Abstract
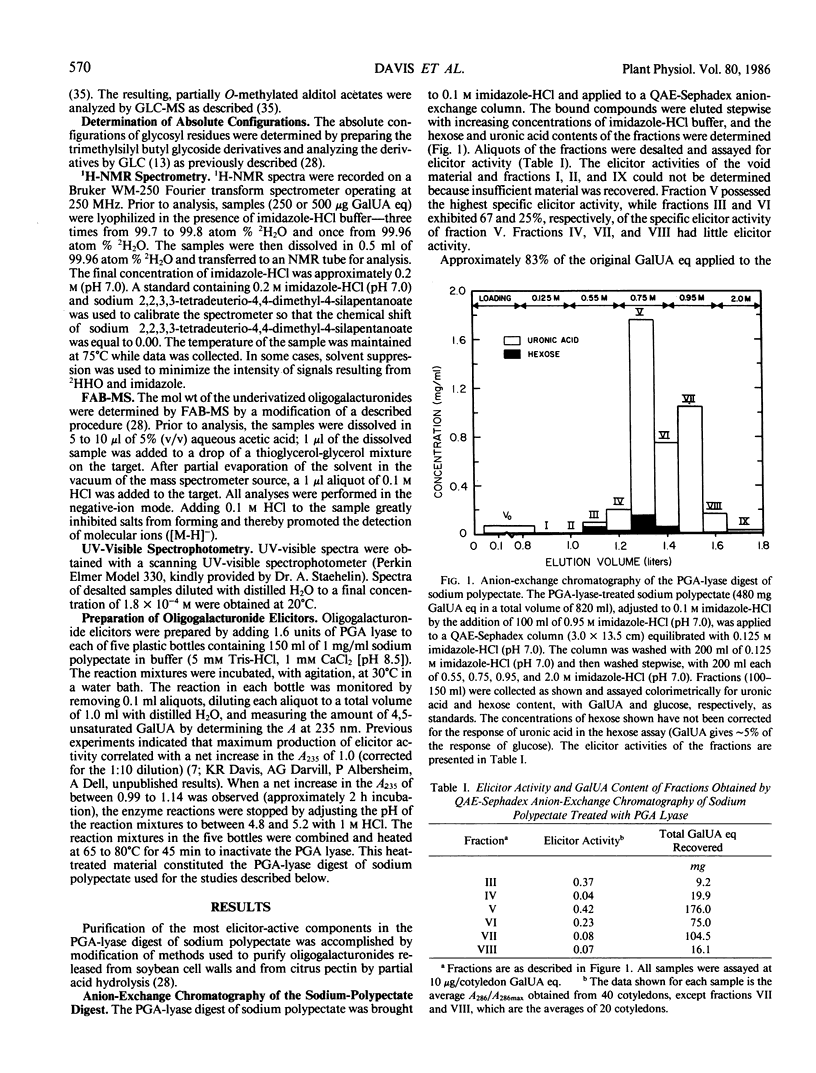
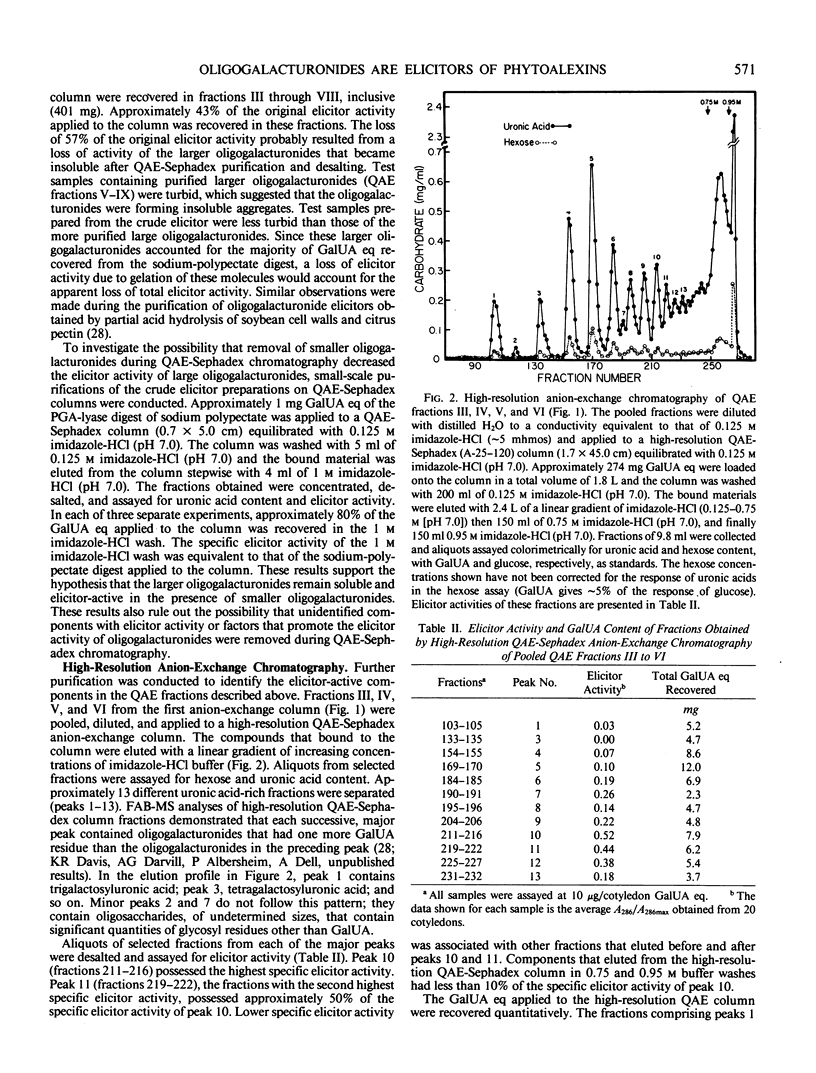
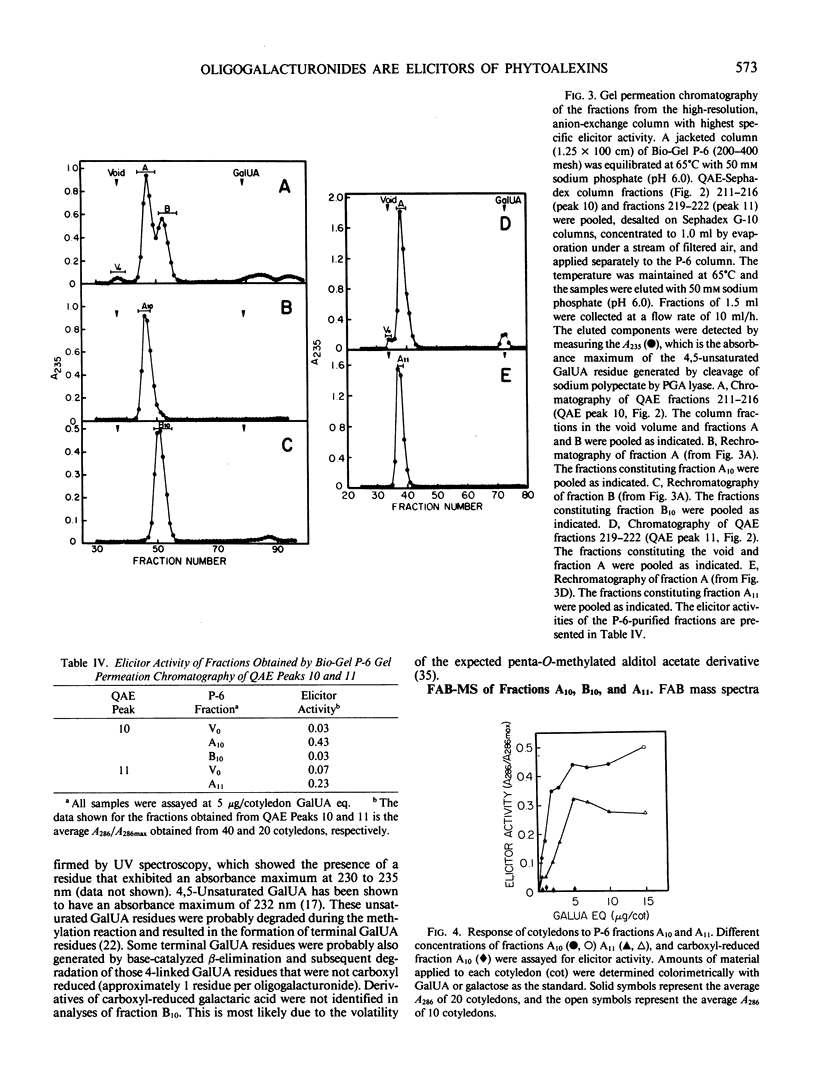
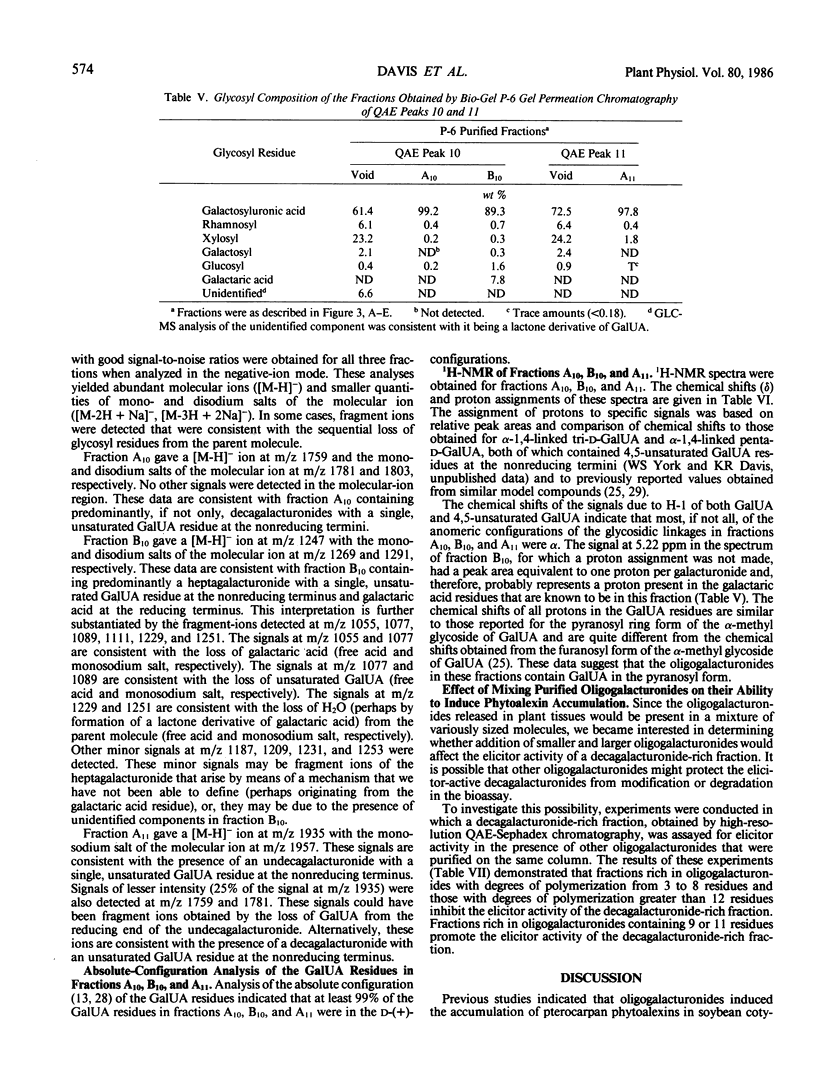
Recent studies have demonstrated that an apparently homogeneous preparation of an α-1,4-d-endopolygalacturonic acid lyase (EC 4.2.2.2) isolated from the phytopathogenic bacterium Erwinia carotovora induced phytoalexin accumulation in cotyledons of soybean (Glycine max [L.] Merr. cv Wayne) and that this pectin-degrading enzyme released heat-stable elicitors of phytoalexins from soybean cell walls, citrus pectin, and sodium polypectate (KR Davis et al. 1984 Plant Physiol 74: 52-60). The present paper reports the purification, by anion-exchange chromatography on QAE-Sephadex columns followed by gel-permeation chromatography on a Bio-Gel P-6 column, of the two fractions with highest specific elicitor activity present in a crude elicitor-preparation obtained by lyase treatment of sodium polypectate. Structural analysis of the fraction with highest specific elicitor activity indicated that the major, if not only, component was a decasaccharide of α-1,4-d-galactosyluronic acid that contained the expected product of lyase cleavage, 4-deoxy-β-l-5-threohexopyranos-4-enyluronic acid (4,5-unsaturated galactosyluronic acid), at the nonreducing terminus. This modified decagalacturonide fraction exhibited half-maximum and maximum elicitor activity at 1 microgram/cotyledon (6 micromolar) and 5 micrograms/cotyledon (32 micromolar) galactosyluronic acid equivalents, respectively. Reducing 90 to 95% of the carboxyl groups of the galactosyluronic acid residues abolished the elicitor activity of the decagalacturonide fraction. The second most elicitor-active fraction contained mostly undeca-α-1,4-d-galactosyluronic acid that contained 4,5-unsaturated galactosyluronic acid at the nonreducing termini. This fraction exhibited half-maximum and maximum elicitor activity at approximately 3 micrograms/cotyledon (17 micromolar) and 6 micrograms/cotyledon (34 micromolar) galactosyluronic acid equivalents, respectively. These results confirm and extend previous observations that oligogalacturonides derived from the pectic polysaccharides of plant cell walls can serve as regulatory molecules that induce phytoalexin accumulation in soybean. These results are consistent with the hypothesis that oligogalacturonides play a role in disease resistance in plants.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumenkrantz N., Asboe-Hansen G. New method for quantitative determination of uronic acids. Anal Biochem. 1973 Aug;54(2):484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- Bruce R. J., West C. A. Elicitation of Casbene Synthetase Activity in Castor Bean : THE ROLE OF PECTIC FRAGMENTS OF THE PLANT CELL WALL IN ELICITATION BY A FUNGAL ENDOPOLYGALACTURONASE. Plant Physiol. 1982 May;69(5):1181–1188. doi: 10.1104/pp.69.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers R. E., Clamp J. R. An assessment of methanolysis and other factors used in the analysis of carbohydrate-containing materials. Biochem J. 1971 Dec;125(4):1009–1018. doi: 10.1042/bj1251009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis K. R., Lyon G. D., Darvill A. G., Albersheim P. Host-Pathogen Interactions : XXV. Endopolygalacturonic Acid Lyase from Erwinia carotovora Elicits Phytoalexin Accumulation by Releasing Plant Cell Wall Fragments. Plant Physiol. 1984 Jan;74(1):52–60. doi: 10.1104/pp.74.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon R. A., Dey P. M., Lamb C. J. Phytoalexins: enzymology and molecular biology. Adv Enzymol Relat Areas Mol Biol. 1983;55:1–136. doi: 10.1002/9780470123010.ch1. [DOI] [PubMed] [Google Scholar]
- English P. D., Maglothin A., Keegstra K., Albersheim P. A Cell Wall-degrading Endopolygalacturonase Secreted by Colletotrichum lindemuthianum. Plant Physiol. 1972 Mar;49(3):293–298. doi: 10.1104/pp.49.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HASEGAWA S., NAGEL C. W. The characterization of an alpha, beta-unsaturated digalacturonic acid. J Biol Chem. 1962 Mar;237:619–621. [PubMed] [Google Scholar]
- Hahn M. G., Darvill A. G., Albersheim P. Host-Pathogen Interactions : XIX. THE ENDOGENOUS ELICITOR, A FRAGMENT OF A PLANT CELL WALL POLYSACCHARIDE THAT ELICITS PHYTOALEXIN ACCUMULATION IN SOYBEANS. Plant Physiol. 1981 Nov;68(5):1161–1169. doi: 10.1104/pp.68.5.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin D. F., West C. A. Characteristics of galacturonic Acid oligomers as elicitors of casbene synthetase activity in castor bean seedlings. Plant Physiol. 1984 Apr;74(4):989–992. doi: 10.1104/pp.74.4.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. C., West C. A. Polygalacturonase from Rhizopus stolonifer, an Elicitor of Casbene Synthetase Activity in Castor Bean (Ricinus communis L.) Seedlings. Plant Physiol. 1981 Apr;67(4):633–639. doi: 10.1104/pp.67.4.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. C., West C. A. Properties of Rhizopus stolonifer Polygalacturonase, an Elicitor of Casbene Synthetase Activity in Castor Bean (Ricinus communis L.) Seedlings. Plant Physiol. 1981 Apr;67(4):640–645. doi: 10.1104/pp.67.4.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon G. D., Albersheim P. Host-Pathogen Interactions : XXI. Extraction of a Heat-Labile Elicitor of Phytoalexin Accumulation from Frozen Soybean Stems. Plant Physiol. 1982 Aug;70(2):406–409. doi: 10.1104/pp.70.2.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil M., Darvill A. G., Fry S. C., Albersheim P. Structure and function of the primary cell walls of plants. Annu Rev Biochem. 1984;53:625–663. doi: 10.1146/annurev.bi.53.070184.003205. [DOI] [PubMed] [Google Scholar]
- Nothnagel E. A., McNeil M., Albersheim P., Dell A. Host-Pathogen Interactions : XXII. A Galacturonic Acid Oligosaccharide from Plant Cell Walls Elicits Phytoalexins. Plant Physiol. 1983 Apr;71(4):916–926. doi: 10.1104/pp.71.4.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlin A. S., Mackie D. M., Dietrich C. P. Evidence for a (1 leads to 4)-linked 4-O-( -L-idopyranosyluronic acid 2-sulfate)-(2-deoxy-2-sulfoamino-D-glucopyranosyl 6-sulfate) sequence in heparin. Long-range H-H coupling in 4-deoxy-hex-4-enopyranosides. Carbohydr Res. 1971 Jun;18(2):185–194. doi: 10.1016/s0008-6215(00)80341-9. [DOI] [PubMed] [Google Scholar]
- Sharp J. K., McNeil M., Albersheim P. The primary structures of one elicitor-active and seven elicitor-inactive hexa(beta-D-glucopyranosyl)-D-glucitols isolated from the mycelial walls of Phytophthora megasperma f. sp. glycinea. J Biol Chem. 1984 Sep 25;259(18):11321–11336. [PubMed] [Google Scholar]
- Sharp J. K., Valent B., Albersheim P. Purification and partial characterization of a beta-glucan fragment that elicits phytoalexin accumulation in soybean. J Biol Chem. 1984 Sep 25;259(18):11312–11320. [PubMed] [Google Scholar]
- Taylor R. L., Conrad H. E. Stoichiometric depolymerization of polyuronides and glycosaminoglycuronans to monosaccharides following reduction of their carbodiimide-activated carboxyl groups. Biochemistry. 1972 Apr 11;11(8):1383–1388. doi: 10.1021/bi00758a009. [DOI] [PubMed] [Google Scholar]
- Weinstein L. I., Hahn M. G., Albersheim P. Host-Pathogen Interactions : XVIII. ISOLATION AND BIOLOGICAL ACTIVITY OF GLYCINOL, A PTEROCARPAN PHYTOALEXIN SYNTHESIZED BY SOYBEANS. Plant Physiol. 1981 Aug;68(2):358–363. doi: 10.1104/pp.68.2.358. [DOI] [PMC free article] [PubMed] [Google Scholar]


