Abstract
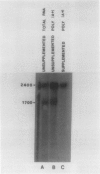
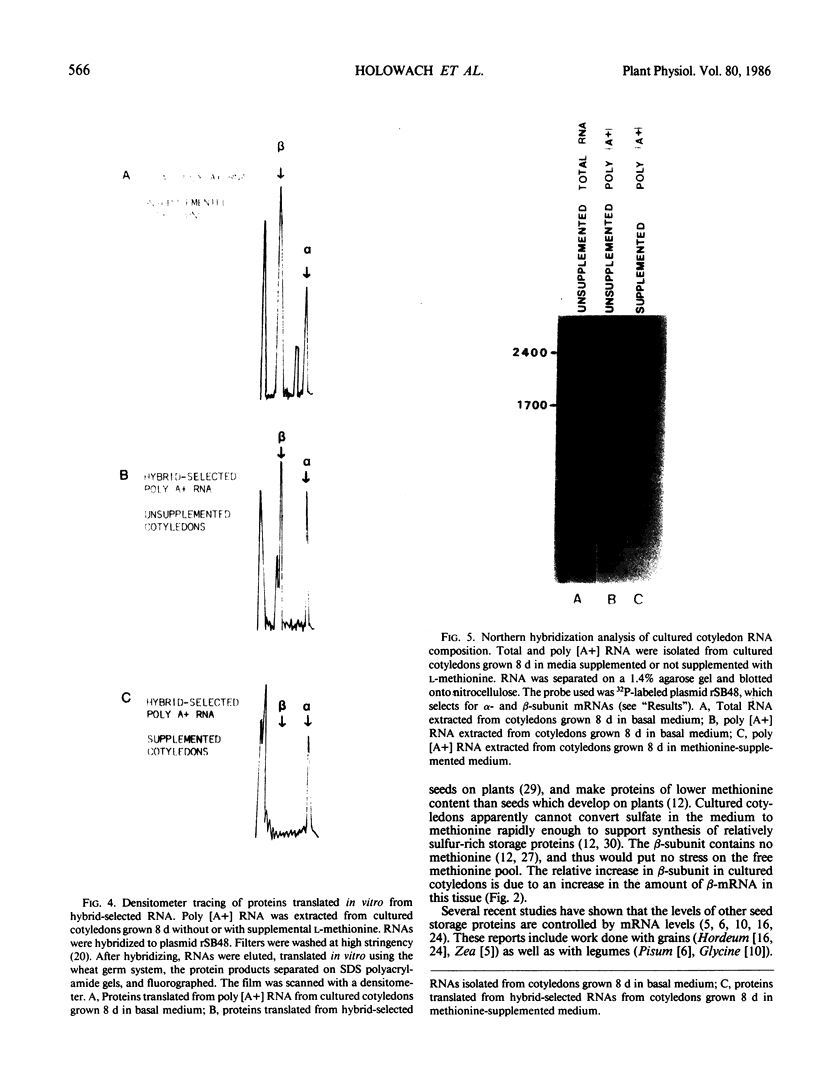
In previous studies (GL Creason et al. 1983 Biochem Biophys Res Commun 117: 658-662; LP Holowach et al. 1984 Plant Physiol 74: 576-583), we have shown that when soybean (Glycine max L. Merrill cv Provar) cotyledons are cultured in medium supplemented with l-methionine, the β-subunit of 7S protein and β-mRNA are absent. We have carried out further studies on the mechanism of the methionine action. In one experiment, cotyledons were cultured for 16 days with or without methionine. After 4 days, some cotyledons were transferred from methionine-supplemented to basal (no methionine) medium and vice versa. In basal medium, β-subunit was detected at 4 days whereas in methionine-supplemented medium, no β-subunit was present. When cotyledons were transferred from basal to methionine-supplemented medium, the β-subunit increased within a 4 day period and then remained constant (on a per cotyledon basis). This result indicated that methionine was not acting by accelerating the degradation of the β-subunit. Four days after transfer from supplemented to basal medium cotyledons contained β-subunit, thus demonstrating that the inhibition was reversible. During this time, the uncombined methionine declined from 7 to 1.5 μmoles methionine per gram fresh weight. When β-mRNA was measured by in vitro translation, functional β-mRNA was absent in tissue that was not accumulating β-subunit. The messenger RNA for the β-subunit had a half-life of about 1 day in the presence of methionine. Hybridization of cotyledon mRNA with cDNA complementary to β-mRNA revealed that the 1700 nucleotide β-mRNA was not present in supplemented cotyledons. Thus, expression of the β-subunit gene is controlled at the level of transcription, RNA processing, or RNA turnover, rather than at the level of translation.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beachy R. N., Jarvis N. P., Barton K. A. Biosynthesis of subunits of the soybean 7S storage protein. J Mol Appl Genet. 1981;1(1):19–27. [PubMed] [Google Scholar]
- Beachy R. N., Thompson J. F., Madison J. T. Isolation of polyribosomes and messenger RNA active in in vitro synthesis of soybean seed proteins. Plant Physiol. 1978 Feb;61(2):139–144. doi: 10.1104/pp.61.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop J. M., Koch G., Evans B., Merriman M. Poliovirus replicative intermediate: structural basis of infectivity. J Mol Biol. 1969 Dec 14;46(2):235–249. doi: 10.1016/0022-2836(69)90419-7. [DOI] [PubMed] [Google Scholar]
- Burr F. A., Burr B. Three mutations in Zea mays affecting zein accumulation: a comparison of zein polypeptides, in vitro synthesis and processing, mRNA levels, and genomic organization. J Cell Biol. 1982 Jul;94(1):201–206. doi: 10.1083/jcb.94.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler P. M., Spencer D., Randall P. J., Higgins T. J. Influence of Sulfur Nutrition on Developmental Patterns of Some Major Pea Seed Proteins and Their mRNAs. Plant Physiol. 1984 Jul;75(3):651–657. doi: 10.1104/pp.75.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creason G. L., Holowach L. P., Thompson J. F., Madison J. T. Exogenous methionine depresses level of mRNA for a soybean storage protein. Biochem Biophys Res Commun. 1983 Dec 28;117(3):658–662. doi: 10.1016/0006-291x(83)91647-9. [DOI] [PubMed] [Google Scholar]
- Danielsson C. E. Seed globulins of the Gramineae and Leguminosae. Biochem J. 1949;44(4):387–400. doi: 10.1042/bj0440387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg R. B., Hoschek G., Ditta G. S., Breidenbach R. W. Developmental regulation of cloned superabundant embryo mRNAs in soybean. Dev Biol. 1981 Apr 30;83(2):218–231. doi: 10.1016/0012-1606(81)90468-1. [DOI] [PubMed] [Google Scholar]
- Holowach L. P., Thompson J. F., Madison J. T. Effects of exogenous methionine on storage protein composition of soybean cotyledons cultured in vitro. Plant Physiol. 1984 Mar;74(3):576–583. doi: 10.1104/pp.74.3.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holowach L. P., Thompson J. F., Madison J. T. Storage Protein Composition of Soybean Cotyledons Grown In Vitro in Media of Various Sulfate Concentrations in the Presence and Absence of Exogenous l-Methionine. Plant Physiol. 1984 Mar;74(3):584–589. doi: 10.1104/pp.74.3.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu F. C., Bennett A. B., Spanswick R. M. Concentrations of sucrose and nitrogenous compounds in the apoplast of developing soybean seed coats and embryos. Plant Physiol. 1984 May;75(1):181–186. doi: 10.1104/pp.75.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabat D., Chappell M. R. Competition between globin messenger ribonucleic acids for a discriminating initiation factor. J Biol Chem. 1977 Apr 25;252(8):2684–2690. [PubMed] [Google Scholar]
- Kreis M., Shewry P. R., Forde B. G., Rahman S., Bahramian M. B., Miflin B. J. Molecular analysis of the effects of the lys 3a gene on the expression of Hor loci in developing endosperms of barley (Hordeum vulgare L.). Biochem Genet. 1984 Apr;22(3-4):231–255. doi: 10.1007/BF00484227. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Murai N., Kemp J. D., Sutton D. W., Murray M. G., Slightom J. L., Merlo D. J., Reichert N. A., Sengupta-Gopalan C., Stock C. A., Barker R. F., Hall T. C. Phaseolin gene from bean is expressed after transfer to sunflower via tumor-inducing plasmid vectors. Science. 1983 Nov 4;222(4623):476–482. doi: 10.1126/science.222.4623.476. [DOI] [PubMed] [Google Scholar]
- Okayama H., Berg P. High-efficiency cloning of full-length cDNA. Mol Cell Biol. 1982 Feb;2(2):161–170. doi: 10.1128/mcb.2.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuler M. A., Ladin B. F., Pollaco J. C., Freyer G., Beachy R. N. Structural sequences are conserved in the genes coding for the alpha, alpha' and beta-subunits of the soybean 7S seed storage protein. Nucleic Acids Res. 1982 Dec 20;10(24):8245–8261. doi: 10.1093/nar/10.24.8245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerton J., Atkins T., Bestwick R. A rapid method for preparation of bacterial plasmids. Anal Biochem. 1983 Aug;133(1):79–84. doi: 10.1016/0003-2697(83)90224-5. [DOI] [PubMed] [Google Scholar]
- Thanh V. H., Shibasaki K. Beta-conglycinin from soybean proteins. Isolation and immunological and physicochemical properties of the monomeric forms. Biochim Biophys Acta. 1977 Feb 22;490(2):370–384. doi: 10.1016/0005-2795(77)90012-5. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zezulka A. Y., Calloway D. H. Nitrogen retention in men fed varying levels of amino acids from soy protein with or without added L-methionine. J Nutr. 1976 Feb;106(2):212–221. doi: 10.1093/jn/106.2.212. [DOI] [PubMed] [Google Scholar]