Abstract
The denervation or activation of the sympathetic nerve in the kidney can affect renal hemodynamics. The sympathetic nervous system regulates the physiological functions of the kidneys. Stimulation of sympathetic efferent nerves affects various parameters related to renal hemodynamics, including sodium excretion, renin secretion, and renal blood flow (RBF). Hence, renal sympathetic fibers may also play an essential role in regulating systemic vascular resistance and controlling blood pressure. In the absence of renal nerves, the hemodynamics response to stimuli is negligible or absent. The effect of renal sympathetic denervation on RBF is dependent on several factors such as interspecies differences, the basic level of nerve activity in the vessels or local density of adrenergic receptor in the vascular bed. The role of renal denervation has been investigated therapeutically in hypertension and related disorders. Hence, the dynamic impact of renal nerves on RBF enables using RBF dynamic criteria as a marker for renal denervation therapy.
Keywords: Renal blood flow, renal sympathetic denervation, renal sympathetic nerve activity
INTRODUCTION
The sympathetic nervous system regulates a wide range of physiological functions within the body. The sympathetic nervous system innervates the kidneys through the vasculature, tubules, and juxtaglomerular apparatus. Since, the kidneys play an important role in adjusting blood pressure, the neural control of the kidneys is critical for regulating the body’s fluid volume, sodium homeostasis, and renin release.[1] It has been suggested that animals’ basal renal sympathetic nerve activity (RSNA) is at a minimum level under normal conditions. However, this activity is raised in pathological conditions, such as hypertension.[2] In addition, the RSNA fluctuations affect sodium reabsorption from renal tubular cells and renin release from juxtaglomerular cells. Due to the involvement of renal adrenergic nerves in regulating renal vascular resistance (RVR) and renal hemodynamics such as renal blood flow (RBF), the kidneys can adapt to both physiologic and pathologic stimulants.[3] The activity of sympathetic nerves of afferent and efferent renal arteries affects RBF and glomerular filtration rate (GFR).[4] Furthermore, stimulating renal efferent nerves change renal hemodynamics by increasing renin secretion, enhancing tubular fluid and electrolyte absorption, and reducing water and sodium excretion.[5] The renal nerves are inactive under normal conditions and based on the steady state measurement of RBF. However, they respond to experimental stimuli or several diseases where the RSNA exceeds the physiological level. In general, the dynamic measurement of RBF indicates that renal nerves are incessantly regulating RBF.[6]
Central sympathetic signals from the kidneys target various organs, such as the heart and lead the peripheral arteries to constrict and increasing of blood pressure.[7] The role of renal denervation has been investigated therapeutically in hypertension, chronic kidney insufficiency, and chronic heart failure (HF) conditions.[8] This review intends to evaluate the effects of renal nerve sympathetic activity or renal sympathetic denervation (RSDN) on RBF in physiological and pathological conditions based on basic and clinical evidences.
SYMPATHETIC RENAL INNERVATION
There are sympathetic inputs and outputs in the kidneys; the efferent sympathetic nerves from the central nervous system (CNS) and the afferent sympathetic nerves from the kidneys to the CNS constitute the sympathetic innervation of the kidneys. The sympathetic nerves innervate the kidneys through a dense network of postganglion neurons. Along the renal artery, preganglionic nerves enter the kidney from the hilus[9] and the branches of the renal sympathetic efferent nerves innervate glomerular arterioles, proximal tubules, and the juxtaglomerular system.[10] Activation of the sympathetic nerve increases the production of noradrenaline (NA) from the nerve terminals and denervation of the kidney causes a significant reduction in NA (by 95%).[11] The release of increased NA has three primary outcomes as follows:
NA stimulates beta-adrenergic receptors (β1-ARs) of juxtaglomerular granular cells, which in turn release renin and increase the activity of the renin-angiotensin-aldosterone system (RAAS)
NA reduces sodium and water excretion by increasing tubular reabsorption
NA reduces RBF and GFR by contracting renal arteries.[11][Figure 1].
Figure 1.
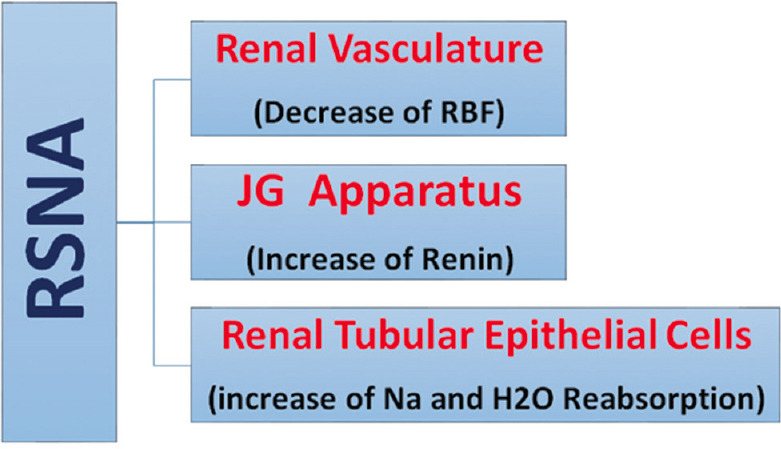
Schematic image of the effect of increased renal sympathetic nerve activity on different parts of the kidney. RSNA = Renal sympathetic nerve activity
The activation of distinct adrenoceptor (ARs) subtypes found on the renal vasculature by the renal sympathetic nervous system mediates adrenergic control of the kidneys. ARs support renal hemodynamic and tubular functions and are found on the renal vasculature, nephrons, and proximal tubules in the kidneys. The α-ARs are the most important regulators of renal vascular tone among the different types of ARs.[12] During an adrenergic response, NA released into the circulation binds to the smooth muscle cells’ α1 receptors, causing the smooth muscle to contract. By mediating catecholamine-induced effects on the ARs type α1 found on the renal vasculature, the renal sympathetic nervous system significantly affects the renal hemodynamics.[12]
Activation of the sympathetic efferent nerves of the kidney can occur in response to reinforced afferent signaling of the sensory nerve fibers of the kidney, which can be induced by various effectors such as renal hypoxia, ischemia, and oxidative stress.[8]
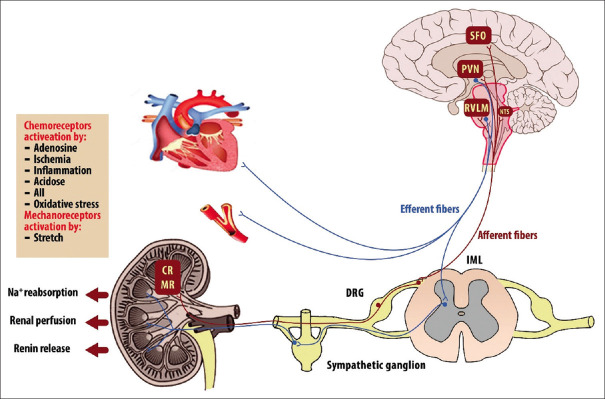
The pelvic area is the primary location of the afferent renal sensory nerves and the pressure in this area defines the activity of the nerves. Thus, as a reno-renal reflex response, enhancement in the urine flow rate raises the firing rate of renal afferent fibers, decreasing efferent RSNA and increasing sodium excretion from urine.[13] The renal afferent fibers are either chemo-sensitive and respond to nociceptive stimuli (such as inflammation, ischemia, acidosis, oxidative stress, adenosine, and angiotensin (Ang) (II)) or are mechano-sensitive (more common in the renal cortex) and respond to stretch.[14] The nervous system centers that received these signals include the nucleus tractus solitaries, paraventricular nucleus (PVN) of the hypothalamus, rostral ventrolateral medulla (RVLM), and subfornical organ.[15-17]
The neuronal activity in sympathetic premotor nuclei in the brain stem and hypothalamus, including RVLM and PVN, determines the degree of RSNA. Preganglionic neurons in the intermediolateral cell column of the spinal cord get input from the neurons in the RVLM; these neurons then project to postganglionic neurons, which in turn project to peripheral organs such the heart, arteries, and kidneys.[18] Figure 2 summarizes the central and peripheral pathways of sympathetic control of the kidney.
Figure 2.
Schematic image of the connections between renal afferent sensory signaling and renal efferent sympathetic outflow on the kidney and other cardiovascular organs, which regulate blood pressure. Renal mechano and chemoreceptor reflexes, which are carried out by renal afferent nerves, regulate the activity of premotor neurons in the rostral ventrolateral medulla and paraventricular nucleus. CR = Renal chemoreceptors; DRG = Dorsal root ganglion; IML = Intermediolateral cell column; MR = Renal mechanoreceptors; NTS = Nucleus tractus solitarius; PVN = Paraventricular nucleus of the hypothalamus; RVLM = Rostral ventrolateral medulla; SFO = Subfornical organ
Activating of renal afferent sensory nerve (by modulation of posterior hypothalamic activity and secretion of oxytocin and vasopressin) affects the sympathetic outflow to highly innervated organs such as the kidneys, heart, and peripheral blood vessels.[19,20] Stimulation of the afferent system activates the cardiovascular regulatory centers in the CNS. The destruction of these nerves (in some diseases) reduces the central sympathetic flow to major organs regulating blood pressure, especially the kidneys, heart, and peripheral arteries.[14]
Renal denervation is believed to be effective in treating numerous diseases that are accompanied by increased sympathetic renal activity, such as chronic and end-stage renal disease, hypertension, cardiac-renal syndrome, left ventricular hypertrophy, and improper fluid retention in HF.[19] In general, afferent sympathetic fibers may also play an essential role in regulating systemic vascular resistance and controlling blood pressure.[20]
RENAL SYMPATHETIC NERVE ACTIVITY AND RENAL BLOOD FLOW
The share of RBF from cardiac output is about 20% at rest, so its regulation plays a vital role in controlling blood pressure.[21] The kidneys have two robust auto-regulation mechanisms for regulating blood pressure, tubule-glomerular feedback, and myogenic response.[22] However, the importance of RSNA in the physiological regulation of RBF is still controversial based on the two findings. The first finding indicated that electrically stimulated renal nerves at different frequencies affect RBF differently, and in the pathophysiological range of RSNA, a significant decrease in RBF was observed.[6] Other findings violate the influence of renal nerves in the physiological regulation of RBF since renal denervation is not affecting basal RBF. However, both of these findings had significant drawbacks.[23] The electrical stimuli inherently cannot distinguish between physiological, pathophysiological, and supraphysiological effects. RSNA recruits special renal postganglionic fibers in response to specific stimuli with different effects.[23] In addition, the particular axons can electively innervate the vessels, juxtaglomerular cells, or tubules, and even axons that innervate juxtaglomerular arteries can be differentiated from those that innervate other renal vessels.[24] Eventually, by changing RSNA, which occurs through either stimulation or denervation, it must overcome powerful autoregulatory mechanisms to affect the steady state of RBF.[23]
It is stated that the vascular system is insensitive to slight changes in RSNA. In experimental models, RSNA was increased progressively by electrical stimulation of the renal nerves in anesthetized cats or dogs[25-27] or reflex activation in conscious dogs.[28,29] At low RSNA levels, only renin release occurred, and then, slightly increased levels have resulted in changes in sodium excretion, and still, RBF alteration was obtained only at much higher levels,[30] indicating that in daily life, changes in RSNA at near resting levels have minimal impact on RBF.[30]
Grady and Bullivant measured RBF during the daily activity in conscious rats, demonstrating that RBF decreased with increasing activity levels; however, this result was not obtained when RSNA was previously blocked with local anesthetics.[31] In alert rabbits, a moderate increase or decrease in RSNA affected RBF. However, sound stress, air-jet stress, and hypoxia increased RSNA by 12%–31%, reducing RBF by 8%–12% compared to controls.[32] In addition, an increase in blood volume, which reduces RSNA by 25%, leads to a 17% increase in RBF.[33] It is also reported that rapid and physiological changes in sympathetic output affect RBF during normal daily activities.[6]
Routine activities such as sleeping or grooming have increased RSNA and concomitantly decreased RBF.[34] Furthermore, a small increase in heart rate and RSNA in unilaterally renal denervated rabbits showed considerable differences in the RBF of innervated and denervated kidneys. These findings suggested that RSNA changes in the physiological range affect RBF, so further research is needed to elucidate the role of renal nerves in the dynamic regulation of resting RBF.[23]
Sympathetic activity has two components: frequency and amplitude. The frequency shows baroreceptor modulation and central generation and the amplitude indicates the number of recruited nerves. Since various afferent stimuli can change these components, changes in the frequency or number of recruited nerves or multiple activation patterns can affect kidney function.[35,36] It is shown that dilatation of a pig’s uterus reduced RBF by sympathetic nerves without altering blood pressure.[37] In Mancia et al.’s experiments, RBF decreased by 8%, 15%, and 19% in the three states of confrontation; without movement, forelimb movement, and hind limb and forelimb movement, respectively.[38] An experiment on conscious baboons demonstrated that RBF decreased in response to psychological stress.[39] Another study found that acute psychological stress in conscious monkeys reduced RBF by increasing RSNA.[40] Furthermore, RSNA increases and RBF decreases in moderate heat stress.[41-43]
RBF decreases in response to a slight increase in RSNA, but whether RBF increases in response to a slight decrease in RSNA is ambiguous. In the alert rabbits, an increase in plasma volume caused a moderate reduction in RSNA (by 25%) and a significant rise in RBF. In contrast, this response was not obtained in the renal denervated animals.[33]
RBF in the cortex and medulla was also decreased after the electrical stimulation of the sympathetic nerves.[44] Stimulation of the renal sympathetic nerve creates a different pattern in medullary perfusion and renal cortex, attributed to the less sensitive medulla in the anesthetized rat.[45] In rabbits, activation of the renal sympathetic nerves resulted in a greater increase in RBF and cortical perfusion than in medullary perfusion.[46,47] They were similar at each stimulation level of perfusion changes in the inner and outer medulla.[48]
In humans, renal function is measured in response to stimuli related to RSNA change instead of direct RSNA assay, while it is impossible to measure RSNA directly.[35] Psychological stress increases the activity of the sympathetic muscle nerve by up to 30% and decreases cortical blood flow by up to 36%.[49] Submerging in water and neck suction increases RBF due to decreased RSNA levels.[50,51] To sum up, it is clear that the stimulation of the sympathetic nerves of the kidney reduces RBF, and many studies proposed that the alterations in RSNA induced by natural behavioral activities had a remarkable effect on RBF [Table 1].
Table 1.
The effect of renal sympathetic nerve activity on renal blood flow
| RSNA in animal or human | RBF | Reference |
|---|---|---|
| Anesthetized cat | Decrease | [25] |
| Anesthetized dog | Decrease | [26] |
| Conscious dog | Decrease | [28,29] |
| Conscious rat | Decrease | [31,34] |
| Conscious rabbit | Decrease | [32] |
| Anesthetized pig | Decrease | [37] |
| Conscious cat | Decrease | [38] |
| Conscious baboon | Decrease | [39,42] |
| Anesthetized rat | Decrease | [43,52] |
| Conscious monkey | Decrease | [40] |
| Human | Decrease | [49] |
RSNA=Renal sympathetic nerve activity; RBF=Renal blood flow
RENAL SYMPATHETIC DENERVATION AND RENAL BLOOD FLOW
The RSDN is performed to determine the nonneurological effects on the kidney. In this case, either the response is weak and difficult to measure or there is no response at all. Studies indicated that RBF increases in alert and resting animals after renal denervation, so RSNA is responsible for supplying the tonic level of renal vasoconstriction.[31,32] Furthermore, GFR was increased in patients with refractory hypertension with bilateral renal denervation.[53] In contrast, there was no difference in RBF between innervated and denervated kidneys in alert and resting rats.[34] Similarly, in anesthetized rats during the 1st h after unilateral renal denervation, no difference in RBF was observed in the denervated and innervated kidneys.[3] Such findings were also detected in rabbits on days 14–21[54] or after 7 weeks.[30] Similarly, there was no change in RBF after administrating an adrenergic blocker (dibenamine) to relaxed and stress-free state patients.[55] In general, the effect of RSNA on RBF differs in anxiety and pathophysiological conditions from calm and restful conditions. Anxiety and pathophysiological conditions reduce RBF, but in calm conditions, there is a slight tonic effect on RBF.[48] The tonic result of basal RSNA on RBF seems to be negligible, and acute surgical denervation has little impact on renal hemodynamics.[48] Overall, the basal renal nerve activity does not affect renal hemodynamics; for example, it is specified that in alert dogs and humans, renal denervation with medication or surgery does not affect RBF,[56,57] and in nondiuretic rats after acute unilateral denervation, renal plasma flow (RPF) remains unchanged in the kidneys.[58]
All stimuli that significantly reduced RBF in renal innervated rabbits, such as air-jet stress, hypoxia, or noise stress, failed to elicit an RBF response after renal denervation.[32] Similarly, following baroreflex alteration of RSNA, the response of RBF was significantly altered in response to change in arterial pressure after administering a calcium antagonist or an Ang II antagonist following renal denervation in rats.[59] In the same way, in conscious cats, RBF responses to confrontation following renal denervation were eliminated.[38] Other studies have shown that acute denervation causes diuresis and natriuresis in anesthetized dogs and rats without significantly affecting renal hemodynamics parameters.[58,60,61] No alteration in RBF was reported with renal denervation performed on unconscious pigs[62] and cats[63,64] and no difference was observed in anesthetized monkeys in renal excretory function after renal denervation.[65] However, in conscious baboons, RBF responses to psychological stress following renal denervation were persisted.[39] The impact of RSDN on RBF at different times after RSDN in patients with resistant hypertension indicated a 20% increase in total blood flow per cardiac cycle and a significant decrease in blood pressure, without any changes in RBF.[66] It is also stated that under normal sympathetic tone, the sympathetic nerve fibers of the kidney have little effect on the dynamic auto-regulation of renal vascular tone and, consequently, on RBF.[3] In a study on a pig model, RBF increased acutely after RSDN and remained at the same acute peak even after a month, while RBF reserve remained lower, and based on these observations, it can be concluded that such changes in RBF parameters can be a valuable biomarker for successful denervation.[67] Hemodynamic measurements in renal arteries of healthy pigs after RSDN, immediately, 3 weeks, and 3 months after RSDN indicated that RBF at rest propends to increment.[68] This results agree with relative increase in RBF after renal denervation in dogs.[69] However, as contradictory results in this regard, in anesthetized nondiuretic rats, RBF and GFR remained unchanged after denervation.[58] Furthermore, some studies have reported that renal basal sympathetic nerve resection in normal dogs and rats does not affect RBF.[70,71] A study on rats determined the regional blood flow in the cortex and medulla of the left kidney, and they did not observe a significant effect on intracortical blood distribution after renal denervation,[72] However, acute unilateral renal denervation increased RBF and RPF without altering GFR. In general, renal denervation did not affect intracortical blood flow distribution and renal hemodynamics.[72] Otherwise, it is suggested that renal denervation causes a rapid (approximately 25%) increase in cortical perfusion in anesthetized rats.[73] In hypertension and congestive HF (CHF) rat model, RSDN increased basal RBF.[70] However, in Sprague Dawley rats (SD), RSDN did not affect RBF.[3] These disagreements may be due to differences among animal species or the RSDN method.[30]
It has been reported that renal denervation does not significantly alter arterial pressure in spontaneously hypertensive rats (SHR) over a short period of 1 h, despite interfering with intrarenal function (such as increasing RBF, dynamic autoregulation of RBF, and variability of RBF).[74] Meanwhile, despite causing systemic hypotension, RSDN does not affect perfusion and renal function at various intervals (directly and after 3 months) and does not alter RBF in patients with hypertension.[75] Hence, it can be deduced that the effect of RSDN is negligible on acute or chronic renal perfusion.[75] However, a case report indicated that RSDN was associated with increased RPF.[76]
In Wistar Kyoto (WKY) and SHR, acute renal denervation under genetic control resulted in continuous diuresis and natriuresis in SHR and not in WKY, and there was no significant change in RBF.[77] Also, in SD and Munich-Wistar (MW) rats, similar to SHR, renal hemodynamics remained unchanged.[77] Acute denervation studies have shown a negligible tonic effect of renal efferent nerves on renal arteries in SHR, WKY, and SD-MW rats.[77] Strain differences have been identified between SHR and WKY in renal excretory response to acute unilateral renal RSDN.[77] Also, the effect of acute renal RSDN on RBF or GFR is not noticeable in normal adult rats in hydroponic, euvolemic, or volume-expanded conditions.[58,78] Table 2 shows the effect of RSDN on RBF in some studies models.
Table 2.
he effect of renal sympathetic denervation on renal blood flow
| RSDN | Model | RBF | References |
|---|---|---|---|
| Transmission blocking drug (xylocaine) | Conscious rat | Increase | [31] |
| Bilateral | Conscious rabbit | Increase | [32] |
| Bilateral | Conscious sheep | Increase | [79] |
| Acute and chronic | Anesthetized rat | No change | [48] |
| - | Anesthetized rat | Increase (cortical RBF) | [73] |
| Chronic (14–21 days) | Rabbit | No change | [54] |
| Chronic (7 weeks) | Rabbit | No change | [30] |
| - | Conscious rat | No change | [34] |
| Adrenergic blocking drug (dibenamine) | Human unstressed | No change | [55] |
| Adrenergic blocking drug (dibenamine) | Anxious human | Increase | [55] |
| Surgical or pharmacological | Conscious dogs and humans | No change | [56,57] |
| Acute unilateral | Nondiuretic rats | No change | [58] |
| - | Rats | No change | [59] |
| - | Conscious cats | No change | [38] |
| Unilateral | Anesthetized rats and dogs | No change | [58,60,61] |
| Acute | Anesthetized pigs | No change | [62] |
| - | Cat | No change | [63,64] |
| Chronic bilateral | Anesthetized monkeys | No change | [65] |
| - | Conscious baboons | No change | [39] |
| Acute unilateral | Rat | Increase | [72] |
| - | Hypertensive patients | No change | [66] |
| Acute | Rat | No change | [3,77] |
| - | Porcine model | Increase | [67] |
| Chronic | Pig | Increase | [68] |
| - | Normal dog | No change | [70,71] |
| Acute | Hypertensive rats | Increase | [70,74] |
| Acute | Congestive heart failure rat | Increase | [70] |
| Acute | Spontaneously hypertensive rats | No change | [77] |
| Acute | Wistar-Kyoto genetic control rats | No change | [77] |
| Chronic | Normotensive rats (Sprague–Dawley strains) | No change | [80] |
| Acute | Volume-expanded Rat | No change | [78] |
| Acute | Hydropenic, euvolemic rat | No change | [58] |
| - | Pacing-induced heart failure rabbit | Increase | [81] |
| Chronic | Resistant hypertension patient | No change | [75] |
RSDN=Renal sympathetic denervation; RBF=Renal blood flow
Overall, there is a degree of uncertainty in these studies. The reasons for the above inconsistent results are not specific, because the studies were performed either under anesthesia or consciously. Factors such as differences between animal species, the method of RSDN, the degree of RSNA required to impact on RBF, final evaluation of renal hemodynamics, and validation of renal denervation are factors that can be involved in these differences.[82] Studies in normal animals presented where basal RSNA was sub-vasoconstrictor, basal RBF and dynamic RBF auto-regulation were not altered by the elimination of basal RSNA by renal denervation.[70] Also, under a number of physiological and pathological circumstances, there may be a change in the functional participation of a1– ARs.[12] In the deoxycorticosterone acetate-salt (DOCA)-salt-hypertensive rats, a local change in the density of α1-ARs may be responsible for the increased responsiveness of the mesenteric vascular bed to α1-AR agonists, and Suzuki et al. discovered that the mesenteric vasculature of DOCA-salt hypertensive rats had increased α1-AR density and affinity.[83] Compared to normotensive WKY rats, SHR rats showed enhanced affinity of the small mesenteric artery α1–AR.[84] Both Dahl salt-sensitive rats and SHR rats showed higher renal densities of α1-AR and α2–AR.[85] Additional research in various salt-related hypertension animal models has shown that a local change in the α1-AR density may be the cause of the increased reactivity of the vasculature to catecholamine.[86] The neurovascular transduction mechanisms may vary as a result of these variations in vascular beds’ sensitivity.[86] Aging modifies the distribution of the vascular α1-AR subtype in humans, which differs from animal models, changes with vessel bed.[87] These discoveries provide possible new therapeutic targets that might be used in a variety of clinical scenarios.
THE SYMPATHETIC NERVOUS ACTIVITY IN PATHOLOGICAL CONDITIONS
Overactive sympathetic nerves are linked to hypertension and numerous cardiometabolic disorders, but the underlying mechanisms are poorly understood.[88] Sympathetic hyperactivity is associated with decreased GFR, RBF, and salt excretion, and this might affect systemic blood pressure. Renal denervation has been demonstrated to be an effective therapeutic method for lowering blood pressure. The relationship between renal sympathetic nerves and the pathophysiology of hypertension, HF, and chronic kidney disease has been highlighted.[89] Based on these phenomena, renal denervation helps lower blood pressure and may be used to treat insulin resistance,[90] obesity-related hypertension,[91] HF,[92] chronic kidney disease,[93] metabolic syndrome,[94] diabetes,[95] and obstructive sleep apnea.[96]
Hypertension
Sympathetic hyperactivity is a common trait in both human and animal models of hypertension. When compared to normotensive people, RSNA in hypertension patients is twice.[97] However, Gattone et al. demonstrated that renal damage is mitigated by sympathetic function suppression irrespective of systemic hypertension.[98] Antiadrenergic, diuretics, Ang-converting enzyme inhibitors (ACEi), AngII receptor blockers (ARBs), calcium-channel blockers, and anti-renin medicines are just a few of the many efficient anti-hypertensive medications that are now on the market. However a significant portion of individuals with essential hypertension are drug-resistant, meaning they are unable to lower their blood pressure despite taking three separate antihypertensive medications at the recommended dose.[99] Renal denervation is a therapeutic option for severe resistant hypertension patients.[100,101] The rise in blood pressure was reduced in the DOCA-salt rat model of hypertension by surgically ablate both efferent and afferent renal neurons.[102] The afferent renal nerve activity in the clipped kidney was increased in the two-kidney-one-clip (2K1C) mouse and rat models, while afferent renal denervation (ARDN) and total renal denervation (TRDN) attenuated the increase in blood pressure.[103,104] The expression of Ang II receptors was assessed in both kidneys of the 2K1C rat model, and the results revealed a significant up-regulation of Ang II receptor mRNA in the clipped kidney; while, renal denervation led to a normalization of their expression in the ischemic kidneys.[105] TRDN reduced the rise of blood pressure during the emerging stage of hypertension in stroke-prone SHR (SHRSP), but such finding was not seen by ARDN.[106] It seems that the suppression of the development of hypertension in SHRSP is a result of the denervation of efferent renal nerves.[106] RSDN is helpful in the pathophysiological circumstances of sympathetically driven hypertension, such as obesity-related hypertension.[107] RSDN, lowered renin production and enhanced RBF in individuals with essential hypertension, indicating that the efferent renal nerves had been successfully targeted.[76] RSDN does not necessarily have antihypertensive effects in several animal models, such as Ang II salt-induced hypertensive rats, Wistar rats, and dogs whose hypertension was brought on by chronic nitric oxide (NO) synthase suppression.[108-110] Both ARDN and TRDN were unable to reduce blood pressure elevation in Ang II or high salt diet-induced hypertensive rats (AngII-salt rats).[111] AngII-salt rats show continually high blood AngII levels despite sympathetic nerve activity and vascular disorders such as arteriosclerosis, endothelial dysfunction, and impairment of vasodilator response to sympathetic suppression.[111] It seems that, RSDN may not lower blood pressure even if it lowers the sympathetic outflow from the brain. In addition, RSDN may be inefficient in lowering blood pressure in the presence of pathophysiological factors linked to the development of vascular diseases, such as advanced age and isolated systolic hypertension.[111] RSDN may be useful in treating certain types of hypertension and offers the potential for more individualized disease management.[112]
Heart failure
Sympathoexcitation is a feature of chronic HF, especially in the heart and kidneys.[113] Renal vasoconstriction, reduced RBF, increased water and salt reabsorption, and renal fibrosis are all brought on by increased RSNA.[114] Following stimulation of the sympathetic nerves that innervate the vasculature, the vasculature (macro-and microcirculations) is susceptible to endothelial cell malfunction, smooth muscle cell hypertrophy, and vasoconstriction. The release of renin from the kidneys, activation of the RAAS, and renal damage are all further enhanced by increased RSNA. RSNA causes pathological changes in the kidneys, which increase blood volume, cause tissue edema, and cause systemic vasoconstriction through Ang II to considerably worsen HF.[115] The success of neuro-hormonal modulators, including beta-blockers, ACEi, ARBs, aldosterone antagonists, diuretics, and neprilysin inhibition, as standards of care to treat CHF is a testimony to the substantial role the SNS plays in worsening HF severity.[116-119] Despite the fact that these pharmacotherapies have been effective in lowering morbidity and early death, pharmacotherapy resistance, unintended side effects, and patient nonadherence to medication regimens[120,121] continue to aggravate HF symptoms over time. Therefore, there is still a clinical unmet need for supplemental or alternative therapy approaches to treat HF. In animal model studies of the CHF, it was found that acute renal denervation in anesthetized rats, increased RBF,[70] so it can be concluded that the renal nerves may apply a tonic vasoconstrictive function in CHF.[122]
DiBona and Sawin investigated the tonic effect of basal RSNA on dynamic autoregulation of RBF in rats, and found that, RSDN increased basal RBF in CHF and SHR but not in SD and WKY rats[70] and notably ameliorated auto-regulation of RBF.[70] In the pacing-induced HF model in rabbits, decreased RBF, increased RVR, increased expression of Ang II receptor type 1 (AT1), and decreased expression of Ang II type II receptor (AT2) in renal cortical arteries, was specified.[81] These alterations were stopped by RSDN before induction of HF. Principally, the results of these animal studies cleared that the activity of renal sympathetic nerves has a deleterious effect on RBF and can be associated with changes in the expression of Ang II receptors so that renal denervation may be effective in the treatment of CHF.[92]
HF is linked to sleep apnea,[123] and RSDN counteracted the decrease of renal hypoperfusion during apnea and the activation of the RAAS in the kidney.[124] An improvement in sodium excretion, an increase in cardiac output, and an improvement in RBF mediating unfavorable responses were all seen in animal models of RSDN after myocardial infarction.[125,126]
Kidney diseases
Another research used an ovine model of hypertensive chronic kidney disease to show the efficacy of RSDN. In comparison to sham controls animal, the hypertensive CKD accompanied with RSDN showed larger improvements in GFR, RBF, and albuminuria 5 months after the ablation.[127] Furthermore, RSDN recovered estimated GFR (eGFR) by changes of intrarenal hemodynamics in CKD patients.[128,129] The eGFR assessments could help to evaluate the exact renal functions.[130]
It has been shown that ischemic acute kidney injury changes renal hemodynamics and is associated with endothelial cell dysfunction brought on by an increase in the formation of reactive oxygen species, which reduces NO availability.[131] Numerous physiological functions of NO in the kidney include the control of RSNA.[132] By reducing NO synthesis may directly increase sympathetic nervous system activity in CKD patients.[133] The glomerular microvasculature becomes more constricted as a result of NO production inhibition and proximal tubular reabsorption decreases.[133] RSDN treatment has stopped these effects.[134] However, RSDN may not be suitable for lowering blood pressure in patients with polycystic kidney disease.[135]
Renal denervation and future challenge
Despite new data demonstrating the large benefits of RSDN, there are still numerous unsolved problems, including responder identification, procedural guidance, effects persistence, and the applicability of clinical outcomes. The identification of responders is a particularly important subject matter. The hypertensive patients who had a baseline plasma renin activity > 0.65 ng/ml/h or a baseline heart rate > 73.5 bpm were more sensitive to RSDN.[136,137] The preference of patients for RSDN is another crucial feature that has to be taken into account in addition to the identification of responders. A nationwide web survey in Japan revealed that the presence of side effects while taking antihypertensive medications, younger patient age, male sex, higher systolic blood pressure (at home or at the office), and poor antihypertensive drug adherence were all significant predictors of preference for RSDN.[138] This should be considered while deciding on a course of antihypertensive treatment. Finally, it is debatable whether renal nerve regeneration impacts the long-term responses to renal denervation. The re-innervation of the renal nerves may start in humans as early as 28 days.[139] Similar events were seen in dogs where, 3–6 months after transplantation, renal autografts were re-innervated.[139] On the basis of enough data, it is envisaged that the therapeutic use of RSDN would proceed completely.
CONCLUSION
Several afferent and central pathways are involved in inducing an increase in RSNA, all of which result in a significant reduction in RBF that is proportional to the increase in RSNA. Without renal nerves, the response to stimuli is minimal or absent. Based on experiments, the effect of RSDN on RBF varies. The dynamic impact of renal nerves on RBF enables using RBF dynamic criteria as a biomarker in renal denervation therapy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
This research was supported by the Isfahan University of Medical Sciences.
REFERENCES
- 1.Schlaich MP, Hering D, Sobotka PA, Krum H, Esler MD. Renal denervation in human hypertension: Mechanisms, current findings, and future prospects. Curr Hypertens Rep. 2012;14:247–53. doi: 10.1007/s11906-012-0264-9. [DOI] [PubMed] [Google Scholar]
- 2.Dibona GF, Sawin LL. Effect of endogenous angiotensin II on the frequency response of the renal vasculature. Am J Physiol Renal Physiol. 2004;287:F1171–8. doi: 10.1152/ajprenal.00201.2004. [DOI] [PubMed] [Google Scholar]
- 3.Salman IM, Sattar MA, Abdullah NA, Ameer OZ, Hussain FB, Hye Khan MA, et al. Renal functional and haemodynamic changes following acute unilateral renal denervation in Sprague Dawley rats. Indian J Med Res. 2010;131:76–82. [PubMed] [Google Scholar]
- 4.Calzavacca P, May CN, Bellomo R. Glomerular haemodynamics, the renal sympathetic nervous system and sepsis-induced acute kidney injury. Nephrol Dial Transplant. 2014;29:2178–84. doi: 10.1093/ndt/gfu052. [DOI] [PubMed] [Google Scholar]
- 5.Girchev R, Bäcker A, Markova P, Kramer HJ. Impaired response of the denervated kidney to endothelin receptor blockade in normotensive and spontaneously hypertensive rats. Kidney Int. 2004;65:982–9. doi: 10.1111/j.1523-1755.2004.00483.x. [DOI] [PubMed] [Google Scholar]
- 6.Schiller AM, Pellegrino PR, Zucker IH. Eppur Si Muove: The dynamic nature of physiological control of renal blood flow by the renal sympathetic nerves. Auton Neurosci. 2017;204:17–24. doi: 10.1016/j.autneu.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mahfoud F, Böhm M, Schmieder R, Narkiewicz K, Ewen S, Ruilope L, et al. Effects of renal denervation on kidney function and long-term outcomes: 3-year follow-up from the global SYMPLICITY registry. Eur Heart J. 2019;40:3474–82. doi: 10.1093/eurheartj/ehz118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kannan A, Medina RI, Nagajothi N, Balamuthusamy S. Renal sympathetic nervous system and the effects of denervation on renal arteries. World J Cardiol. 2014;6:814–23. doi: 10.4330/wjc.v6.i8.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Esler M. The 2009 Carl Ludwig Lecture: Pathophysiology of the human sympathetic nervous system in cardiovascular diseases: The transition from mechanisms to medical management. J Appl Physiol (1985) 2010;108:227–37. doi: 10.1152/japplphysiol.00832.2009. [DOI] [PubMed] [Google Scholar]
- 10.Sobotka PA, Mahfoud F, Schlaich MP, Hoppe UC, Böhm M, Krum H. Sympatho-renal axis in chronic disease. Clin Res Cardiol. 2011;100:1049–57. doi: 10.1007/s00392-011-0335-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hering D, Esler MD, Krum H, Mahfoud F, Böhm M, Sobotka PA, et al. Recent advances in the treatment of hypertension. Expert Rev Cardiovasc Ther. 2011;9:729–44. doi: 10.1586/erc.11.71. [DOI] [PubMed] [Google Scholar]
- 12.Kazi RN. Differential role of renal alpha 1 adreno receptors subtypes in renal vasculature in normotensive and hypertensive conditions subjected to high dietary salt load. Biomed Pharmac J. 2021;14:343–50. [Google Scholar]
- 13.Xu J, Hering D, Sata Y, Walton A, Krum H, Esler MD, et al. Renal denervation: Current implications and future perspectives. Clin Sci (Lond) 2014;126:41–53. doi: 10.1042/CS20120581. [DOI] [PubMed] [Google Scholar]
- 14.Patel HC, Hayward C, Vassiliou V, Patel K, Howard JP, Di Mario C. Renal denervation for the management of resistant hypertension. Integr Blood Press Control. 2015;8:57–69. doi: 10.2147/IBPC.S65632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takahashi M, Tanaka J. Serotonin release in the subfornical organ area induced by sodium and water intake in the rat. Physiol Behav. 2016;164:123–8. doi: 10.1016/j.physbeh.2016.04.037. [DOI] [PubMed] [Google Scholar]
- 16.Dampney RA, Michelini LC, Li DP, Pan HL. Regulation of sympathetic vasomotor activity by the hypothalamic paraventricular nucleus in normotensive and hypertensive states. Am J Physiol Heart Circ Physiol. 2018;315:H1200–14. doi: 10.1152/ajpheart.00216.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumagai H, Oshima N, Matsuura T, Iigaya K, Imai M, Onimaru H, et al. Importance of rostral ventrolateral medulla neurons in determining efferent sympathetic nerve activity and blood pressure. Hypertens Res. 2012;35:132–41. doi: 10.1038/hr.2011.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mahfoud F, Schlaich MP, Lobo MD. Device therapy of hypertension. Circ Res. 2021;128:1080–99. doi: 10.1161/CIRCRESAHA.121.318091. [DOI] [PubMed] [Google Scholar]
- 19.Schlaich MP, Sobotka PA, Krum H, Whitbourn R, Walton A, Esler MD. Renal denervation as a therapeutic approach for hypertension: Novel implications for an old concept. Hypertension. 2009;54:1195–201. doi: 10.1161/HYPERTENSIONAHA.109.138610. [DOI] [PubMed] [Google Scholar]
- 20.Papademetriou V, Doumas M, Tsioufis K. Renal sympathetic denervation for the treatment of difficult-to-control or resistant hypertension. Int J Hypertens 2011. 2011:196518. doi: 10.4061/2011/196518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meyer E. A review of renal protection strategies. South Afr J Anaesth Analg. 2015;21:5–8. [Google Scholar]
- 22.Carlström M, Wilcox CS, Arendshorst WJ. Renal autoregulation in health and disease. Physiol Rev. 2015;95:405–511. doi: 10.1152/physrev.00042.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schiller AM, Pellegrino PR, Zucker IH. Renal nerves dynamically regulate renal blood flow in conscious, healthy rabbits. Am J Physiol Regul Integr Comp Physiol. 2016;310:R156–66. doi: 10.1152/ajpregu.00147.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DiBona GF. Neural control of the kidney: Functionally specific renal sympathetic nerve fibers. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1517–24. doi: 10.1152/ajpregu.2000.279.5.R1517. [DOI] [PubMed] [Google Scholar]
- 25.Coote JH, Johns EJ, Macleod VH, Singer B. Effect of renal nerve stimulation, renal blood flow and adrenergic blockade on plasma renin activity in the cat. J Physiol. 1972;226:15–36. doi: 10.1113/jphysiol.1972.sp009971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holdaas H, DiBona GF, Kiil F. Effect of low-level renal nerve stimulation on renin release from nonfiltering kidneys. Am J Physiol. 1981;241:F156–61. doi: 10.1152/ajprenal.1981.241.2.F156. [DOI] [PubMed] [Google Scholar]
- 27.Osborn JL, DiBona GF, Thames MD. Beta-1 receptor mediation of renin secretion elicited by low-frequency renal nerve stimulation. J Pharmacol Exp Ther. 1981;216:265–9. [PubMed] [Google Scholar]
- 28.Nelson LD, Osborn JL. Neurogenic control of renal function in response to graded nonhypotensive hemorrhage in conscious dogs. Am J Physiol. 1993;264:R661–7. doi: 10.1152/ajpregu.1993.264.4.R661. [DOI] [PubMed] [Google Scholar]
- 29.Miki K, Hayashida Y, Tajima F, Iwamoto J, Shiraki K. Renal sympathetic nerve activity and renal responses during head-up tilt in conscious dogs. Am J Physiol. 1989;257:R337–43. doi: 10.1152/ajpregu.1989.257.2.R337. [DOI] [PubMed] [Google Scholar]
- 30.Barrett CJ, Navakatikyan MA, Malpas SC. Long-term control of renal blood flow: What is the role of the renal nerves? Am J Physiol Regul Integr Comp Physiol. 2001;280:R1534–45. doi: 10.1152/ajpregu.2001.280.5.R1534. [DOI] [PubMed] [Google Scholar]
- 31.Grady HC, Bullivant EM. Renal blood flow varies during normal activity in conscious unrestrained rats. Am J Physiol. 1992;262:R926–32. doi: 10.1152/ajpregu.1992.262.5.R926. [DOI] [PubMed] [Google Scholar]
- 32.Malpas SC, Evans RG. Do different levels and patterns of sympathetic activation all provoke renal vasoconstriction? J Auton Nerv Syst. 1998;69:72–82. doi: 10.1016/s0165-1838(98)00010-1. [DOI] [PubMed] [Google Scholar]
- 33.Leonard BL, Navakatikyan MA, Malpas SC. Differential regulation of the oscillations in sympathetic nerve activity and renal blood flow following volume expansion. Auton Neurosci. 2000;83:19–28. doi: 10.1016/S0165-1838(00)00103-X. [DOI] [PubMed] [Google Scholar]
- 34.Yoshimoto M, Sakagami T, Nagura S, Miki K. Relationship between renal sympathetic nerve activity and renal blood flow during natural behavior in rats. Am J Physiol Regul Integr Comp Physiol. 2004;286:R881–7. doi: 10.1152/ajpregu.00105.2002. [DOI] [PubMed] [Google Scholar]
- 35.Malpas SC, Leonard BL. Neural regulation of renal blood flow: A re-examination. Clin Exp Pharmacol Physiol. 2000;27:956–64. doi: 10.1046/j.1440-1681.2000.03386.x. [DOI] [PubMed] [Google Scholar]
- 36.DiBona GF, Jones SY. Analysis of renal sympathetic nerve responses to stress. Hypertension. 1995;25:531–8. doi: 10.1161/01.hyp.25.4.531. [DOI] [PubMed] [Google Scholar]
- 37.Vacca G, Battaglia A, Grossini E, Mary DA, Molinari C, Surico N. Changes in regional blood flow in response to distension of the uterus in anaesthetised pigs. J Auton Nerv Syst. 1997;66:7–14. doi: 10.1016/s0165-1838(97)00039-8. [DOI] [PubMed] [Google Scholar]
- 38.Mancia G, Baccelli G, Zanchetti A. Regulation of renal circulation during behavioral changes in the cat. Am J Physiol. 1974;227:536–42. doi: 10.1152/ajplegacy.1974.227.3.536. [DOI] [PubMed] [Google Scholar]
- 39.Smith OA, Hohimer AR, Astley CA, Taylor DJ. Renal and hindlimb vascular control during acute emotion in the baboon. Am J Physiol. 1979;236:R198–205. doi: 10.1152/ajpregu.1979.236.3.R198. [DOI] [PubMed] [Google Scholar]
- 40.Forsyth RP. Regional blood-flow changes during 72-hour avoidance schedules in the monkey. Science. 1971;173:546–8. doi: 10.1126/science.173.3996.546. [DOI] [PubMed] [Google Scholar]
- 41.Wilson TE. Renal sympathetic nerve, blood flow, and epithelial transport responses to thermal stress. Auton Neurosci. 2017;204:25–34. doi: 10.1016/j.autneu.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 42.Eisman MM, Rowell LB. Renal vascular response to heat stress in baboons –Role of renin-angiotensin. J Appl Physiol Respir Environ Exerc Physiol. 1977;43:739–46. doi: 10.1152/jappl.1977.43.4.739. [DOI] [PubMed] [Google Scholar]
- 43.Kenney MJ, Musch TI. Senescence alters blood flow responses to acute heat stress. Am J Physiol Heart Circ Physiol. 2004;286:H1480–5. doi: 10.1152/ajpheart.00857.2003. [DOI] [PubMed] [Google Scholar]
- 44.Hermansson K, Källskog O, Wolgast M. Effect of renal nerve stimulation on the activity of the tubuloglomerular feedback mechanism. Acta Physiol Scand. 1984;120:381–5. doi: 10.1111/j.1748-1716.1984.tb07398.x. [DOI] [PubMed] [Google Scholar]
- 45.Walkowska A, Badzyńska B, Kompanowska-Jezierska E, Johns EJ, Sadowski J. Effects of renal nerve stimulation on intrarenal blood flow in rats with intact or inactivated NO synthases. Acta Physiol Scand. 2005;183:99–105. doi: 10.1111/j.1365-201X.2004.01376.x. [DOI] [PubMed] [Google Scholar]
- 46.Eppel GA, Malpas SC, Denton KM, Evans RG. Neural control of renal medullary perfusion. Clin Exp Pharmacol Physiol. 2004;31:387–96. doi: 10.1111/j.1440-1681.2004.04003.x. [DOI] [PubMed] [Google Scholar]
- 47.Leonard BL, Evans RG, Navakatikyan MA, Malpas SC. Differential neural control of intrarenal blood flow. Am J Physiol Regul Integr Comp Physiol. 2000;279:R907–16. doi: 10.1152/ajpregu.2000.279.3.R907. [DOI] [PubMed] [Google Scholar]
- 48.Johns EJ, Kopp UC, DiBona GF. Neural control of renal function. Compr Physiol. 2011;1:731–67. doi: 10.1002/cphy.c100043. [DOI] [PubMed] [Google Scholar]
- 49.Middlekauff HR, Nguyen AH, Negrao CE, Nitzsche EU, Hoh CK, Natterson BA, et al. Impact of acute mental stress on sympathetic nerve activity and regional blood flow in advanced heart failure: Implications for 'triggering'adverse cardiac events. Circulation. 1997;96:1835–42. doi: 10.1161/01.cir.96.6.1835. [DOI] [PubMed] [Google Scholar]
- 50.van Tilborg KA, Rabelink TJ, van Rijn HJ, Boomsma F, Koomans HA. Arterial baroreflex control of renal hemodynamics in humans. Circulation. 1994;90:1883–90. doi: 10.1161/01.cir.90.4.1883. [DOI] [PubMed] [Google Scholar]
- 51.Van Tilborg KA, Rabelink TJ, Koomans HA. Naloxone inhibits renal hemodynamic effect of head-out water immersion in humans. Kidney Int. 1995;48:860–5. doi: 10.1038/ki.1995.362. [DOI] [PubMed] [Google Scholar]
- 52.Massett MP, Johnson DG, Kregel KC. Cardiovascular and sympathoadrenal responses to heat stress following water deprivation in rats. Am J Physiol. 1996;270:R652–9. doi: 10.1152/ajpregu.1996.270.3.R652. [DOI] [PubMed] [Google Scholar]
- 53.Krum H, Schlaich M, Whitbourn R, Sobotka PA, Sadowski J, Bartus K, et al. Catheter-based renal sympathetic denervation for resistant hypertension: A multicentre safety and proof-of-principle cohort study. Lancet. 2009;373:1275–81. doi: 10.1016/S0140-6736(09)60566-3. [DOI] [PubMed] [Google Scholar]
- 54.Ramchandra R, Barrett CJ, Guild SJ, Malpas SC. Is the chronically denervated kidney supersensitive to catecholamines? Am J Physiol Regul Integr Comp Physiol. 2002;282:R603–10. doi: 10.1152/ajpregu.00404.2001. [DOI] [PubMed] [Google Scholar]
- 55.Brod J. Regulation of renal function. Chekh Fiziol. 1952;1:274–300. [PubMed] [Google Scholar]
- 56.Hollenberg NK, Adams DF, Solomon H, Chenitz WR, Burger BM, Abrams HL, et al. Renal vascular tone in essential and secondary hypertension: Hemodynamic and angiographic responses to vasodilators. Medicine (Baltimore) 1975;54:29–44. doi: 10.1097/00005792-197501000-00002. [DOI] [PubMed] [Google Scholar]
- 57.Sadowski J, Kurkus J, Gellert R. Denervated and intact kidney responses to saline load in awake and anesthetized dogs. Am J Physiol. 1979;237:F262–7. doi: 10.1152/ajprenal.1979.237.4.F262. [DOI] [PubMed] [Google Scholar]
- 58.Bello-Reuss E, Colindres RE, Pastoriza-Muñoz E, Mueller RA, Gottschalk CW. Effects of acute unilateral renal denervation in the rat. J Clin Invest. 1975;56:208–17. doi: 10.1172/JCI108069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Takishita S, Muratani H, Sesoko S, Teruya H, Tozawa M, Fukiyama K, et al. Short-term effects of angiotensin II blockade on renal blood flow and sympathetic activity in awake rats. Hypertension. 1994;24:445–50. doi: 10.1161/01.hyp.24.4.445. [DOI] [PubMed] [Google Scholar]
- 60.Abildgaard U, Holstein-Rathlou NH, Leyssac PP. Effect of renal nerve activity on tubular sodium and water reabsorption in dog kidneys as determined by the lithium clearance method. Acta Physiol Scand. 1986;126:251–7. doi: 10.1111/j.1748-1716.1986.tb07812.x. [DOI] [PubMed] [Google Scholar]
- 61.DiBona GF, Rios LL. Renal nerves in compensatory renal response to contralateral renal denervation. Am J Physiol. 1980;238:F26–30. doi: 10.1152/ajprenal.1980.238.1.F26. [DOI] [PubMed] [Google Scholar]
- 62.Ciccone CD, Zambraski EJ. Effects of acute renal denervation on kidney function in deoxycorticosterone acetate-hypertensive swine. Hypertension. 1986;8:925–31. doi: 10.1161/01.hyp.8.10.925. [DOI] [PubMed] [Google Scholar]
- 63.Matsukawa K, Wall PT, Wilson LB, Mitchell JH. Neurally mediated renal vasoconstriction during isometric muscle contraction in cats. Am J Physiol. 1992;262:H833–8. doi: 10.1152/ajpheart.1992.262.3.H833. [DOI] [PubMed] [Google Scholar]
- 64.Johns EJ. Role of the renal nerves in modulating renin release during pressure reduction at the feline kidney. Clin Sci (Lond) 1985;69:185–95. doi: 10.1042/cs0690185. [DOI] [PubMed] [Google Scholar]
- 65.Peterson TV, Chase NL, Gray DK. Renal effects of volume expansion in the renal-denervated nonhuman primate. Am J Physiol. 1984;247:H960–6. doi: 10.1152/ajpheart.1984.247.6.H960. [DOI] [PubMed] [Google Scholar]
- 66.Delacroix S, Chokka RG, Nelson AJ, Wong DT, Sidharta S, Pederson SM, et al. Renal sympathetic denervation increases renal blood volume per cardiac cycle: A serial magnetic resonance imaging study in resistant hypertension. Int J Nephrol Renovasc Dis. 2017;10:243–9. doi: 10.2147/IJNRD.S131220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tsioufis C, Papademetriou V, Dimitriadis K, Tsiachris D, Thomopoulos C, Park E, et al. Catheter-based renal sympathetic denervation exerts acute and chronic effects on renal hemodynamics in swine. International journal of cardiology. 2013;168:987–92. doi: 10.1016/j.ijcard.2012.10.038. [DOI] [PubMed] [Google Scholar]
- 68.Verloop WL, Hubens LE, Spiering W, Doevendans PA, Goldschmeding R, Bleys RL, et al. The effects of renal denervation on renal hemodynamics and renal vasculature in a porcine model. PLoS One. 2015;10:e0141609. doi: 10.1371/journal.pone.0141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Slick GL, Aguilera AJ, Zambraski EJ, DiBona GF, Kaloyanides GJ. Renal neuroadrenergic transmission. Am J Physiol. 1975;229:60–5. doi: 10.1152/ajplegacy.1975.229.1.60. [DOI] [PubMed] [Google Scholar]
- 70.DiBona GF, Sawin LL. Effect of renal denervation on dynamic autoregulation of renal blood flow. Am J Physiol Renal Physiol. 2004;286:F1209–18. doi: 10.1152/ajprenal.00010.2004. [DOI] [PubMed] [Google Scholar]
- 71.Just A, Wittmann U, Ehmke H, Kirchheim HR. Autoregulation of renal blood flow in the conscious dog and the contribution of the tubuloglomerular feedback. J Physiol. 1998;506(Pt 1):275–90. doi: 10.1111/j.1469-7793.1998.275bx.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Veress AT, Chong CK, Sonnenberg H. Effect of acute unilateral renal denervation on intrarenal haemodynamics and urinary excretion in rats before and during hypervolaemia. Clin Sci (Lond) 1982;62:457–64. doi: 10.1042/cs0620457. [DOI] [PubMed] [Google Scholar]
- 73.Kompanowska-Jezierska E, Walkowska A, Johns EJ, Sadowski J. Early effects of renal denervation in the anaesthetised rat: Natriuresis and increased cortical blood flow. J Physiol. 2001;531:527–34. doi: 10.1111/j.1469-7793.2001.0527i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Moreira NJ, Dos Santos F, Moreira ED, Farah D, de Souza LE, da Silva MB, et al. Acute renal denervation normalizes aortic function and decreases blood pressure in spontaneously hypertensive rats. Sci Rep. 2020;10:21826. doi: 10.1038/s41598-020-78674-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ott C, Janka R, Schmid A, Titze S, Ditting T, Sobotka PA, et al. Vascular and renal hemodynamic changes after renal denervation. Clin J Am Soc Nephrol. 2013;8:1195–201. doi: 10.2215/CJN.08500812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schlaich MP, Sobotka PA, Krum H, Lambert E, Esler MD. Renal sympathetic-nerve ablation for uncontrolled hypertension. N Engl J Med. 2009;361:932–4. doi: 10.1056/NEJMc0904179. [DOI] [PubMed] [Google Scholar]
- 77.Rudd MA, Grippo RS, Arendshorst WJ. Acute renal denervation produces a diuresis and natriuresis in young SHR but not WKY rats. Am J Physiol Renal Physiol. 1986;251:F655–17. doi: 10.1152/ajprenal.1986.251.4.F655. [DOI] [PubMed] [Google Scholar]
- 78.Bello-Reuss E, Pastoriza-Muńoz E, Colindres RE. Acute unilateral renal denervation in rats with extracellular volume expansion. Am J Physiol. 1977;232:F26–32. doi: 10.1152/ajprenal.1977.232.1.F26. [DOI] [PubMed] [Google Scholar]
- 79.Fan L, Mukaddam-Daher S, Gutkowska J, Nuwayhid BS, Quillen EW., Jr Renal perfusion pressure and renin secretion in bilaterally renal denervated sheep. Can J Physiol Pharmacol. 1994;72:782–7. doi: 10.1139/y94-111. [DOI] [PubMed] [Google Scholar]
- 80.Fernández-Repollet E, Silva-Netto CR, Colindres RE, Gottschalk CW. Role of renal nerves in maintaining sodium balance in unrestrained conscious rats. Am J Physiol. 1985;249:F819–26. doi: 10.1152/ajprenal.1985.249.6.F819. [DOI] [PubMed] [Google Scholar]
- 81.Clayton SC, Haack KK, Zucker IH. Renal denervation modulates angiotensin receptor expression in the renal cortex of rabbits with chronic heart failure. Am J Physiol Renal Physiol. 2011;300:F31–9. doi: 10.1152/ajprenal.00088.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.DiBona GF, Kopp UC. Neural control of renal function. Physiol Rev. 1997;77:75–197. doi: 10.1152/physrev.1997.77.1.75. [DOI] [PubMed] [Google Scholar]
- 83.Suzuki S, Takata Y, Kubota S, Ozaki S, Kato H. Characterization of the alpha-1 adrenoceptors in the mesenteric vasculature from deoxycorticosterone-salt hypertensive rats: Studies on vasoconstriction, radioligand binding and postreceptor events. J Pharmacol Exp Ther. 1994;268:576–83. [PubMed] [Google Scholar]
- 84.Nyborg NC, Bevan JA. Increased alpha-adrenergic receptor affinity in resistance vessels from hypertensive rats. Hypertension. 1988;11:635–8. doi: 10.1161/01.hyp.11.6.635. [DOI] [PubMed] [Google Scholar]
- 85.el Attari A, Qing W, Ben-Ishay D, Parini A, Dausse JP. Alpha-adrenoceptor properties in rat strains sensitive or resistant to salt-induced hypertension. Fundam Clin Pharmacol. 1989;3:483–95. doi: 10.1111/j.1472-8206.1989.tb00683.x. [DOI] [PubMed] [Google Scholar]
- 86.Caveney SW, Taylor DA, Fleming WW. Examination by radioligand binding of the alpha1 adrenoceptors in the mesenteric arterial vasculature during the development of salt-sensitive hypertension. Naunyn Schmiedebergs Arch Pharmacol. 1997;356:374–82. doi: 10.1007/pl00005065. [DOI] [PubMed] [Google Scholar]
- 87.Rudner XL, Berkowitz DE, Booth JV, Funk BL, Cozart KL, D'Amico EB, et al. Subtype specific regulation of human vascular alpha(1)-adrenergic receptors by vessel bed and age. Circulation. 1999;100:2336–43. doi: 10.1161/01.cir.100.23.2336. [DOI] [PubMed] [Google Scholar]
- 88.Osborn JW, Tyshynsky R, Vulchanova L. Function of renal nerves in kidney physiology and pathophysiology. Annu Rev Physiol. 2021;83:429–50. doi: 10.1146/annurev-physiol-031620-091656. [DOI] [PubMed] [Google Scholar]
- 89.Schlaich MP, Krum H, Sobotka PA, Esler MD. Renal denervation and hypertension. Am J Hypertens. 2011;24:635–42. doi: 10.1038/ajh.2011.35. [DOI] [PubMed] [Google Scholar]
- 90.Huggett RJ, Scott EM, Gilbey SG, Stoker JB, Mackintosh AF, Mary DA. Impact of type 2 diabetes mellitus on sympathetic neural mechanisms in hypertension. Circulation. 2003;108:3097–101. doi: 10.1161/01.CIR.0000103123.66264.FE. [DOI] [PubMed] [Google Scholar]
- 91.Lohmeier TE, Iliescu R. The sympathetic nervous system in obesity hypertension. Curr Hypertens Rep. 2013;15:409–16. doi: 10.1007/s11906-013-0356-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sobotka PA, Krum H, Böhm M, Francis DP, Schlaich MP. The role of renal denervation in the treatment of heart failure. Curr Cardiol Rep. 2012;14:285–92. doi: 10.1007/s11886-012-0258-x. [DOI] [PubMed] [Google Scholar]
- 93.Schmieder RE, Mahfoud F, Schmid A, Ditting T, Veelken R, Uder M, et al. Does renal denervation stopp renal function decline in treatment resistant hypertension: Results of a pilot study. Circulation. 2013;128:A17557. [Google Scholar]
- 94.Schlaich M, Straznicky N, Lambert E, Lambert G. Metabolic syndrome: A sympathetic disease? Lancet Diabetes Endocrinol. 2015;3:148–57. doi: 10.1016/S2213-8587(14)70033-6. [DOI] [PubMed] [Google Scholar]
- 95.Straznicky NE, Grima MT, Sari CI, Eikelis N, Lambert EA, Nestel PJ, et al. Neuroadrenergic dysfunction along the diabetes continuum: A comparative study in obese metabolic syndrome subjects. Diabetes. 2012;61:2506–16. doi: 10.2337/db12-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Narkiewicz K, Somers VK. Sympathetic nerve activity in obstructive sleep apnoea. Acta Physiol Scand. 2003;177:385–90. doi: 10.1046/j.1365-201X.2003.01091.x. [DOI] [PubMed] [Google Scholar]
- 97.Esler M. The sympathetic system and hypertension. Am J Hypertens. 2000;13:99S–105S. doi: 10.1016/s0895-7061(00)00225-9. [DOI] [PubMed] [Google Scholar]
- 98.Gattone VH, 2nd, Evan AP, Overhage JM, Severs WB. Developing renal innervation in the spontaneously hypertensive rat: Evidence for a role of the sympathetic nervous system in renal damage. J Hypertens. 1990;8:423–8. doi: 10.1097/00004872-199005000-00005. [DOI] [PubMed] [Google Scholar]
- 99.Calhoun DA, Jones D, Textor S, Goff DC, Murphy TP, Toto RD, et al. Resistant hypertension: diagnosis, evaluation, and treatment. A scientific statement from the American Heart Association professional education committee of the council for high blood pressure research. Hypertension. 2008;51:1403–19. doi: 10.1161/HYPERTENSIONAHA.108.189141. [DOI] [PubMed] [Google Scholar]
- 100.Thorp AA, Schlaich MP. Device-based approaches for renal nerve ablation for hypertension and beyond. Front Physiol. 2015;6:193. doi: 10.3389/fphys.2015.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nishi EE, Bergamaschi CT, Campos RR. The crosstalk between the kidney and the central nervous system: The role of renal nerves in blood pressure regulation. Exp Physiol. 2015;100:479–84. doi: 10.1113/expphysiol.2014.079889. [DOI] [PubMed] [Google Scholar]
- 102.Banek CT, Knuepfer MM, Foss JD, Fiege JK, Asirvatham-Jeyaraj N, Van Helden D, et al. Resting afferent renal nerve discharge and renal inflammation: Elucidating the role of afferent and efferent renal nerves in deoxycorticosterone acetate salt hypertension. Hypertension. 2016;68:1415–23. doi: 10.1161/HYPERTENSIONAHA.116.07850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ong J, Kinsman BJ, Sved AF, Rush BM, Tan RJ, Carattino MD, et al. Renal sensory nerves increase sympathetic nerve activity and blood pressure in 2-kidney 1-clip hypertensive mice. J Neurophysiol. 2019;122:358–67. doi: 10.1152/jn.00173.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Milanez MI, Veiga AC, Martins BS, Pontes RB, Bergamaschi CT, Campos RR, et al. Renal sensory activity regulates the γ-aminobutyric acidergic inputs to the paraventricular nucleus of the hypothalamus in goldblatt hypertension. Front Physiol. 2020;11:601237. doi: 10.3389/fphys.2020.601237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nishi EE, Lopes NR, Gomes GN, Perry JC, Sato AY, Naffah-Mazzacoratti MG, et al. Renal denervation reduces sympathetic overactivation, brain oxidative stress, and renal injury in rats with renovascular hypertension independent of its effects on reducing blood pressure. Hypertens Res. 2019;42:628–40. doi: 10.1038/s41440-018-0171-9. [DOI] [PubMed] [Google Scholar]
- 106.Ikeda S, Shinohara K, Kashihara S, Matsumoto S, Yoshida D, Nakashima R, et al. Contribution of afferent renal nerve signals to acute and chronic blood pressure regulation in stroke-prone spontaneously hypertensive rats. Hypertens Res. 2023;46:268–79. doi: 10.1038/s41440-022-01091-z. [DOI] [PubMed] [Google Scholar]
- 107.Mendoza MF, Kachur SM, Lavie CJ. Hypertension in obesity. Curr Opin Cardiol. 2020;35:389–96. doi: 10.1097/HCO.0000000000000749. [DOI] [PubMed] [Google Scholar]
- 108.King AJ, Osborn JW, Fink GD. Splanchnic circulation is a critical neural target in angiotensin II salt hypertension in rats. Hypertension. 2007;50:547–56. doi: 10.1161/HYPERTENSIONAHA.107.090696. [DOI] [PubMed] [Google Scholar]
- 109.Kline RL, Mercer PF. Functional reinnervation and development of supersensitivity to NE after renal denervation in rats. Am J Physiol. 1980;238:R353–8. doi: 10.1152/ajpregu.1980.238.5.R353. [DOI] [PubMed] [Google Scholar]
- 110.Granger J, Novak J, Schnackenberg C, Williams S, Reinhart GA. Role of renal nerves in mediating the hypertensive effects of nitric oxide synthesis inhibition. Hypertension. 1996;27:613–8. doi: 10.1161/01.hyp.27.3.613. [DOI] [PubMed] [Google Scholar]
- 111.Foss JD, Fiege J, Shimizu Y, Collister JP, Mayerhofer T, Wood L, et al. Role of afferent and efferent renal nerves in the development of AngII-salt hypertension in rats. Physiol Rep. 2018;6:e13602. doi: 10.14814/phy2.13602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Iliescu R, Lohmeier TE, Tudorancea I, Laffin L, Bakris GL. Renal denervation for the treatment of resistant hypertension: Review and clinical perspective. Am J Physiol Renal Physiol. 2015;309:F583–94. doi: 10.1152/ajprenal.00246.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chen WJ, Liu H, Wang ZH, Liu C, Fan JQ, Wang ZL, et al. The impact of renal denervation on the progression of heart failure in a canine model induced by right ventricular rapid pacing. Front Physiol. 2019;10:1625. doi: 10.3389/fphys.2019.01625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.DiBona GF. Dynamic analysis of patterns of renal sympathetic nerve activity: Implications for renal function. Exp Physiol. 2005;90:159–61. doi: 10.1113/expphysiol.2004.029215. [DOI] [PubMed] [Google Scholar]
- 115.Sharp TE, 3rd, Lefer DJ. Renal denervation to treat heart failure. Annu Rev Physiol. 2021;83:39–58. doi: 10.1146/annurev-physiol-031620-093431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bozkurt B, Aguilar D, Deswal A, Dunbar SB, Francis GS, Horwich T, et al. Contributory risk and management of comorbidities of hypertension, obesity, diabetes mellitus, hyperlipidemia, and metabolic syndrome in chronic heart failure: A scientific statement from the American Heart Association. Circulation. 2016;134:e535–78. doi: 10.1161/CIR.0000000000000450. [DOI] [PubMed] [Google Scholar]
- 117.Remme WJ. Neurohormonal modulation in heart failure: ACE inhibition and beyond. Eur Heart J. 1995;16(Suppl N):73–8. doi: 10.1093/eurheartj/16.suppl_n.73. [DOI] [PubMed] [Google Scholar]
- 118.Braunwald E. The path to an angiotensin receptor antagonist-neprilysin inhibitor in the treatment of heart failure. J Am Coll Cardiol. 2015;65:1029–41. doi: 10.1016/j.jacc.2015.01.033. [DOI] [PubMed] [Google Scholar]
- 119.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized aldactone evaluation study investigators. N Engl J Med. 1999;341:709–17. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 120.Rossignol P, Dobre D, McMurray JJ, Swedberg K, Krum H, van Veldhuisen DJ, et al. Incidence, determinants, and prognostic significance of hyperkalemia and worsening renal function in patients with heart failure receiving the mineralocorticoid receptor antagonist eplerenone or placebo in addition to optimal medical therapy: Results from the eplerenone in mild patients hospitalization and survival study in heart failure (EMPHASIS-HF) Circ Heart Fail. 2014;7:51–8. doi: 10.1161/CIRCHEARTFAILURE.113.000792. [DOI] [PubMed] [Google Scholar]
- 121.Fitzgerald AA, Powers JD, Ho PM, Maddox TM, Peterson PN, Allen LA, et al. Impact of medication nonadherence on hospitalizations and mortality in heart failure. J Card Fail. 2011;17:664–9. doi: 10.1016/j.cardfail.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 122.Schiller AM, Pellegrino PR, Zucker IH. The renal nerves in chronic heart failure: Efferent and afferent mechanisms. Front Physiol. 2015;6:224. doi: 10.3389/fphys.2015.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Parish JM, Somers VK. Obstructive sleep apnea and cardiovascular disease. Mayo Clin Proc. 2004;79:1036–46. doi: 10.4065/79.8.1036. [DOI] [PubMed] [Google Scholar]
- 124.Linz D, Hohl M, Nickel A, Mahfoud F, Wagner M, Ewen S, et al. Effect of renal denervation on neurohumoral activation triggering atrial fibrillation in obstructive sleep apnea. Hypertension. 2013;62:767–74. doi: 10.1161/HYPERTENSIONAHA.113.01728. [DOI] [PubMed] [Google Scholar]
- 125.Villarreal D, Freeman RH, Johnson RA, Simmons JC. Effects of renal denervation on postprandial sodium excretion in experimental heart failure. Am J Physiol. 1994;266:R1599–604. doi: 10.1152/ajpregu.1994.266.5.R1599. [DOI] [PubMed] [Google Scholar]
- 126.Masaki H, Imaizumi T, Harasawa Y, Takeshita A. Dynamic arterial baroreflex in rabbits with heart failure induced by rapid pacing. Am J Physiol. 1994;267:H92–9. doi: 10.1152/ajpheart.1994.267.1.H92. [DOI] [PubMed] [Google Scholar]
- 127.Singh RR, Sajeesh V, Booth LC, McArdle Z, May CN, Head GA, et al. Catheter-based renal denervation exacerbates blood pressure fall during hemorrhage. J Am Coll Cardiol. 2017;69:951–64. doi: 10.1016/j.jacc.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 128.Hering D, Marusic P, Duval J, Sata Y, Head GA, Denton KM, et al. Effect of renal denervation on kidney function in patients with chronic kidney disease. Int J Cardiol. 2017;232:93–7. doi: 10.1016/j.ijcard.2017.01.047. [DOI] [PubMed] [Google Scholar]
- 129.Kiuchi MG, Maia GL, de Queiroz Carreira MA, Kiuchi T, Chen S, Andrea BR, et al. Effects of renal denervation with a standard irrigated cardiac ablation catheter on blood pressure and renal function in patients with chronic kidney disease and resistant hypertension. Eur Heart J. 2013;34:2114–21. doi: 10.1093/eurheartj/eht200. [DOI] [PubMed] [Google Scholar]
- 130.Ghadian A, Einollahi B, Ebrahimi M, Javanbakht M, Asadi M, Kazemi R. Renal function markers in single-kidney patients after percutaneous nephrolithotomy: A pilot study. J Res Med Sci. 2022;27:17. doi: 10.4103/jrms.jrms_880_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Basile DP, Yoder MC. Renal endothelial dysfunction in acute kidney ischemia reperfusion injury. Cardiovasc Hematol Disord Drug Targets. 2014;14:3–14. doi: 10.2174/1871529x1401140724093505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Eppel GA, Denton KM, Malpas SC, Evans RG. Nitric oxide in responses of regional kidney perfusion to renal nerve stimulation and renal ischaemia. Pflugers Arch. 2003;447:205–13. doi: 10.1007/s00424-003-1149-1. [DOI] [PubMed] [Google Scholar]
- 133.Kaur J, Young BE, Fadel PJ. Sympathetic overactivity in chronic kidney disease: Consequences and mechanisms. Int J Mol Sci. 2017;18:1682. doi: 10.3390/ijms18081682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bruck H, Gössl M, Spitthöver R, Schäfers RF, Kohnle M, Philipp T, et al. The nitric oxide synthase inhibitor L-NMMA potentiates noradrenaline-induced vasoconstriction: Effects of the alpha2-receptor antagonist yohimbine. J Hypertens. 2001;19:907–11. doi: 10.1097/00004872-200105000-00011. [DOI] [PubMed] [Google Scholar]
- 135.Li S, Hildreth CM, Rahman AA, Barton SA, Wyse BF, Lim CK, et al. Renal denervation does not affect hypertension or the renin-angiotensin system in a rodent model of juvenile-onset polycystic kidney disease: Clinical implications. Sci Rep. 2021;11:14286. doi: 10.1038/s41598-021-93575-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Böhm M, Mahfoud F, Townsend RR, Kandzari DE, Pocock S, Ukena C, et al. Ambulatory heart rate reduction after catheter-based renal denervation in hypertensive patients not receiving anti-hypertensive medications: data from SPYRAL HTN-OFF MED, a randomized, sham-controlled, proof-of-concept trial. Eur Heart J. 2019;40:743–51. doi: 10.1093/eurheartj/ehy871. [DOI] [PubMed] [Google Scholar]
- 137.Mahfoud F, Townsend RR, Kandzari DE, Kario K, Schmieder RE, Tsioufis K, et al. Changes in plasma renin activity after renal artery sympathetic denervation. J Am Coll Cardiol. 2021;77:2909–19. doi: 10.1016/j.jacc.2021.04.044. [DOI] [PubMed] [Google Scholar]
- 138.Kario K, Kagitani H, Hayashi S, Hanamura S, Ozawa K, Kanegae H. A Japan nationwide web-based survey of patient preference for renal denervation for hypertension treatment. Hypertens Res. 2022;45:232–40. doi: 10.1038/s41440-021-00760-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gazdar AF, Dammin GJ. Neural degeneration and regeneration in human renal transplants. N Engl J Med. 1970;283:222–4. doi: 10.1056/NEJM197007302830502. [DOI] [PubMed] [Google Scholar]