(10):1243‐1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cai J, Chen L, Zhang Z, et al. Genome‐wide mapping of 5‐hydroxymethylcytosines in circulating cell‐free DNA as a non‐invasive approach for early detection of hepatocellular carcinoma. Gut. 2019;68(12):2195‐2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Haupt S, Caramia F, Klein SL, Rubin JB, Haupt Y. Sex disparities matter in cancer development and therapy. Nat Rev Cancer. 2021;21(6):393‐407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pizzato M, Li M, Vignat J, et al. The epidemiological landscape of thyroid cancer worldwide: GLOBOCAN estimates for incidence and mortality rates in 2020. Lancet Diabetes Endocrinol. 2022;10(4):264‐272. [DOI] [PubMed] [Google Scholar]
- 25. Han D, Lu X, Shih AH, et al. A highly sensitive and robust method for genome‐wide 5hmC profiling of rare cell populations. Mol Cell. 2016;63(4):711‐719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yuan S‐X, Wang J, Yang F, et al. Long noncoding RNA DANCR increases stemness features of hepatocellular carcinoma by derepression of CTNNB1. Hepatology. 2016;63(2):499‐511. [DOI] [PubMed] [Google Scholar]
- 27. Loda A, Collombet S, Heard E. Gene regulation in time and space during X‐chromosome inactivation. Nat Rev Mol Cell Biol. 2022;23(4):231‐249. [DOI] [PubMed] [Google Scholar]
- 28. Dunford A, Weinstock DM, Savova V, et al. Tumor‐suppressor genes that escape from X‐inactivation contribute to cancer sex bias. Nat Genet. 2017;49(1):10‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tukiainen T, Villani A‐C, Yen A, et al. Landscape of X chromosome inactivation across human tissues. Nature. 2017;550(7675):244‐248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sadagopan A, Nasim IT, Li J, Achom M, Zhang C‐Z, Viswanathan SR. Somatic XIST activation and features of X chromosome inactivation in male human cancers. Cell Syst. 2022;13(11):932‐944.e5. [DOI] [PubMed] [Google Scholar]
- 31. Yildirim E, Kirby JE, Brown DE, et al. Xist RNA is a potent suppressor of hematologic cancer in mice. Cell. 2013;152(4):727‐742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yu K, Fan J, Ding X, et al. Association study of a functional copy number variation in the WWOX gene with risk of gliomas among Chinese people. Int J Cancer. 2014;135(7):1687‐1691. [DOI] [PubMed] [Google Scholar]
- 33. Jia D, Wei L, Guo W, et al. Genome‐wide copy number analyses identified novel cancer genes in hepatocellular carcinoma. Hepatology. 2011;54(4):1227‐1236. [DOI] [PubMed] [Google Scholar]
- 34. Park C, Kim J‐I, Hong SN, et al. A copy number variation in PKD1L2 is associated with colorectal cancer predisposition in Korean population. Int J Cancer. 2017;140(1):86‐94. [DOI] [PubMed] [Google Scholar]
- 35. Zhou C‐C, Yang F, Yuan S‐X, et al. Systemic genome screening identifies the outcome associated focal loss of long noncoding RNA PRAL in hepatocellular carcinoma. Hepatology. 2016;63(3):850‐863. [DOI] [PubMed] [Google Scholar]
- 36. Wang K, Lim HY, Shi S, et al. Genomic landscape of copy number aberrations enables the identification of oncogenic drivers in hepatocellular carcinoma. Hepatology. 2013;58(2):706‐717. [DOI] [PubMed] [Google Scholar]
- 37. Makhlouf M, Ouimette J‐F, Oldfield A, Navarro P, Neuillet D, Rougeulle C. A prominent and conserved role for YY1 in Xist transcriptional activation. Nat Commun. 2014;5:4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Forbes A, Wilkinson ML, Iqbal MJ, Johnson PJ, Williams R. Response to cyproterone acetate treatment in primary hepatocellular carcinoma is related to fall in free 5 alpha‐dihydrotestosterone. Eur J Cancer Clin Oncol. 1987;23(11):1659‐1664. [DOI] [PubMed] [Google Scholar]
- 39. Matsuura B, Taniguchi Y, Ohta Y. Effect of antiandrogen treatment on chemical hepatocarcinogenesis in rats. J Hepatol. 1994;21(2):187‐193. [DOI] [PubMed] [Google Scholar]
- 40. Ma W‐L, Hsu C‐L, Yeh C‐C, et al. Hepatic androgen receptor suppresses hepatocellular carcinoma metastasis through modulation of cell migration and anoikis. Hepatology. 2012;56(1):176‐185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ma W‐L, Jeng L‐B, Lai H‐C, Liao P‐Y, Chang C. Androgen receptor enhances cell adhesion and decreases cell migration via modulating β1‐integrin‐AKT signaling in hepatocellular carcinoma cells. Cancer Lett. 2014;351(1):64‐71. [DOI] [PubMed] [Google Scholar]
- 42. Arnold AP. X chromosome agents of sexual differentiation. Nat Rev Endocrinol. 2022;18(9):574‐583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Brockdorff N, Bowness JS, Wei G. Progress toward understanding chromosome silencing by Xist RNA. Genes Dev. 2020;34(11‐12):733‐744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pan J, Fang S, Tian H, et al. lncRNA JPX/miR‐33a‐5p/Twist1 axis regulates tumorigenesis and metastasis of lung cancer by activating Wnt/β‐catenin signaling. Mol Cancer. 2020;19(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Aguilar R, Spencer KB, Kesner B, et al. Targeting Xist with compounds that disrupt RNA structure and X inactivation. Nature. 2022;604(7904):160‐166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Richart L, Picod‐Chedotel M‐L, Wassef M, et al. XIST loss impairs mammary stem cell differentiation and increases tumorigenicity through Mediator hyperactivation. Cell. 2022;185(12):2164‐2183.e25. [DOI] [PubMed] [Google Scholar]
- 47. Yang X, Zhang S, He C, et al. METTL14 suppresses proliferation and metastasis of colorectal cancer by down‐regulating oncogenic long non‐coding RNA XIST. Mol Cancer. 2020;19(1):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jones AN, Tikhaia E, Mourão A, Sattler M. Structural effects of m6A modification of the Xist A‐repeat AUCG tetraloop and its recognition by YTHDC1. Nucleic Acids Res. 2022;50(4):2350‐2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wang D, Tang L, Wu Y, et al. Abnormal X chromosome inactivation and tumor development. Cell Mol Life Sci. 2020;77(15):2949‐2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Li C, Wan L, Liu Z, et al. Long non‐coding RNA XIST promotes TGF‐beta‐induced epithelial‐mesenchymal transition by regulating miR‐367/141‐ZEB2 axis in non‐small‐cell lung cancer. Cancer Lett. 2018;418:185‐195. [DOI] [PubMed] [Google Scholar]
- 51. Shen Y, Lin Y, Liu K, et al. XIST: a meaningful long noncoding RNA in NSCLC process. Curr Pharm Des. 2021;27(11):1407‐1417. [DOI] [PubMed] [Google Scholar]
- 52. Zhuang LK, Yang YT, Ma X, et al. MicroRNA‐92b promotes hepatocellular carcinoma progression by targeting Smad7 and is mediated by long non‐coding RNA XIST. Cell Death Dis. 2016;7(4):e2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chen Z, Qi L, Fu H, Ma L. Long non‐coding RNA X‐inactive specific transcript suppresses the progression of hepatocellular carcinoma through microRNA‐221‐3p‐targeted regulation of O6‐methylguanine‐DNA methyltransferase. Bioengineered. 2022;13(5):14013‐14027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Dong Z, Yang J, Zheng F, Zhang Y. The expression of lncRNA XIST in hepatocellular carcinoma cells and its effect on biological function. J BUON. 2020;25(5):2430‐2437. [PubMed] [Google Scholar]
- 55. Liu L, Jiang H, Pan H, Zhu X. LncRNA XIST promotes liver cancer progression by acting as a molecular sponge of miR‐200b‐3p to regulate ZEB1/2 expression. J Int Med Res. 2021;49(5):3000605211016211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Liu WG, Xu Q. Long non‐coding RNA XIST promotes hepatocellular carcinoma progression by sponging miR‐200b‐3p. Eur Rev Med Pharmacol Sci. 2019;23(22):9857‐9862. [DOI] [PubMed] [Google Scholar]
- 57. Ohhata T, Yamazawa K, Miura‐Kamio A, et al. Dynamics of transcription‐mediated conversion from euchromatin to facultative heterochromatin at the Xist promoter by Tsix. Cell Rep. 2021;34(13):108912. [DOI] [PubMed] [Google Scholar]
- 58. Sun S, Del Rosario BC, Szanto A, Ogawa Y, Jeon Y, Lee JT. Jpx RNA activates Xist by evicting CTCF. Cell. 2013;153(7):1537‐1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 The correlation between the methylation level and the relative expression of XIST in hepatocellular carcinoma (HCC) tissues using the Shiny Methylation Analysis Resource Tool (SMART) date. The correlation between the methylation level and the relative expression of XIST in 118 female (A) and in 244 male (C) HCC tissues (probe:cg12653510). The correlation between the methylation level and the relative expression of XIST in 113 female (B) and in 235 male (D) HCC tissues (probe:cg03554089). The correlation of female date was measured by Pearson correlation analysis. The correlation of male date was measured by Spearman correlation analysis.
Figure S2 The prognosis of patients with higher expression of XIST are better from the database of Kaplan–Meier plotter. (A) The overall survival of 118 female liver cancer patients. (B) The recurrence‐free survival of 106 female liver cancer patients. (C) The overall survival of 246 male liver cancer patients. (D) The recurrence‐free survival of 210 male liver cancer patients.
Figure S3 XIST suppresses hepatocellular carcinoma (HCC)‐1016 cell proliferation. (A) Relative expression level of XIST in XIST‐silenced HCC‐1016 cells compared with control cells determined by real‐time polymerase chain reaction (PCR). (B) Proliferation of HCC‐1016 cells assessed by CCK8 assay. XIST silencing promote cell proliferation. (C) EdU immunofluorescence staining of HCC‐1016 cells. (D) The percentage of EdU‐positive nuclei.
Figure S4 Representative pictures of the livers (arrows depict tumours) from diethylnitrosamine (DEN)‐administered male mice.
Figure S5 TET2 and YY1 could not binding to the promoter region of XIST in male adjacent normal liver tissues by chromatin immunoprecipitation (ChIP)‐qPCR. (A) Bar plot representing qPCR values of the ChIP results in male adjacent normal liver tissues. (B) XIST was quantified using DNA agarose gel electrophoresis.
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Data Availability Statement
Data supporting this study will be made available upon reasonable request.


