Abstract
BACKGROUND:
Vitamin D deficiency (VDD) is highly prevalent across the globe. Cholecalciferol (Vitamin D3) fails to attain sufficient serum concentrations of 25-hydroxyvitamin D (25(OH)D) in a significant proportion of supplemented individuals. Calcifediol (25-hydroxyvitamin D3) is less studied in healthy adults and its effects on 25(OH)D, parathyroid hormone (PTH), and 1,25-dihydroxyvitamin D (1,25(OH)2D) at higher doses are not well known.
MATERIALS AND METHODS:
The study was an open-label, interventional trial recruiting consecutive participants with VDD who were allocated to receive either 2 capsules (50 μg-group) or 1 capsule (25 μg-group) daily doses of calcifediol. Baseline assessment included clinicodemographic parameters, dietary calcium, calcemic (calcium, inorganic phosphate, albumin, alkaline phosphatase, urine spot calcium/creatinine), and hormonal parameters (25(OH)D, PTH, and 1,25(OH)2D). Participants were followed up at 4 and 8 weeks with repeat assessments of calcemic and hormonal parameters.
RESULTS:
There were 64 participants, 35 (50 μg-group) and 29 (25 μg-group), without any significant difference in any of the baseline parameters. 97.1% participants in the 50 μg-group (at 4 and 8 weeks) and 93.1% (at 4 weeks) and 96.5% (at 8 weeks) in the 25 μg-group attained 25(OH)D sufficiency (≥30 ng/ml) with calcifediol. The mean serum 25(OH)D was 84.0 ± 27.7 ng/ml in the 50 μg-group and 58.0 ± 23.6 ng/ml in the 25 μg-group group at 4 weeks, which later rose to 94.3 ± 21.8 ng/ml and 76.0 ± 16.4 ng/ml, respectively, at 8 weeks. PTH levels decreased in both groups at both time points. 1,25(OH)2D rose significantly in both groups at 4 and 8 weeks but was not significantly different between both groups. There was no case of incident hypercalcemia or symptomatic nephrolithiasis.
CONCLUSION:
Calcifediol is a safe and efficacious alternative for oral Vitamin D supplementation in young adults. Increment in 25(OH)D levels is rapid and dose-dependent.
Keywords: 1, 25-dihydroxyvitamin D; calcifediol; 25-hydroxyvitamin D; parathyroid hormone; Vitamin D supplementation
Introduction
Calcifediol, or 25-hydroxyvitamin D3, is a metabolite of Vitamin D that helps attain Vitamin D sufficiency faster and at a higher level. Although cholecalciferol (D3) and ergocalciferol (D2) are the most common therapeutic options used for Vitamin D supplementation, there exist several barriers to achieving an optimal 25-hydroxyvitamin D (25(OH)D) level with these preparations. Further, there are diverse determinants of the response of 25(OH)D levels following supplementation in a given individual, resulting in considerable inter-individual variation.[1,2] These include the efficiency of intestinal absorption, body fat or adiposity, and cytochrome genes regulating Vitamin D metabolism.[3,4,5] The baseline 25(OH)D is another important determinant of the eventual concentration attained following supplementation.[6] Apart from the variability in response, the overall efficacy of D3 supplementation seems suboptimal, as the pooled mean difference of the level of 25(OH)D attained following supplementation was found to be approximately 37 nmol/l (approximately 15 ng/ml) in a recent meta-analysis.[4] The final mean levels of 25(OH)D varied between 46 and 123 nmol/l with an average of 81.3 nmol/l (approximately 32 ng/ml). In another study, even doses as high as 2000 IU/d of Vitamin D3 were unable to correct the deficiency in 25% of subjects.[7] In randomized controlled trials where doses of 400 IU/d (corresponding to the recommended dietary allowance for young adults) or 800 IU/d (higher than the recommended allowance for older adults) were supplemented, nearly 50% of the individuals failed to attain the desired level of 30 ng/ml, even following intervention for approximately 2 years.[8] The response to Vitamin D3 supplementation is graded and varies with the dose supplemented, but adequate levels (30–50 ng/ml) were attained in a proportion of the intervened population, only at doses exceeding 2000–4000 IU/d.[8] These data point toward the existing shortcomings both in terms of eventually attained the levels and the proportion of patients attaining sufficiency following intervention with Vitamin D3 supplementation.
While cholecalciferol is the most commonly used formulation, 1,25-dihydroxyvitamin D (1,25(OH)2D) (the active metabolite of Vitamin D) use is limited to specific populations, such as those with chronic renal insufficiency or hypoparathyroidism (characterized by inactivation of 1-α-hydroxylase), owing to the higher incidence of adverse effects and the need for regular and stringent monitoring. Among the other available metabolites of Vitamin D, calcifediol is the one with good potential benefits but is less studied, with limited use in clinical settings.[9] Calcifediol is a highly potent oral formulation of Vitamin D, with good oral bioavailability, leading to higher and faster elevation in serum 25(OH)D concentrations. Importantly, it has a linear dose-response curve, such that the influence of baseline 25(OH)D level on the final 25(OH)D concentrations is much less marked with its use as opposed to Vitamin D3. Although the liver and some extra-hepatic sites are equipped with the 25-α-hydroxylase enzyme, the conversion of Vitamin D3 (cholecalciferol) to calcifediol is not always optimal and influenced by several variables. Hence, calcifediol has been suggested to be used more extensively in clinical practice.[1,10]
The current study was planned to evaluate the effects of calcifediol supplementation in two varying doses for 2 months on the dynamic alterations in various biochemical and hormonal parameters of bone and mineral metabolism.
Materials and Methods
The study was conducted by the Department of Endocrinology Post Graduate Institute of Medical Education and Research (PGIMER), Chandigarh. Participants were recruited at the outpatient department and assessed for eligibility. The inclusion criteria included adults with Vitamin D deficiency (VDD) (25(OH)D <30 ng/ml) at baseline who provided consent. Those with chronic kidney disease, pregnancy, or lactation, already on Vitamin D supplementation or with 25(OH)D levels exceeding 30 ng/ml, hypercalcemia (>10.2 mg/dl), primary hyperparathyroidism, chronic glucocorticoid therapy (>5 mg/d in prior 3 months), HIV or other known immunodeficiencies, active malignancy, psychiatric illness, or other immunosuppressive treatment were excluded from the study. Since it was a pilot study, the proposed minimum sample size was 30, and ultimately, 64 participants fulfilling the inclusion criteria were enrolled.
The study design was an open-label, interventional trial in consecutively recruited participants who were allocated to either of the two treatment arms. The first arm (50 μg-group) included participants who were prescribed 50 μg calcifediol daily (2 capsules) and the second arm (25 μg-capsule group) comprised participants who received 25 μg calcifediol (1 capsule) daily. Study capsules were kindly gifted by the Dishman Carbogen Amcis Ltd. Written, informed consent was obtained from the participants and the study was an offshoot of the DIVE study, which was approved by the Institutional Ethics Committee, PGIMER (PGI/IEC/2021/SPL-1256).
Biochemical and hormonal investigations
Baseline clinical details of participants, including demographic parameters, anthropometry, and comorbidities, were recorded. Participants fulfilling the eligibility criteria mentioned above were recruited in the study. Baseline investigations included calcemic parameters, urine calcium creatinine clearance ratio (CCCR), and hormonal parameters like 25(OH)D, intact parathyroid hormone (iPTH), and 1,25(OH)2D. Albumin-corrected calcium was used for the analysis. Calcemic parameters were estimated using COBAS 8000 Analyser Roche Diagnostics, Germany. Serum 25(OH)D and iPTH were measured by electro-chemiluminescence assay (ECLIA, COBAS 8000, Roche Diagnostics, Germany) with a coefficient of variation of 6%–8%. 1,25(OH)2D was measured by chemiluminescence assay (CLIA, Diasorin Liasion). The CCCR was estimated using a fully automated biochemical analyzer, Em 200, Transasia). Dietary calcium was calculated by recording average daily calcium intake (from all food items as well as from dairy products alone), using the DietCal version 10.0 AIIMS, New Delhi.
Clinical visits and assessment of adherence
Participants in both arms were supplemented for 8 weeks. The follow-up protocol consisted of a re-assessment of the calcemic and hormonal parameters at 4 and 8 weeks from the time of recruitment into the study. Reinforcement for daily supplementation was done by the team at the time of enrolment, telephonic conversations or messaging, and at each follow-up visit. Compliance was assessed by capsule counting at each follow-up visit. Adverse events, if any, were also recorded at each follow-up visit.
Statistical analysis
Statistical analysis was performed using the statistical package Graph pad software (Prism version 9.0, San Diego, California, USA). Quantitative variables were checked for normality using the Kolmogorov–Smirnov test and classified as parametric and nonparametric. The paired t-test was used to compare the means of values during follow-up in both the intervention arms and the unpaired t-test was used to compare the mean values between both intervention arms at any given point in time. Change in values of the hormonal parameters was evaluated in both treatment arms using the Wilcoxon matched-pairs test. Mann–Whitney U-test was used to compare the delta changes in both groups for different parameters. A P < 0.05 was considered significant.
Results
There was a total of 64 participants, 35 in the 50 μg-group and 29 in the 25-μg group, who were recruited into the study, intervened with calcifediol, and followed up for 8 weeks. The participant inclusion and follow-up protocol are summarized in Supplementary Figures 1 (436.7KB, tif) and 2 (244.2KB, tif) . The baseline parameters of both groups are depicted in Table 1. The mean age of the participants was comparable in both groups, and nearly one-third were males in either of the groups. Baseline biochemical, hormonal, and dietary calcium intake were also comparable between both groups.
Table 1.
Baseline clinic-demographic, anthropometric, and biochemical parameters of the participants
| Parameter | 50 µg-group (n=35) | 25 µg-group (n=29) | P | Normal range |
|---|---|---|---|---|
| Age (years) | 40.1±12.1 | 35.2±13.1 | 0.11 | - |
| Gender (% males) | 34.2 | 34.4 | 0.86 | - |
| Height (cm) | 161.7±6.8 | 159.8±9.2 | 0.42 | - |
| Weight (kg) | 72.3±20.2 | 64.2±13.3 | 0.07 | - |
| BMI (kg/m2) | 27.0±6.8 | 24.6±6.3 | 0.13 | 18.0–22.9 |
| Average dietary calcium intake from total diet (mg) | 868±271.6 | 936.7±273.3 | 0.37 | 600 |
| Average dietary calcium intake from dairy products alone (mg) | 626.8±252.0 | 692.2±257.1 | 0.33 | |
| Ca (mg/dL) | 9.1±0.3 | 9.1±0.4 | 0.93 | 8.6–10.2 |
| PO4 (mg/dL) | 3.4±0.4 | 3.7±0.7 | 0.11 | 2.7–4.5 |
| Albumin (g/dL) | 4.7±0.7 | 4.5±0.3 | 0.33 | 3.4–4.8 |
| ALP (IU/L) | 91.1±27.0 | 100.4±31.9 | 0.24 | 42–128 |
| 25(OH)D (ng/mL) | 16.9±6.3 | 18.7±7.4 | 0.29 | 11.1–42.9 |
| PTH (pg/mL) | 69.4±33.1 | 67.0±29.6 | 0.73 | 15–65 |
| CCCR | 0.11±0.09 | 0.12±0.07 | 0.49 | <0.20 |
| 1,25 (OH) 2D (pmol/L) | 119.5±51.9 | 118.9±40.3 | 0.91 | 47–190 |
BMI: Body mass index, CCCR: Calcium creatinine clearance ratio, ALP: Alkaline phosphatase, PTH: Parathyroid hormone, 25(OH)D: 25-hydroxyvitamin D, 1,25(OH)2D: 1,25-dihydroxyvitamin D, PO4: Phosphate
The follow-up hormonal parameters are represented in Figures 1-4. Figures 1 and 2 depict the dynamics of various biochemical and hormonal parameters in the 50 μg-group and Figures 3 and 4 depict the changes in various biochemical and hormonal parameters in the 25 μg-group.
Figure 1.
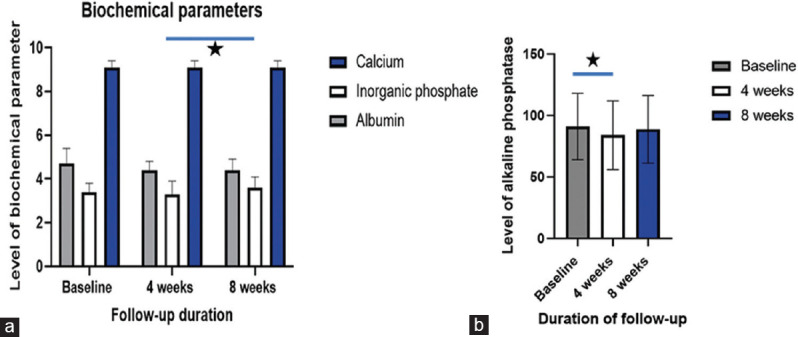
Alterations in calcemic including calcium and inorganic phosphate (a) and alkaline phosphatase (b) following supplementation with 50 μg calcifediol over 8 weeks follow-up, Asterisk denotes significance (p<0.05)
Figure 4.
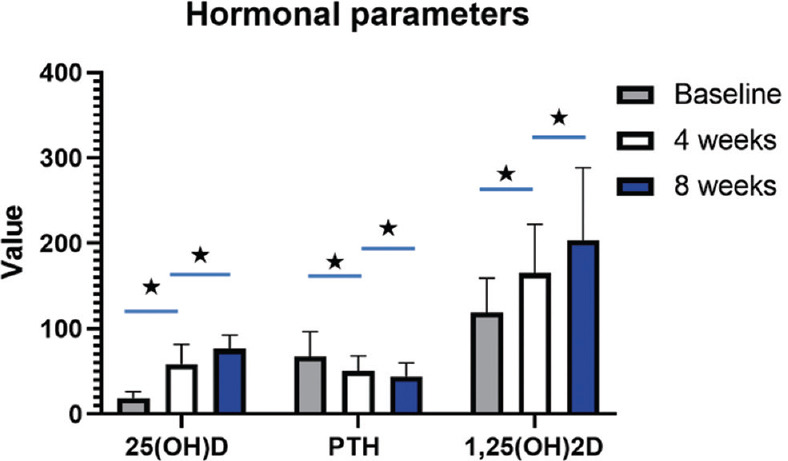
Serum concentration time graphs depicting alterations in hormonal parameters such as 25-hydroxyvitamin D, Parathyroid hormone, and 1,25-dihydroxyvitamin D following supplementation with 25 μg calcifediol over 8 weeks follow-up. PTH: Parathyroid hormone, 25(OH)D: 25-hydroxyvitamin D, 1,25(OH)2D: 1,25-dihydroxyvitamin D, Asterisk denotes significance (p<0.05)
Figure 2.
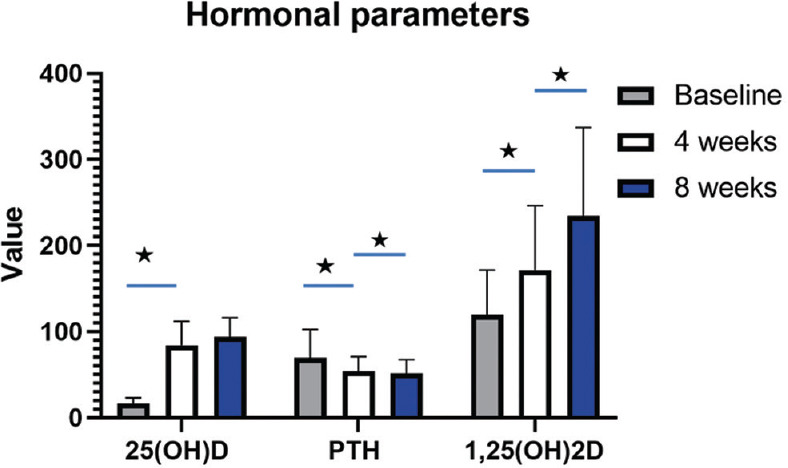
Serum concentration time graphs depicting alterations in hormonal parameters such as 25-hydroxyvitamin D, parathyroid hormone, and 1,25-dihydroxyvitamin D following supplementation with 50 μg calcifediol over 8 weeks follow-up. PTH: Parathyroid hormone, 25(OH)D: 25-hydroxyvitamin D, 1,25(OH)2D: 1,25-dihydroxyvitamin D, Asterisk denotes significance (p<0.05)
Figure 3.
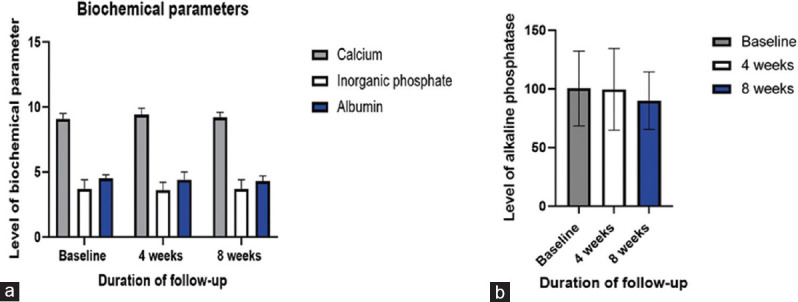
Alterations in calcemic including calcium, inorganic phosphate (a) and alkaline phosphatase (b) following supplementation with 25 μg calcifediol over 8 weeks follow-up, Asterisk denotes significance (p<0.05)
The median rise in 25(OH)D was 406.5% (interquartile range [IQR]: 240.5–557.5) at 4 weeks and 16.9% (IQR: −4.3–45) at 8 weeks in the 50 μg-group. Correspondingly, the median parathyroid hormone (PTH) levels fell by 20.8% (IQR: −35.4–1.5) at 4 weeks and 9.8% (IQR: −19.3–9.3) at 8 weeks. The median rise in 25(OH)D was 184.5% (IQR: 90.6–381.5) at 4 weeks and 36.7% (IQR: 17.4%–82.6%) at 8 weeks in the 25 μg-group. The PTH fell by a median of 24.6% (IQR: −60.8–11.3) at 4 weeks and 3.9% (IQR: −36.2–38.6) at 8 weeks in the 25 μg-group. The change in serum concentration of 25(OH)D was significantly higher in the 50 μg-group as compared to the 25 μg-group at 4 weeks (P = 0.0002) and 8 weeks (P = 0.03). The fall in PTH was not significant between both groups at 4 weeks (P = 0.50), or at 8 weeks (P = 0.66). The median rise in 1,25(OH) 2D was 37.0% (IQR: 4.3–74.9) at 4 weeks and 31.2% (IQR: 10.3–59.8) at 8 weeks in the 50 μg-group, whereas it was 33.1% (IQR: 16.5–74.4) at 4 weeks and 23.7% (IQR: −3.5–61.7) at 8 weeks in the 1-capsule group. Both changes were not significantly different between both groups (P = 0.64 at 4 weeks and 0.55 at 8 weeks). The alterations in serum concentrations of various biochemical and hormonal parameters are summarized in Table 2 and Supplementary Figure 3 (731.7KB, tif) .
Table 2.
Biochemical and hormonal parameters in both treatment arms following intervention with calcifediol
| Parameter | 50 µg-group (n=35) | 25 µg-group (n=29) | P |
|---|---|---|---|
| 4 weeks | |||
| Ca (mg/dL) | 9.1±0.3 | 9. 4±0.5 | 0.71 |
| PO4 (mg/dL) | 3.3±0.6 | 3.6±0.6 | 0.23 |
| Albumin (g/dL) | 4.4±0.4 | 4.4±0.6 | 0.63 |
| ALP (IU/L) | 84.0±27.9 | 99.7±34.8 | 0.06 |
| 25(OH)D (ng/mL) | 84.0±27.7 | 58.0±23.6 | 0.0002 |
| PTH (pg/mL) | 54.4±16.4 | 50.5±17.6 | 0.37 |
| 1,25 (OH)2D (pg/mL) | 171.5±74.8 | 165.3±56.7 | 0.67 |
| 8 weeks | |||
| Ca (mg/dL) | 9.1±0.3 | 9.2±0.4 | 0.98 |
| PO4 (mg/dL) | 3.6±0.5 | 3.7±0.7 | 0.58 |
| Albumin (g/dL) | 4.4±0.5 | 4.3±0.4 | 0.67 |
| ALP (IU/L) | 88.8±27.5 | 90.0±24.5 | 0.91 |
| 25(OH)D (ng/mL) | 94.3±21.8 | 76.0±16.4 | 0.001 |
| PTH (pg/mL) | 51.6±16.0 | 43.9±16.0 | 0.06 |
| 1,25 (OH)2D (pg/mL) | 234.5±102.8 | 203.3±85.3 | 0.18 |
ALP: Alkaline phosphatase, PTH: Parathyroid hormone, PO4: Phosphate, 25(OH)D: 25-hydroxyvitamin D, 1,25(OH)2D: 1,25-dihydroxyvitamin D
All except 1 subject (97.1%) attained sufficiency of Vitamin D with levels of exceeding 30 ng/ml at 4-and 8-week follow-up in the 50 μg-group. On the other hand, in the 25 μg-group, all except 2 participants (93.1%) attained sufficiency at 4 weeks and all except 1 participant (96.5%) were sufficient for Vitamin D at 8 weeks. Serum PTH did not normalize (<65 pg/ml) in 20% (n = 7) of participants at 4 weeks and 11.4% (n = 4) at 8 weeks in the 50 μg-group. Similarly, the rate of nonnormalization of PTH was 13.7% (n = 4) at 4 weeks and 10.3% (n = 3) at 8 weeks in the 25 μg-group.
Hypercalciuria was prevalent in 5.7% (two subjects) in the 50 μg and 10.3% (three subjects) in the 25 μg-group at 8 weeks, respectively, but was only marginally raised. There was no case of hypercalcemia or incident of symptomatic nephrolithiasis in any of the participants.
Discussion
The current study provides information on the dynamic alterations in multiple calcemic and hormonal parameters following supplementation with two varying doses of calcifediol in participants with VDD. We found that both doses of calcifediol were efficacious in significantly raising the levels of Vitamin D as early as 4 weeks. The levels of 25(OH)D increased further till 8 weeks, but the change was much lower and not significantly higher than the concentration attained at 4 weeks. However, PTH levels decreased and 1,25(OH)2D levels increased as early as 4 weeks and continued till 8 weeks, even after the 25(OH)D level plateaued. The proportion of participants attaining sufficiency of 25(OH)D exceeded 93% in either of the intervention arms at both 4 and 8 weeks. There was neither a significant increase (beyond the upper range of normal) in serum calcium, inorganic phosphate, nor alkaline phosphatase (ALP) in either of the intervention arms nor any instance of nephrolithiasis, except a significant fall in ALP at 4 weeks in the 50 μg-group. These lines of evidence point towards the fact that even a 25 μg daily dose of calcifediol is effective and safe in raising the level of 25(OH)D in individuals with VDD in as early as 4 weeks. Further, the dose-response of 25(OH)D to calcifediol is dose-dependent, with higher mean levels attained with 50 μg compared to the 25 μg dose.
Vitamin D has numerous skeletal and extra-skeletal benefits and mediates several genomic and nongenomic biological pathways.[11,12,13] There is overwhelming evidence in favor of an association between VDD and a score of nonskeletal parameters. However, there are negative results obtained from intervention studies (with Vitamin D and cardiovascular disease, cancer, and diabetes mellitus). Therefore, correction of VDD is important and imperative.[11] Cholecalciferol and ergocalciferol (D2) are the most commonly used oral formulations for the correction of VDD. However, several factors impede the attainment of Vitamin D sufficiency after supplementation with these formulations. This is reflected by evidence from large-scale population studies, which reveal the high burden of uncorrected VDD even following supplementation (~25%–50% in various settings).[7,8] Hence, there exists a need for optimizing the use of alternatives such as calcifediol for the correction of prevalent VDD, which needs further investigation. The current study was aimed at evaluating the efficacy and safety of calcifediol supplementation in adults with VDD over 8-week duration.
Apart from the fact that calcifediol is more hydrophilic and already hydroxylated, thereby offering obvious advantages in raising Vitamin D levels in individuals with malabsorption or hepatic dysfunction, it is also more potent and raises Vitamin D levels rapidly.[14,15] The bioavailability of calcifediol is enhanced by the fact that it has a higher binding affinity to Vitamin D binding protein and a higher rate of intestinal absorption.[16] The fact that it is a step closer to the generation of 1,25(OH)2D, the active metabolite, also contributes to its potency. Calcifediol use has been previously reported in a dose-response study with a smaller patient population (n = 59) where older adults (≥65 years) were administered doses of 5, 10, and 15 μg.[17] Levels of Vitamin D attained were between 30 and 40 ng/ml in the 10 and 15 μg dose groups at 4 and 8 weeks, respectively. Further, daily, weekly, or bolus doses of calcifediol have been reported to have more potency than Vitamin D3.[14] The commonly used doses of calcifediol are 0.667 mg monthly, 20 μg daily, or 140 μg weekly. However, bolus dosing is physiologically less effective because higher dosing activates 24-α-hydroxylase (CYP24A1), which hinders the formation of 1,25(OH)2D, the active metabolite, in both renal, skeletal, and extra-renal tissues. This inhibition can last for weeks or longer.[18] Hence, daily dosing is preferable, not only to elevate 25(OH)D levels but also to maximize the concentration of 1,25(OH)2D. Although the rise in 25(OH)D is more rapid, reliable, and stable with calcifediol as compared to cholecalciferol (Vitamin D3), the actual rise is usually up to 75–100 nmol/l, which corresponds to 30–40 ng/ml. However, whether higher doses of calcifediol could be effective in attaining higher levels of 25(OH)D (to the range of 50ng/ml or more which is recommended for extraskeletal benefits), is not well known.
In the current study, higher doses (25 μg and 50 μg) were supplemented in participants with VDD. The proportion of participants attaining Vitamin D sufficiency exceeded 93%, irrespective of low dose (25 μg-group) or high dose (50 μg-group) in as early as 4 weeks after intervention. Further, the median rise exhibited a dose-dependent pattern, with a higher median rise in the 50 μg-group (nearly 2.2 times) at 4 weeks, as compared to the 25 μg-group. Later, the median rise was lower in the 50 μg-group as compared to the 25 μg-group, probably because the absolute levels of Vitamin D at 4 weeks were higher in the 50 μg-group, leading to a lesser increment at 8 weeks. Regarding the dynamic changes in the other hormonal parameters, the PTH was found to decrease significantly by 4 weeks and continued to decline till 8 weeks duration. Correspondingly, there was a significant increase in the 1,25(OH)2D levels at 4 weeks, which continued to rise even later, till 8 weeks. Though the study did not have a third intervention arm with Vitamin D3 supplementation, the approximate dose conversion of 25 μg and 50 μg daily calcifediol doses correspond to 1000 IU and 2000 IU of Vitamin D3, respectively.[19] The current study proves the efficacy as well as safety of Vitamin D supplementation in young adults at doses of calcifediol equivalent to 2000 IU daily vitamin D3. The estimated average requirement and recommended dietary allowance for Vitamin D is usually 600–800 IU/d and most adults attain levels of approximately 20 ng/ml with this.[8] A level of nearly 30 ng/ml requires 1800–4000 IU/d, but the current study suggests that at much lower doses of calcifediol (nearly half), higher Vitamin D level could be attained rapidly and reliably.
In terms of adverse events, 4.6% developed hypercalciuria overall, following supplementation with calcifediol. Hypercalciuria depends upon several factors, including age, geographical location, dietary calcium intake, salt intake, natriuresis, and drugs. Hence, there is no unequivocal definition for hypercalciuria and reported cutoffs have varied from 0.21 to 0.70 or even higher.[20,21,22] In a previous study from India, the cutoff was found to be 0.13,[21] whereas in another study, up to 0.35 was considered normal.[20] Hence, it is reasonable to regard all participants with CCCR of 0.3 or lower as normal, especially when there was no increase in urine calcium excretion in them following supplementation. In the current study, using a cutoff of >0.3 to define high urine calcium excretion, the hypercalciuria prevalence was of 4.6%.
The strengths of our study include a good number of participants, near-complete adherence to medication, and comparison of dose-response of various parameters to 2 doses of daily oral calcifediol, both of which are higher than the previously used doses. Assessment of safety following calcifediol supplementation by clinical and biochemical estimation of various calcemic and hormonal parameters is another strength of the study. The limitations include a short duration of follow-up and the noninclusion of a control group with cholecalciferol (Vitamin D3) supplementation.
The evidence generated by the current study suggests the utility and safety of 25 μg calcifediol daily supplementation as sufficient to attain 25(OH)D levels in the range not only desired for skeletal but for extraskeletal benefits. Hence, we propose that for participants with an indication for using higher doses of Vitamin D supplementation, such as in malabsorption, obesity, chronic liver or renal disease, or in case of concurrent use of drugs that increase the metabolism of Vitamin D, a daily dose of 25 μg calcifediol can be both effective and safe.
Conclusion
Calcifediol is a safe and efficacious alternative for oral Vitamin D supplementation in young adults. Increment in 25(OH)D levels is rapid (within 4 weeks) and sustained. The response of 25(OH)D and 1,25(OH)2D are dose-dependent.
Financial support and sponsorship
Funding for the study was obtained from Dishman Carbogen Amcis Ltd, Ahmedabad, India.
Conflicts of interest
The authors except MFH have no conflicts of interest to declare. MFH reported grants from Carbogen- Amcis and Solius Inc; Consultant, personal fees from Biogenia Consultant, Sanofi Speaker Bureau, Faes Farma Consultant, non-financial support from Ontometrics Consultant, Hyper Hypera Pharma Speaker Bureau, Pulse Pharmaceuticals pvt, Ltd. Speaker Bureau, and Menarini India Private Limited.
Flow diagram depicting the recruitment, treatment allocation, and follow-up of participants in the study. 25(OH)D: 25-hydroxyvitamin D
Flow diagram depicting the time points and duration of follow-up
Serum concentration time graphs depicting alterations in hormonal parameters, with (a) showing 25(OH)D levels, (b) showing PTH levels and (c) showing 1,25(OH)2D levels following supplementation in both groups at different time points. PTH: Parathyroid hormone, 25(OH)D: 25-hydroxyvitamin D, 1,25(OH)2D: 1,25-dihydroxyvitamin D
Acknowledgment
We wish to acknowledge Dr. Peter Mueller and Dr. Scott Miller for their valuable scientific inputs, Dishman Carbogen Amcis Ltd, Ahmedabad, India (Mr. Deep Patel), for kindly gifting the capsules and Ms. Pamela for co-ordination of the project.
References
- 1.Cesareo R, Falchetti A, Attanasio R, Tabacco G, Naciu AM, Palermo A. Hypovitaminosis D: is it time to consider the use of calcifediol? Nutrients. 2019;11:1016. doi: 10.3390/nu11051016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Richter K, Breitner S, Webb AR, Huth C, Thorand B, Kift R, et al. Influence of external, intrinsic and individual behaviour variables on serum 25 (OH) D in a German survey. J Photochem Photobiol B: Biology. 2014;140:120–9. doi: 10.1016/j.jphotobiol.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 3.Gozdzik A, Zhu J, Wong BY, Fu L, Cole DE, Parra EJ. Association of vitamin D binding protein (VDBP) polymorphisms and serum 25 (OH) D concentrations in a sample of young Canadian adults of different ancestry. J Steroid Biochem Mol Biol. 2011;127:405–12. doi: 10.1016/j.jsbmb.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 4.Shab-Bidar S, Bours S, Geusens PP, Kessels AG, van den Bergh JP. Serum 25 (OH) D response to vitamin D3 supplementation: a meta-regression analysis. Nutrition. 2014;30:975–85. doi: 10.1016/j.nut.2013.12.020. [DOI] [PubMed] [Google Scholar]
- 5.Gallagher JC, Yalamanchili V, Smith LM. The effect of vitamin D supplementation on serum 25OHD in thin and obese women. J Steroid Biochem Mol Biol. 2013;136:195–200. doi: 10.1016/j.jsbmb.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao LJ, Zhou Y, Bu F, Travers-Gustafson D, Ye A, Xu X, Hamm L, et al. Factors predicting vitamin D response variation in non-Hispanic white postmenopausal women. J Clin Endocrinol Metab. 2012;97:2699–705. doi: 10.1210/jc.2011-3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yao P, Sun L, Lu L, Ding H, Chen X, Tang L, et al. Effects of genetic and nongenetic factors on total and bioavailable 25 (OH) D responses to vitamin D supplementation. J Clin Endocrinol Metab. 2017;102:100–10. doi: 10.1210/jc.2016-2930. [DOI] [PubMed] [Google Scholar]
- 8.Bischoff-Ferrari HA, Shao A, Dawson-Hughes B, Hathcock J, Giovannucci E, Willett WC. Benefit–risk assessment of vitamin D supplementation. Osteoporos Int. 2010;21:1121–32. doi: 10.1007/s00198-009-1119-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vieth R. Vitamin D supplementation: cholecalciferol, calcifediol, and calcitriol. Eur J Clin Nutr. 2020;74:1493–7. doi: 10.1038/s41430-020-0697-1. [DOI] [PubMed] [Google Scholar]
- 10.Bouillon R, Antonio L, Olarte OR. Calcifediol (25OH Vitamin D3) Deficiency: A Risk Factor from Early to Old Age. Nutrients. 2022;14:1168. doi: 10.3390/nu14061168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bouillon R, Manousaki D, Rosen C, Trajanoska K, Rivadeneira F, Richards JB. The health effects of vitamin D supplementation: Evidence from human studies. Nat Rev Endocrinol. 2022;18:96–110. doi: 10.1038/s41574-021-00593-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marino R, Misra M. Extra-skeletal effects of vitamin D. Nutrients. 2019;11:1460. doi: 10.3390/nu11071460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wimalawansa SJ. Non-musculoskeletal benefits of vitamin D. J Steroid Biochem Mol Biol. 2018;175:60–81. doi: 10.1016/j.jsbmb.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 14.Jetter A, Egli A, Dawson-Hughes B, Staehelin HB, Stoecklin E, Goessl R, et al. Pharmacokinetics of oral vitamin D3 and calcifediol. Bone. 2014;59:14–19. [PubMed] [Google Scholar]
- 15.Quesada-Gomez JM, Bouillon R. Is calcifediol better than cholecalciferol for vitamin D supplementation? Osteoporos Int. 2018;29:1697–711. doi: 10.1007/s00198-018-4520-y. [DOI] [PubMed] [Google Scholar]
- 16.Cesareo R, Falchetti A, Attanasio R, Tabacco G, Naciu AM, Palermo A. Hypovitaminosis D: is it time to consider the use of calcifediol? Nutrients. 2019;11:1016. doi: 10.3390/nu11051016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vaes AM, Tieland M, de Regt MF, Wittwer J, van Loon LJ, de Groot LC. Dose–response effects of supplementation with calcifediol on serum 25-hydroxyvitamin D status and its metabolites: a randomized controlled trial in older adults. Clin Nutr. 2018;37:808–14. doi: 10.1016/j.clnu.2017.03.029. [DOI] [PubMed] [Google Scholar]
- 18.Mazess RB, Bischoff-Ferrari HA, Dawson-Hughes B. Vitamin D: bolus is bogus—a narrative review. JBMR plus. 2021;5:e10567. doi: 10.1002/jbm4.10567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bischoff-Ferrari HA, Dawson-Hughes B, Stöcklin E, Sidelnikov E, Willett WC, Edel JO, et al. Oral supplementation with 25 (OH) D3 versus vitamin D3: effects on 25 (OH) D levels, lower extremity function, blood pressure, and markers of innate immunity. J Bone Miner Res. 2012;27:160–9. doi: 10.1002/jbmr.551. [DOI] [PubMed] [Google Scholar]
- 20.Garg MK, Marwaha RK, Khadgawat R, Ramot R, Obroi AK, Mehan N, et al. Efficacy of vitamin D loading doses on serum 25-hydroxy vitamin D levels in school going adolescents: an open label non-randomized prospective trial. J Pediatr Endocrinol Metab. 2013;26:515–3. doi: 10.1515/jpem-2012-0390. [DOI] [PubMed] [Google Scholar]
- 21.Marwaha RK, Garg MK, Dang N, Mithal A, Narang A, Chadha A, et al. Reference range of random urinary calcium creatinine ratio in North Indian children and adolescents. Ann Pediatr Endocrinol Metab. 2019;24:34–40. doi: 10.6065/apem.2019.24.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pal M, Datta S, Pradhan AK, Ghosh T, Ganguly A, Basu S, et al. Determination of upper reference value of urinary calcium-creatinine ratio for the paediatric population in Burdwan district. Adv Biol Chem. 2013;3:455–9. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Flow diagram depicting the recruitment, treatment allocation, and follow-up of participants in the study. 25(OH)D: 25-hydroxyvitamin D
Flow diagram depicting the time points and duration of follow-up
Serum concentration time graphs depicting alterations in hormonal parameters, with (a) showing 25(OH)D levels, (b) showing PTH levels and (c) showing 1,25(OH)2D levels following supplementation in both groups at different time points. PTH: Parathyroid hormone, 25(OH)D: 25-hydroxyvitamin D, 1,25(OH)2D: 1,25-dihydroxyvitamin D


