Take Home Message
Higher hospital volumes for radical prostatectomy are associated with a lower risk of postoperative urinary incontinence (RP-UI). There is wide variation in RP-UI outcomes per hospital, even among high-volume hospitals. An increase in hospital volume alone is not sufficient to reduce the incidence of RP-UI.
Keywords: Outcomes, Prostate cancer, Radical prostatectomy, Urinary incontinence, Volume-outcome relationship
Abstract
Background
On the basis of previous analyses of the incidence of urinary incontinence (UI) after radical prostatectomy (RP), the hospital RP volume threshold in the Netherlands was gradually increased from 20 per year in 2017, to 50 in 2018 and 100 from 2019 onwards.
Objective
To evaluate the impact of hospital RP volumes on the incidence and risk of UI after RP (RP-UI).
Design, setting, and participants
Patients who underwent RP during 2016–2020 were identified in the claims database of the largest health insurance company in the Netherlands. Incontinence was defined as an insurance claim for ≥1 pads/d.
Outcome measurements and statistical analysis
The relationship between hospital RP volume (HV) and RP-UI was assessed via multivariable analysis adjusted for age, comorbidity, postoperative radiotherapy, and lymph node dissection.
Results and limitations
RP-UI incidence nationwide and by RP volume category did not decrease significantly during the study period, and 5-yr RP-UI rates varied greatly among hospitals (19–85%). However, low-volume hospitals (≤120 RPs/yr) had a higher percentage of patients with RP-UI and higher variation in comparison to high-volume hospitals (>120 RPs/yr). In comparison to hospitals with low RP volumes throughout the study period, the risk of RP-UI was 29% lower in hospitals shifting from the low-volume to the high-volume category (>120 RPs/yr) and 52% lower in hospitals with a high RP volume throughout the study period (>120 RPs/yr for 5 yr).
Conclusions
A focus on increasing hospital RP volumes alone does not seem to be sufficient to reduce the incidence of RP-UI, at least in the short term. Measurement of outcomes, preferably per surgeon, and the introduction of quality assurance programs are recommended.
Patient summary
In the Netherlands, centralization of surgery to remove the prostate (RP) because of cancer has not yet improved the occurrence of urinary incontinence (UI) after surgery. Hospitals performing more than 120 RP operations per year had better UI outcomes. However, there was a big difference in UI outcomes between hospitals.
1. Introduction
Prostate cancer (PCa) is the most common cancer diagnosis among men [1]. The average 10-yr survival rate for patients with localized PCa is high and radical prostatectomy (RP) offers only a modest survival benefit in comparison to observation in selected patients [2], [3], [4]. RP may result in severe functional side effects, such as urinary incontinence (UI) and sexual dysfunction [5]. The risk of UI is higher after RP than after other treatments such as external beam radiotherapy and brachytherapy [6]. Factors that may influence UI after RP (RP-UI) are well established [7]. Certain biological and patient factors, including older age, higher body mass index, pre-existing lower urinary tract symptoms (LUTS), lower membranous urethral length (MUL), and functional bladder changes, have a negative impact on RP-UI [8]. In addition to patient and tumor characteristics, the RP volume per surgeon plays a role in the variation in outcomes for this procedure [9], [10], [11].
Several studies have shown a positive correlation between favorable perioperative and functional outcomes and the hospital annual RP volume [12], [13]. Although some countries have specialized PCa centers with high patient volumes, many European countries have not yet implemented policies to centralize RP [9]. In the Netherlands, a centralization policy for RPs has emerged. A significant driver for this centralization was our previous Dutch nationwide study based on claims data, which demonstrated that the risk of RP-UI was 30% lower in hospitals conducting more than 100 RPs/yr in comparison to hospitals performing fewer RPs [14]. These results led to a stepwise increase in the national hospital volume standard for RP from 20 RPs/yr up to 2017, to 50 in 2018 and 100 from 2019 onwards. The rationale behind this increase was predicated on the assumption that an increase in the minimum annual RP volume would yield better functional and oncological outcomes.
The aim of the present population-based study was to use claims data to evaluate the impact of hospital RP volumes on the incidence and risk of RP-UI.
2. Patients and methods
2.1. Study approval
Data collection and analyses for this study were performed under the strict privacy rules and regulations of the Dutch government and health insurance companies. Patients included in the analyses could not be identified, so no informed consent or ethics approval was necessary.
2.2. Study aims
The aims of the study were to assess (1) the trend for RP volumes per hospital, (2) the incidence of RP-UI at a national level, (3) variations in RP-UI incidence among hospitals, and (4) the risk of RP-UI.
2.3. Primary outcome
The primary outcome for the study was RP-UI. We defined UI as in our previous study [14]: (1) the use of one or more incontinence pads per day 12–15 mo after RP and/or (2) surgery for RP-UI within 15 mo after RP.
2.4. Study design
2.4.1. RP volumes per hospital
Publicly available data from the website of the Dutch Health Institute [15] were used to analyze the annual RP volume per hospital.
2.4.2. Incidence of RP-UI at a national level
A historical cohort of PCa patients undergoing RP between January 1, 2016 and December 31, 2020 and with claims for incontinence pads or surgery was identified from the claims database of the largest Dutch health insurer (Zilveren Kruis). Patients meeting the following criteria were excluded from the study: those who switched to another health insurer, deceased patients, patients with claims data indicating the use of incontinence pads 1–4 mo before RP, and patients insured by de Friesland, an insurance company label whose reimbursement policy did not provide full coverage for incontinence pads.
UI was analyzed by dividing the total number of patients with claims for incontinence pads by the total number of RP patients for each year (frequency and percentage). Differences were compared using a χ2 test.
2.4.3. Variation in RP-UI incidence among hospitals
The average 5-yr UI incidence rate per hospital was first determined and then the average number of RPs performed annually at each hospital was calculated for two time periods: 2016–2018 (period 1) and 2019–2020 (period 2). Second, we recalibrated the optimal cutoff level for low-volume (LV) and high volume (HV) hospitals (see below). Third, hospitals were categorized as follows: (1) LV1-LV2 = LV in both time periods, (2) LV1-HV2 = LV in period 1 and HV in period 2; and (3) HV1-HV2 = HV in both time periods. For each hospital volume category, the RP-UI rates for period 1 and period 2 were calculated. Patients who underwent RP in hospitals that discontinued RP from 2019 onwards were excluded.
2.4.4. Risk of RP-UI
The impact of hospital RP volume was evaluated by developing an (explanatory) multivariable logistic regression model [16]. The incidence of RP-UI was the primary outcome (dependent variable) and was dichotomized.
For all patients, data on patient characteristics (age at RP and comorbidity), treatment (RP date, lymph node dissection at RP, radiotherapy within 15 mo after RP, and hospital), and RP-UI outcome were collected from claims databases. Comorbidity was based on the ICD-10 code and specified as a diagnosis for each patient (eg, endocrine, heart, nervous system diseases). Case-mix variables were included as independent variables to adjust the effect of hospital volume category on RP-UI incidence.
Models were developed and the optimal threshold value was determined as follows. First, all potential confounders for which data were available (hospital volume [LV1-LV2, LV1-HV2, and HV1-HV2], age [continuous], radiotherapy [yes vs no], lymph node dissection [yes vs no], interaction variable between radiotherapy and lymph node dissection [yes vs no], and comorbidities [per diagnosis: yes vs no]) were separately tested in multivariate backwards analysis (p < 0.05) against the primary outcome (RP-UI). The variables were included as case-mix (independent) variables to adjust the effect of hospital volume category on RP-UI on the basis of an a priori hypothesized causal relationship [7], [17]. We used lymph node dissection as a proxy for tumor stage.
Second, relevant and significant confounders (p < 0.05) were included in the multivariable logistic regression model. Third, we recalibrated the cutoff value of 100 RPs/yr to distinguish LV and HV hospitals from our previous study [14] by performing sensitivity analyses using predefined cutoffs of 100, 110, 120, 125, and 130 RPs/yr. All analyses were performed in SAS v9.4 (SAS Institute, Cary, NC, USA).
3. Results
3.1. RP volumes per hospital
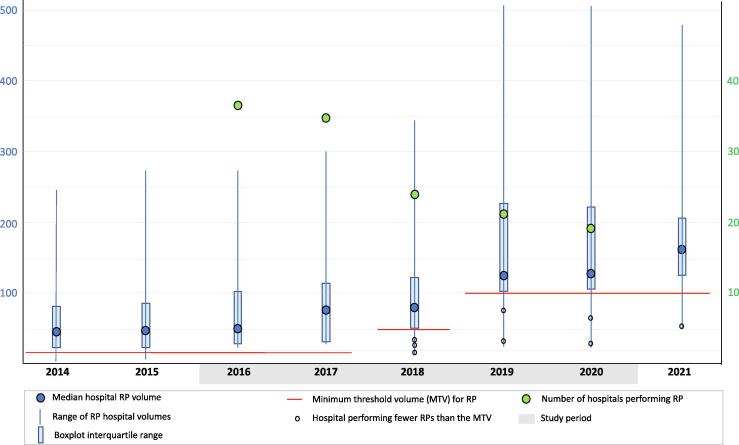
The median annual RP volume per hospital increased from 51 in 2016 to 136 in 2020 and 161 in 2021 (Fig. 1). The annual RP volume varied widely among hospitals and increased over time as a limited number of HV hospitals emerged. The number of hospitals performing RP decreased from 37 in 2016 to 19 in 2020.
Fig. 1.
Boxplots of the number of radical prostatectomy (RP) procedures per hospital per year showing the median, range, interquartile range, and number of hospitals performing RP.
3.2. RP-UI incidence at a national level
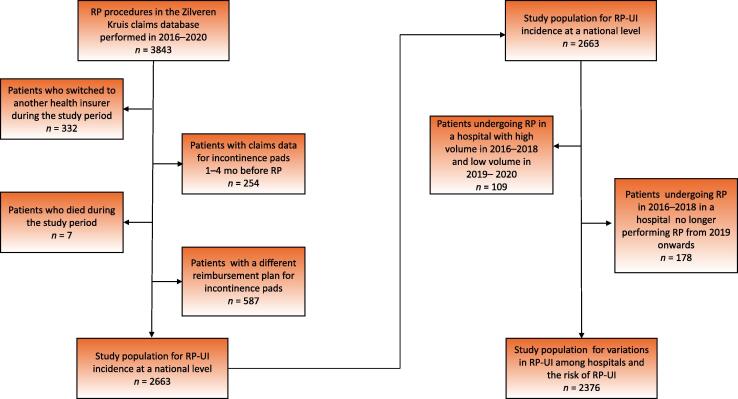
In total, data for 3843 patients who underwent RP during 2016–2020 were retrieved from the database. Data for four groups of patients were excluded (Fig. 2). The study population for analysis of RP-UI incidence at the national level included 2663 primary RP patients (mean age 66.7 yr, standard deviation 6.0).
Fig. 2.
Flow chart of the study populations for assessing the incidence of RP-UI at a national level, variations in RP-UI incidence among hospitals, and the risk of RP-UI. RP = radical prostatectomy; RP-UI = urinary incontinence after RP.
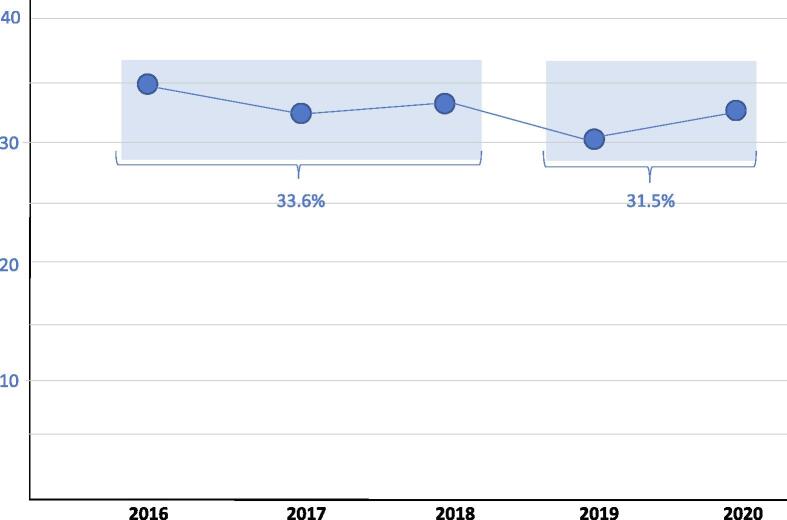
At a national level, RP-UI incidence varied between 30.6% and 34.9% over the study period (Fig. 3). The RP-UI rate was 33.6% during 2016–2018 and decreased to 31.5% in 2019–2020 (difference not significant). The overall RP-UI incidence rate for 2016–2020 was 32.6%.
Fig. 3.
Mean proportion of patients with urinary incontinence 12–15 mo after radical prostatectomy (RP) by year. Differences in the rate of urinary incontinence after RP at a national level were not significant (χ2 = 1.38, p > 0.05).
3.3. Incidence of RP-UI among hospitals
Patient and treatment characteristics and outcomes per hospital for the subpopulation of 2376 patients (Fig. 2) are shown in Table 1. On the basis of goodness of fit and optimal dispersion of the number of hospitals among categories, the cutoff for LV versus HV in the analysis was set at 120 RPs/yr (Supplementary Table 1).
Table 1.
RP volume category, patient characteristics, and RP-UI incidence by hospital during the study period (2016–2020)
| Hospital | Volume | Mean | Mean proportion of patients (%) |
||
|---|---|---|---|---|---|
| category | age (yr) | RT | LND | RP-UI | |
| 1 | LV1-LV2 | 68.0 | 0 | 61.5 | 46.2 |
| 2 | LV1-LV2 | 68.6 | 7.7 | 69.2 | 84.6 |
| 3 | LV1-LV2 | 66.5 | 17.4 | 8.7 | 82.6 |
| 4 | LV1-LV2 | 67.5 | 8.9 | 20.4 | 33.1 |
| 5 | LV1-LV2 | 65.7 | 1.8 | 41.1 | 53.6 |
| 6 | LV1-LV2 | 64.9 | 9.0 | 25.6 | 36.1 |
| 7 | LV1-HV2 | 69.0 | 7.1 | 47.3 | 32.1 |
| 8 | LV1-HV2 | 67.0 | 13.8 | 44.8 | 37.9 |
| 9 | LV1-HV2 | 66.0 | 4.8 | 38.1 | 36.5 |
| 10 | LV1-HV2 | 66.1 | 6.0 | 44.6 | 41.0 |
| 11 | LV1-HV2 | 67.1 | 5.1 | 19.3 | 35.2 |
| 12 | LV1-HV2 | 67.3 | 17.6 | 54.1 | 39.2 |
| 13 | LV1-HV2 | 67.9 | 5.2 | 31.0 | 32.8 |
| 14 | HV1-HV2 | 66.4 | 7.7 | 50.7 | 32.9 |
| 15 | HV1-HV2 | 65.9 | 4.6 | 40.7 | 19.4 |
| 16 | HV1-HV2 | 66.4 | 4.0 | 50.7 | 44.7 |
| 17 | HV1-HV2 | 67.1 | 6.8 | 54.4 | 26.6 |
| 18 | HV1-HV2 | 66.9 | 5.9 | 53.5 | 23.3 |
HV = high RP volume; HV1 = HV during 2016–2018; HV2 = HV during 2019–2020; LV = low RP volume; LV1 = LV during 2016–2018; LV2 = LV during 2019–2020; LND = lymph node dissection; RP = radical prostatectomy; RT = radiotherapy; RP-UI = urinary incontinence after RP.
There was considerable variation in the 5-yr mean RP-UI incidence rate per hospital, ranging from 19.4% to 84.6%. Notably, the LV1-LV2 group showed wide variation. However, even in the HV1-HV2 group, significant hospital variation was observed, ranging from 19.4% to 44.7%.
LV1-LV2 hospitals had the highest mean RP-UI incidence (43.1%; Table 2) and the highest variation (±30.2%). HV1-HV2 hospitals consistently had the lowest RP-UI incidence, with a rate of 28.2% (±11.6%) in 2016–2018 that decreased to 27.9% (±12.1%) in 2019–2020. LV1-HV2 hospitals had lower RP-UI rates than LV1-LV2 hospitals and higher rates than HV1-HV2 hospitals, at 38.3% (±8.4%) in 2016–2018 and 33.6% (±6.3%) in 2019–2020. In all hospital volume categories, RP-UI incidence did not decrease significantly from the first period to the second period.
Table 2.
Percentage of patients with RP-UI by hospital RP volume category
| Hospital volume | Mean RP-UI incidence ± SD (%) |
Hospitals | Patients | |
|---|---|---|---|---|
| category | 2016–2018 | 2019–2020 | (n) | (n) |
| LV1-LV2 | 43.1 ± 27.0 | 40.6 ± 30.2 | 6 | 395 |
| LV1-HV2 | 38.3 ± 8.4 | 33.6 ± 6.3 | 7 | 653 |
| HV1-HV2 | 28.2 ± 11.6 | 27.9 ± 12.1 | 5 | 1328 |
HV = high RP volume (>120 RPs/yr); HV1 = HV during 2016–2018; HV2 = HV during 2019–2020; LV = low RP volume (≤120 RPs/yr); LV1 = LV during 2016–2018; LV2 = LV during 2019–2020; RP = radical prostatectomy; SD = standard deviation; RP-UI = urinary incontinence after RP.
3.4. Relationship between hospital volume and RP-UI risk
The final multivariable model showed that hospital volume category was independently correlated with RP-UI incidence. More precisely, patients undergoing RP in HV1-HV2 hospitals were 52% less likely to experience RP-UI than patients in LV1-LV2 hospitals (adjusted odds ratio [aOR] 0.48, 95% confidence interval [CI] 0.38–0.62; Table 3), while patients undergoing RP in LV1-HV2 hospitals were 29% less likely to suffer from RP-UI (aOR 0.71, 95% CI 0.55–0.92). Age (aOR 1.05, 95% CI 1.03–1.06) and lymph node dissection (aOR 1.34, 95% CI 1.12–1.61) also contributed to the model (Table 3). None of the other variables significantly improved this core model.
Table 3.
Multivariable logistic regression and adjusted odds ratio for the incidence of urinary incontinence after RP a
| Parameter | Effect estimate ± SE | p value | Odds ratio (95% CI) |
|---|---|---|---|
| Age at RP | 0.05 ± 0.01 | <0.0001 | 1.05 (1.03–1.06) |
| Lymph node dissection | 0.28 ± 0.09 | 0.003 | 1.34 (1.12–1.61) |
| Radiotherapy b | 0.26 ± 0.17 | 0.91 | 1.294 (0.921–1.81) |
| HV1-HV2 vs LV1-LV2 | −0.36 ± 0.06 | <0.0001 | 0.48 (0.38–0.62) |
| LV1-HV2 vs LV1-LV2 | 0.01 ± 0.07 | 0.88 | 0.71 (0.55–0.92) |
| Intercept | −3.85 ± 0.53 | <0.0001 |
CI = confidence interval; HV = high RP volume; HV1 = HV during 2016–2018; HV2 = HV during 2019–2020; LV = low RP volume; LV1 = LV during 2016–2018; LV2 = LV during 2019–2020; RP = radical prostatectomy; SE = standard error.
For the final model, total concordance was 61.3%, with χ2 = 8.20 (Hosmer and Lemeshow test; p = 0.42).
Not included in the final model.
4. Discussion
Implementation of volume thresholds of 50 RPs/yr in 2018 and 100 RPs/yr from 2019 onwards in the Netherlands led to a notable reduction in the number of hospitals conducting RPs (from 37 to 19). There was also a significant increase in the median number of RPs performed annually per hospital (from 51 to 136). At a national level, RP-UI rates decreased marginally from 33.6% to 31.5%. The wide variation in hospital 5-yr RP-UI rates (19–85%) may have contributed to this nonsignificant decrease.
After adjustment for relevant confounding factors, hospital volume was the most predictive factor for RP-UI. Patients who underwent RP in hospitals that transitioned from LV to HV had 29% lower likelihood of experiencing RP-UI than patients in LV1-LV2 hospitals. Patients who underwent RP in hospitals with consistently high RP volumes throughout the study period had 52% lower risk of RP-UI. The proportion of patients undergoing RP in a HV hospital (>120 RPs/yr) increased from 33.5% in 2016 to 86.9% in 2020 [15].
Although the risk of RP-UI was lower for patients in HV hospitals, the RP-UI incidence at a national level did not decrease significantly after centralization. This might be attributable to other factors, such as an increase in the age of patients undergoing RP over the study period. The proportion of patients aged >70 yr was 30.5% in 2016–2018 and 37.6% in 2019–2020, and our risk model demonstrated that the risk of RP-UI increases with age. Other unmeasured factors, such as volume per surgeon and tumor stage, may have also contributed to the nonsignificant reduction in RP-UI at a national level. Further studies are needed to evaluate the impact of these factors and to study other functional and oncological outcomes over time.
4.1. Studies on RP-UI
Our study revealed an overall annual RP-UI rate of 33% for a continence definition of <1 pad/d. Relevant (single center) studies in the literature have reported RP-UI rates of approximately 10% [14], [18], [19], [20], [21], [22], [23], [24], [25]. Many of these studies defined continence as “no leak” or “no pads” (Table 4). Our results are closest to findings reported by Haglind et al [18] from a multicenter study in Sweden (n = 1847) in which the RP-UI rate was 20–21%. The comparatively higher national RP-UI rate (33%) in our study may be explained by study differences, as the study by Haglind et al did not encompass all hospitals, used patient-reported questionnaires, followed a prospective design, and excluded patients aged >75 yr [18].
Table 4.
Overview of peer-reviewed evidence on 12-mo UI following radical prostatectomy
| Reference and study period | Cohort | Patients (n) |
Age (yr) |
UI at 12 mo (%) |
DoC | Data used | Surgical method |
|---|---|---|---|---|---|---|---|
| Zorn [19] 2003–2005 |
Single center | 300 | 59.4 | 10 | 0 pads | NR | RARP |
| Shikanov [20] 2003–2008 |
Single center | 380 | 58.1 | 18 | <1 pad | Open interview | RARP |
| Patel [21] 2008–2009 |
Single surgeon | 1100 | 58 | 2.6 | 0 pads | NR | RARP |
| Novara [22] 2005–2009 |
Single center | 308 | 61.6 | 10 | No leak | ICIQ-UI | RARP |
| Haglind [18] 2008–2011 |
14 centers | 1847 | 63 | 21.3 | <1 pad | Standardized questionnaire |
RARP |
| Haglind [18] 2008–2011 |
14 centers | 778 | 63 | 20.2 | <1 pad | Standardized questionnaire |
RRP |
| Coughlin [23] 2010–2015 |
Single center | 157 | 35–70 | 10 | 0 pads | NR | RARP/RRP |
| Schepens [14] 2014–2015 |
Nationwide | 1590 | 65 | 26.0 | <1 pad | Claims data | NR |
| Sauer [24] 2014–2018 |
Single center | 133 | 65 | 17 | ≤1 dry pad | Standardized questionnaire |
RARP/RRP |
| Lee [25] 2004–2015 |
Single center | 1691 | ≤70 | 11.4 | <1 pad | PROs at assessment | RARP/RRP |
| Lee [25] 2004–2015 |
Single center | 610 | >70 | 18.5 | <1 pad | PROs at assessment | RARP/RRP |
| Present study 2016–2020 |
30% of national population |
2663 | 66.8 | 32.6 | <1 pad | Claims data | NR |
DoC = definition of continence; ICIQ-UI = International Consultation on Incontinence Questionnaire-Urinary Incontinence; NR = not reported; PROs = patient-reported outcomes; RARP = robot-assisted radical prostatectomy; RRP = retropubic radical prostatectomy; UI = urinary incontinence.
4.2. Studies on the impact of hospital and surgeon volumes on RP-UI
Our previous nationwide study revealed that the risk of RP-UI was 30% lower for patients undergoing RP in hospitals with an annual volume of >100 RPs [14]. The current study, in which we recalibrated the threshold to 120 RPs/yr, confirms this volume–RP-UI relationship. However, our study could not identify the optimal volume threshold for RP, as the volume threshold was chosen for methodological reasons. Another study confirmed that a high annual caseload (>200 RPs/year) was associated with lower RP-UI incidence [10]. However, Nossiter et al [26] found no difference in RP-UI between LV and HV hospital cohorts in the UK, which might be explained by lower hospital RP volumes in the UK than in the Netherlands and/or the use of patient questionnaires in their study.
Our explanatory model demonstrated that hospital volume and age are important factors for RP-UI risk, but not all the variance in RP-UI incidence could be explained. A systematic review of the association between volume and outcome after RP revealed that both higher hospital volume and higher surgeon volume improved outcomes such as postoperative UI [10]. Other studies confirmed that an increase in surgeon volume—but not hospital volume—was associated with improvement in outcomes such as long-term postoperative UI [10], [27]. Begg et al [27] concluded that surgeons who performed relatively poorly with respect to postoperative complications also performed poorly with respect to late urinary complications, and long-term RP-UI varied from 16% for high-volume to 20% for low-volume surgeons.
4.3. Studies on variation in RP-UI outcomes
Heterogeneity in RP-UI outcomes has been described [28], [29] but few studies have reported on the underlying factors. A Swedish population-based study concluded that the most important factor influencing RP-UI heterogeneity was surgeon experience, which accounted for 42% of the heterogeneity observed [30]. High-volume surgeons generally their improve technique via experience [31]. However, even within a group of high-volume surgeons, substantial variation in outcome has been observed [32]. Performance per surgeon could not be evaluated in the present study.
We found wide variation in the incidence of RP-UI among hospitals. While some HV centers reported remarkably high RP-UI rates, the highest variation was observed in LV hospitals. Therefore, it is possible that surgeon-volume relationships, which we could not measure, contributed in part to the variation in RP-UI rates we observed. Further studies should evaluate which additional measures are needed to reduce variation and to identify the optimal volume threshold per hospital and per surgeon.
4.4. Studies on reducing the risk of RP-UI
As observed for other cancer surgeries, simply increasing the minimum volume standard without also measuring outcomes in quality assurance programs (QAPs) may not be sufficient to improve the quality of care [33]. QAPs are structured programs in which health care employees critically review the outcomes of their patients and continuously analyze and discuss these results in order to improve outcomes. Cathcart et al [34] evaluated the effects of a QAP for RP and found that 3-mo UI scores improved significantly for all but one surgeon who had low RP-UI rates at study initiation. Improving patient outcomes via short quality-improvement cycles is easier in settings in which a higher volume of patients is treated. For a low volume of patients, it can take years to measure a difference in outcomes after a change in practice.
We expect that at a national level the risk of RP-UI can be further reduced by (1) measuring outcomes at a per-surgeon level, (2) increasing the focus on audit and feedback via QAPs, and (3) applying per-surgeon RP volume thresholds.
4.5. Study strengths
The primary strength of our study lies in its novelty, as the first study to assess the trend in RP-UI incidence over time at a national level, interhospital variations, and the impact of hospital RP volumes on the incidence of RP-UI. The study strength stems from the extensive and representative nature of the data used. The source for our data was the database of the largest Dutch health insurance company, covering 27% of all RPs performed in the Dutch population during the study period. Most other studies on RP-UI are based on a single center or even a single surgeon. Patients insured by Zilveren Kruis are representative of the entire Dutch population [35], so the current results can be extrapolated to the total Dutch population.
4.6. Study limitations
Several limitations should be mentioned. First, we could not include all the variables identified in previous research as potential confounders, such as body mass index, LUTS, MUL, surgical technique, surgeon volume, and surgeon experience [27], [11], [36]. Second, it was not possible to verify whether patients actually used the incontinence pads for which they claimed reimbursement. A recent study comparing RP-UI incidence derived from two sources (patient-reported outcome measure vs claims data) revealed comparable rates, with claims data slightly underestimating actual RP-UI rates [37]. Therefore, we do not suspect that the claims database as the source for the study data severely affected our results. Third, no distinction between laparoscopic and robot-assisted surgical approaches was possible. However, surgical approach was not associated with RP-UI incidence in a recent study [17] and almost all RPs in the Netherlands were performed with robotic assistance during the study period.
5. Conclusions
The hospital RP volume threshold in the Netherlands has increased and the number of hospitals performing RP has thus decreased. Therefore, the average number of RPs per hospital increased. In all hospital volume categories, RP-UI incidence did not decrease significantly over the study period. However, LV1-LV2 hospitals had the highest percentage of patients with RP-UI and the highest variation.
In comparison to LV1-LV2 hospitals, the risk of RP-UI was 29% lower for patients undergoing RP in LV1-HV2 hospitals (≤120 RPs/yr during 2016-2018 and >120 RPs/yr during 2019-2020), and 52% lower for patients undergoing RP in HV1-HV2 hospitals (>120 RPs/yr for 5 yrs).
However, at a national level the RP-UI incidence did not significantly decrease. This could be influenced by the increasing age of patients at RP, the large variation in RP-UI rates per hospital, the absence of QAPs, and variables that could not be included in this study, such as surgeon experience and surgeon volume. Measurement of outcomes at a per-surgeon level and a greater focus on audit and feedback are recommended to improve outcomes relevant for patients.
Author contributions: Maike H.J. Schepens had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Schepens, van Hooff, van Limbeek.
Acquisition of data: Schepens, van der Galiën.
Analysis and interpretation of data: Schepens, van Hooff, van Limbeek.
Drafting of the manuscript: Schepens.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: van der Galiën, van Limbeek, van Hooff, Schepens.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: None.
Other: None.
Financial disclosures: Maike H.J. Schepens certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.
Acknowledgments: We thank Ferdinand Roelfsema and Barbara Kolff van Oosterwijk for critical reviews of the manuscript.
Associate Editor: Roderick van den Bergh
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.euros.2023.09.014.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 2.Wilt T.J., Vo T.N., Langsetmo L., et al. Radical prostatectomy or observation for clinically localized prostate cancer: extended follow-up of the Prostate Cancer Intervention Versus Observation Trial (PIVOT) Eur Urol. 2020;77:713–724. doi: 10.1016/j.eururo.2020.02.009. [DOI] [PubMed] [Google Scholar]
- 3.Neal D.E., Metcalfe C., Donovan J.L., et al. Ten-year Mortality, disease progression, and treatment-related side effects in men with localised prostate cancer from the ProtecT randomised controlled trial according to treatment received. Eur Urol. 2020;77:320–330. doi: 10.1016/j.eururo.2019.10.030. [DOI] [PubMed] [Google Scholar]
- 4.Hamdy F.C., Donovan J.L., Lane J.A., et al. Fifteen-year outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med. 2023;388:1547–1558. doi: 10.1056/NEJMoa2214122. [DOI] [PubMed] [Google Scholar]
- 5.Vernooij R.W.M., Cremers R.G.H.M., Jansen H., et al. Urinary incontinence and erectile dysfunction in patients with localized or locally advanced prostate cancer: a nationwide observational study. Urol Oncol. 2020;38:735.e17–735.e25. doi: 10.1016/j.urolonc.2020.05.022. [DOI] [PubMed] [Google Scholar]
- 6.Albkri A., Girier D., Mestre A., Costa P., Droupy S., Chevrot A. Urinary incontinence, patient satisfaction, and decisional regret after prostate cancer treatment: a French national study. Urol Int. 2018;100:50–56. doi: 10.1159/000484616. [DOI] [PubMed] [Google Scholar]
- 7.Lardas M., Grivas N., Debray T.P.A., et al. Patient- and tumour-related prognostic factors for urinary incontinence after radical prostatectomy for nonmetastatic prostate cancer: a systematic review and meta-analysis. Eur Urol Focus. 2022;8:674–689. doi: 10.1016/j.euf.2021.04.020. [DOI] [PubMed] [Google Scholar]
- 8.Heesakkers J., Farag F., Bauer R.M., Sandhu J., De Ridder D., Stenzl A. Pathophysiology and contributing factors in postprostatectomy incontinence: a review. Eur Urol. 2017;71:936–944. doi: 10.1016/j.eururo.2016.09.031. [DOI] [PubMed] [Google Scholar]
- 9.Van den Broeck T., Oprea-Lager D., Moris L., et al. A systematic review of the impact of surgeon and hospital caseload volume on oncological and nononcological outcomes after radical prostatectomy for nonmetastatic prostate cancer. Eur Urol. 2021;80:531–545. doi: 10.1016/j.eururo.2021.04.028. [DOI] [PubMed] [Google Scholar]
- 10.Leow J.J., Leong E.K., Serrell E.C., et al. Systematic review of the volume-outcome relationship for radical prostatectomy. Eur Urol Focus. 2018;4:775–789. doi: 10.1016/j.euf.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 11.Thompson J.E., Egger S., Böhm M., et al. Superior quality of life and improved surgical margins are achievable with robotic radical prostatectomy after a long learning curve: a prospective single-surgeon study of 1552 consecutive cases. Eur Urol. 2014;65:521–531. doi: 10.1016/j.eururo.2013.10.030. [DOI] [PubMed] [Google Scholar]
- 12.Groeben C., Koch R., Baunacke M., Wirth M.P., Huber J. High volume is the key for improving in-hospital outcomes after radical prostatectomy: a total population analysis in Germany from 2006 to 2013. World J Urol. 2017;35:1045–1053. doi: 10.1007/s00345-016-1982-4. [DOI] [PubMed] [Google Scholar]
- 13.Gershman B., Meier S.K., Jeffery M.M., et al. Redefining and contextualizing the hospital volume-outcome relationship for robot-assisted radical prostatectomy: implications for centralization of care. J Urol. 2017;198:92–99. doi: 10.1016/j.juro.2017.01.067. [DOI] [PubMed] [Google Scholar]
- 14.Schepens M.H.J., Ziedses des Plantes C.M.P., Somford D.M., et al. Hoe vaak incontinentie na radicale prostatectomie? [Incidence of incontinence after radical prostatectomy using claims-based data] Ned Tijdschr Geneeskd. 2018;162:D2294. [PubMed] [Google Scholar]
- 15.Health Institute Netherlands. Prostate carcinoma indicators. https://www.zorginzicht.nl/kwaliteitsinstrumenten/porstaatcarcinoom-indicatoren.
- 16.Sainani K.L. Explanatory versus predictive modeling. PM R. 2014;6:841–844. doi: 10.1016/j.pmrj.2014.08.941. [DOI] [PubMed] [Google Scholar]
- 17.Baunacke M., Schmidt M.L., Thomas C., et al. Long-term functional outcomes after robotic vs. retropubic radical prostatectomy in routine care: a 6-year follow-up of a large German health services research study. World J Urol. 2020;38:1701–1709. doi: 10.1007/s00345-019-02956-8. [DOI] [PubMed] [Google Scholar]
- 18.Haglind E., Carlsson S., Stranne J., et al. Urinary incontinence and erectile dysfunction after robotic versus open radical prostatectomy: a prospective, controlled, nonrandomised trial. Eur Urol. 2015;68:216–225. doi: 10.1016/j.eururo.2015.02.029. [DOI] [PubMed] [Google Scholar]
- 19.Zorn K.C., Gofrit O.N., Orvieto M.A., Mikhail A.A., Zagaja G.P., Shalhav A.L. Robotic-assisted laparoscopic prostatectomy: functional and pathologic outcomes with interfascial nerve preservation. Eur Urol. 2007;51:755–762. doi: 10.1016/j.eururo.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 20.Shikanov S.A., Zorn K.C., Zagaja G.P., Shalhav A.L. Trifecta outcomes after robotic-assisted laparoscopic prostatectomy. Urology. 2009;74:619–623. doi: 10.1016/j.urology.2009.02.082. [DOI] [PubMed] [Google Scholar]
- 21.Patel V.R., Coelho R.F., Chauhan S., et al. Continence, potency and oncological outcomes after robotic-assisted radical prostatectomy: early trifecta results of a high-volume surgeon. BJU Int. 2010;106:696–702. doi: 10.1111/j.1464-410X.2010.09541.x. [DOI] [PubMed] [Google Scholar]
- 22.Novara G., Ficarra V., D’elia C., et al. Evaluating urinary continence and preoperative predictors of urinary continence after robot assisted laparoscopic radical prostatectomy. J Urol. 2010;184:1028–1033. doi: 10.1016/j.juro.2010.04.069. [DOI] [PubMed] [Google Scholar]
- 23.Coughlin G.D., Yaxley J.W., Chambers S.K., et al. Robot-assisted laparoscopic prostatectomy versus open radical retropubic prostatectomy: 24-month outcomes from a randomised controlled study. Lancet Oncol. 2018;19:1051–1060. doi: 10.1016/S1470-2045(18)30357-7. [DOI] [PubMed] [Google Scholar]
- 24.Sauer M., Tennstedt P., Berliner C., et al. Predictors of short and long term urinary incontinence after radical prostatectomy in prostate MRI: significance and reliability of standardized measurements. Eur J Radiol. 2019;120:108668. doi: 10.1016/j.ejrad.2019.108668. [DOI] [PubMed] [Google Scholar]
- 25.Lee Y.J., Jung J.W., Lee S., et al. Contemporary trends in radical prostatectomy and predictors of recovery of urinary continence in men aged over 70 years: comparisons between cohorts aged over 70 and less than 70 years. Asian J Androl. 2020;22:280–286. doi: 10.4103/aja.aja_62_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nossiter J., Morris M., Cowling T.E., et al. Hospital volume and outcomes after radical prostatectomy: a national population-based study using patient-reported urinary continence and sexual function. Prostate Cancer Prostat Dis. 2023;26:264–270. doi: 10.1038/s41391-021-00443-z. [DOI] [PubMed] [Google Scholar]
- 27.Begg C.B., Riedel E.R., Bach P.B., et al. Variations in morbidity after radical prostatectomy. N Engl J Med. 2002;346:1138–1144. doi: 10.1056/NEJMsa011788. [DOI] [PubMed] [Google Scholar]
- 28.Vickers A., Savage C., Bianco F., et al. Cancer control and functional outcomes after radical prostatectomy as markers of surgical quality: analysis of heterogeneity between surgeons at a single cancer center. Eur Urol. 2011;59:317–322. doi: 10.1016/j.eururo.2010.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carlsson S., Berglund A., Sjoberg D., et al. Effects of surgeon variability on oncologic and functional outcomes in a population-based setting. BMC Urol. 2014;14:25. doi: 10.1186/1471-2490-14-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nyberg M., Sjoberg D.D., Carlsson S.V., et al. Surgeon heterogeneity significantly affects functional and oncological outcomes after radical prostatectomy in the Swedish LAPPRO trial. BJU Int. 2021;127:361–368. doi: 10.1111/bju.15238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prystowsky J.B., Bordage G., Feinglass J.M. Patient outcomes for segmental colon resection according to surgeon’s training, certification, and experience. Surgery. 2002;132:663–670. doi: 10.1067/msy.2002.127550. [DOI] [PubMed] [Google Scholar]
- 32.Bianco F.J., Jr, Riedel E.R., Begg C.B., Kattan M.W., Scardino P.T. Variations among high volume surgeons in the rate of complications after radical prostatectomy: further evidence that technique matters. J Urol. 2005;173:2099–2103. doi: 10.1097/01.ju.0000158163.21079.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simunovic M., Urbach D., Major D., et al. Assessing the volume-outcome hypothesis and region-level quality improvement interventions: pancreas cancer surgery in two Canadian provinces. Ann Surg Oncol. 2010;17:2537–2544. doi: 10.1245/s10434-010-1114-0. [DOI] [PubMed] [Google Scholar]
- 34.Cathcart P., Sridhara A., Ramachandran N., Briggs T., Nathan S., Kelly J. Achieving quality assurance of prostate cancer surgery during reorganisation of cancer services. Eur Urol. 2015;68:22–29. doi: 10.1016/j.eururo.2015.02.028. [DOI] [PubMed] [Google Scholar]
- 35.van der Lee A.P.M., Önsesveren I., Wierdsma A.I., et al. The impact of antipsychotic formulations on time to medication discontinuation in patients with schizophrenia: a Dutch registry-based retrospective cohort study. CNS Drugs. 2021;35:451–460. doi: 10.1007/s40263-021-00802-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Auffenberg G.B., Qi J., Dunn R.L., Linsell S., et al. Evaluation of patient- and surgeon-specific variations in patient-reported urinary outcomes 3 months after radical prostatectomy from a statewide improvement collaborative. JAMA Surg. 2021;156:e206359. doi: 10.1001/jamasurg.2020.6359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baas D, Reitsma J, van Gerwen L, et al. P055: Correlation of healthcare insurance claims data for absorbing pads with PROMs as measure for urinary incontinence one year after radical prostatectomy. Presented at the 14th European Multidisciplinary Congress on Urological Cancers (EMUC 2022). https://www.postersessiononline.eu/173580348_eu/congresos/EMUC2022/aula/-P_55_EMUC2022.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.