Summary
As a small animal that recapitulates many fundamental aspects of human disease, Drosophila lends itself to probing the biological activity of molecules and drug candidates. Here, we present a protocol to build a drug-testing pipeline in Drosophila. We describe steps for generating synchronous populations of Bicaudal C mutants by genetic crossing and wild-type fly culturing for controlled compound administration and exemplary phenotypic assays.
For complete details on the use and execution of this protocol, please refer to Millet-Boureima et al.,1 Millet-Boureima et al.,2 and Gamberi et al.3
Subject areas: Developmental biology, Genetics, Model Organisms
Graphical abstract
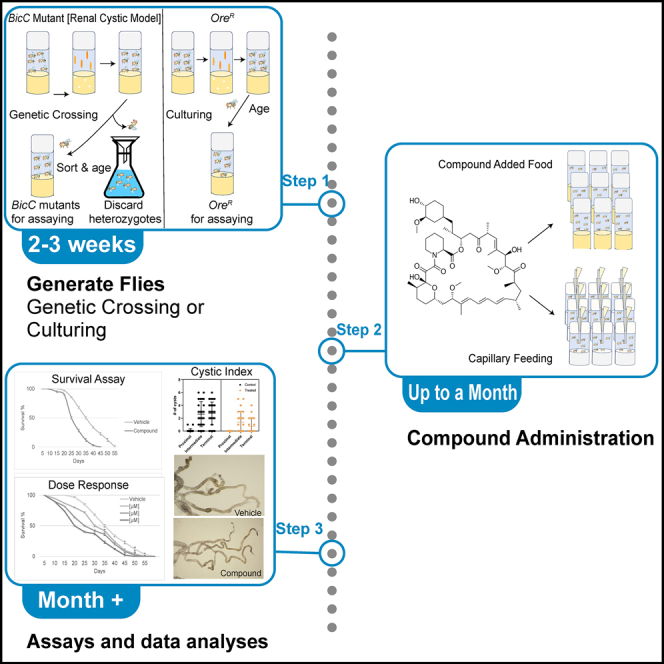
Highlights
-
•
Protocol for a drug-testing pipeline in Drosophila
-
•
Generation of synchronous fly populations by genetic crossing and culturing
-
•
Measuring the cystic index, survival rate, and negative geotaxis as phenotypic readouts
-
•
Applicable to dose-response and structure-activity relationship studies in Drosophila
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
As a small animal that recapitulates many fundamental aspects of human disease, Drosophila lends itself to testing the biological activity of molecules and drug candidates. Here, we present a protocol to build a drug-testing pipeline in Drosophila. We describe steps for generating synchronous populations of Bicaudal C mutants by genetic crossing and wild-type fly culturing for controlled compound administration and exemplary phenotypic assays.
Before you begin
Proper planning is crucial to sustain a constant supply of synchronous flies of the appropriate genotype(s) for testing. Before starting, expand the population of the desired fly lines and maintain as amplified culture sets thereafter. Generation times can be genotype-specific and must be factored-in to obtain synchronous testing populations. For large scale genetic crosses, sufficient parental individuals can be harvested from amplified cultures in a few days. To avoid overpopulation of the culturing vials which may affect fertility and metabolic parameters, the number of flies in each vial should not exceed a number that would cover the surface area of the food and should be transferred into fresh vials as soon as enough viable larvae are present. Considering that the progeny of genotypes harboring balancer chromosomes is partly viable, it is best to titrate the number of adults and the time they are left “seeding” to ensure comparable larval density, oxygenation, and nutrition in each vial. Similarly, the number of vials or culturing bottles needed to obtain the experimental population(s) is sized after determining the routine yields of the desired genotype(s).
Institutional permissions
Drosophila is innocuous. The research strains cultured in the laboratory are contained to prevent release in the wild. All discarded material is autoclaved or frozen before disposal. Please consult and adhere to the current institutional requirements.
Culture media preparation
Cornmeal food
Timing: 90–120 min
Note: This recipe yields ∼450 10 mL culture vials. It can be scaled to maintain enough time to cook the flours, dissolve agar completely and ensure consistent moisture levels (see note after step 6 below).
Note: To promote even and fast cooking, we use an electrical range top and a pot with bottom cladding yielding high thermal conductivity.
Note: Food preparation is best done in a locale separate from where flies are handled to prevent accidental food contamination.
Note: For simplicity, culture (10 mL) and compound-testing (2 mL) vials will be hereby referred to as “large” and “small” respectively.
-
1.Component preparation 15–30 min.
-
a.Prepare five trays with 100 large vials each or small vials as needed.
-
b.Pour five liters of deionized water into a pot and begin heating on an electric range.
-
c.Weigh all dry food ingredients except methylparaben and place in a large bowl.
-
d.Wearing a particulate mask, weigh methylparaben and set it aside.
-
i.Once done, remove methylparaben particles by wiping the scale and the surrounding area with damp tissue paper to be discarded as incinerated or chemical waste.Note: Methylparaben is considered hazardous (2012 OSHA Hazard Communication Standard, 29 CFR 1910, 1200) or dangerous (Globally Harmonized System). Prolonged ingestion may affect the endocrine system. Thus, minimize particulate spreading e.g., by weighing in a fume hood.
-
i.
-
e.Measure out the wet ingredients (corn syrup/molasses, propionic acid) into separate containers.
-
f.Once the water in the pot is warm:
-
i.Gradually add ∼ 700 mL to rehydrate the dry ingredients and mix to obtain a smooth pancake-like batter. Set the mixture aside.
-
ii.Add ∼ 20 mL of warm water to dilute the dense corn syrup/molasses to easy pouring consistency.
-
i.
-
g.Prepare a 65°C water bath to cool the food mixture.Note: To ensure agar remains dissolved, molten food should be dispensed between 55°C and 65°C. Lacking a large water bath, a basin with lukewarm water can also be used to cool down aliquots of molten food.
-
a.
-
2.Cooking 20–25 min.
-
a.Once the water from step 1b reaches 90°C, slowly add the batter from step 1f i while manually stirring. An overhead motorized stirrer could be used at 50–60 rpm, ensuring that the entire volume of cooking food is stirred.
-
b.Cook mixture for 15 min with frequent (manual) or continuous (motorized) stirring.Note: Stirring prevents settling of the flours and agar to the bottom of the pot, where they could burn.
-
c.Pour the pre-diluted corn syrup/molasses into the boiling mixture and stir for five more minutes. Turn off the heat.
-
d.Add methylparaben and mix thoroughly by stirring.
-
e.Add propionic acid and mix thoroughly by stirring.
-
f.Cool the mixture to 55°C–65°C in the water bath from step 1g.
-
a.
-
3.Pouring 30 min.
-
a.Pour 10 mL of cooled mixture into each large vial and 2 mL into each small vial (see note below).
-
a.
-
4.If preparing colored food to test drug consumption. If not, skip directly to step 5.
-
a.Set aside 50–100 mL of ready-to-pour food.
-
b.Add a dark, commercial food dye until the food has a deep hue (Figure 1, also see note in the key resources table section). Depending on the available products, the amounts needed may vary.
-
c.Dispense into vials as in 3a.
-
a.
Note: For culturing vials, food dispensing can be done manually with skill or using manual or peristaltic dispensers suitable for dense fluids (e.g., Pump for Media Dispensing from Filamatic, Automated Fly Food Dispenser from Gilson, and Droso-filler from Genesee). When preparing vials for drug and compound supplementation, volume accuracy is essential to maintain reproducible concentration of the added testing compounds. A wide bore tipped repeater pipettor or a regular 5–25 mL pipette do not clog easily and produce satisfactory results.
-
5.Cover completed trays with cloth netting (20 × 20 mesh size or higher) to prevent stray flies from contaminating the vials and wait about 1 h to reduce moisture condensation (optional for small vials).
-
a.Cap all vials.
-
b.Let the food solidify and dry at room temperature overnight.
-
c.Store in plastic bags at 4°C for two weeks (optimal).
-
a.
Note: Large vials can be used up to four weeks, but only for short term cultures because of lower moisture levels.
-
6.Clean up 5–10 min.
-
a.Remove stray waste from the surrounding area.
-
b.Wash the dishes used and clean the surfaces where cooking took place.
-
a.
Note: Moisture content and cooking time are important. When scaling this protocol, or substituting the source of the flour components, one must determine (empirically) the appropriate cooking time to yield digestible and properly moist food. Food should adhere to the walls of the vials throughout use, i.e., up to four weeks for long-term stock culture collections, and when unused, have a subtly shiny surface with no accumulated superficial moisture that could trap adult flies. Excess wetness could also reduce oxygenation that may hinder larvae as they colonize the food below the surface. Food moisture also promotes microbial growth, which decreases shelf life and may affect fly growth and health. In food, water is present in different bonding states,4 which complicates water content determination. While several methods exist to measure water content in food,5,6 none is rapid enough for use to monitor cooking time and/or practical in the settings of a small lab. Because the cross-section of the pot impacts on the evaporation rate and the dry ingredients may have variable moisture levels, the water amounts may need to be adjusted. Here, we report amounts we find optimal for a pot with a diameter of 25 cm.
CRITICAL: Consistent culturing is paramount for reproducibility. Diet, hence food composition, influences several Drosophila biological parameters, including lifespan,7,8,9,10,11,12 egg laying,13 metabolism,14,15 sleep and activity levels,13,16 and several phenotypes.10,11,17,18,19 Therefore, we emphasize the need for synchronous culture testing of all genotypes and conditions. For slow-developing lines, one may have to set up staggered cultures to simultaneously harvest adult flies for testing. While we have optimized this food recipe for all the fly lines used in our laboratory, other recipes may be equally effective in different settings, as long as they are verified to reproducibly yield vials of good quality (e.g., nutrient and moisture content). Pre-made food mixes and commercially produced Drosophila ready-to-use vials could also be used.
Figure 1.
Dyed cornmeal food compared to regular food
Agar vial preparation
Timing: 30–40 min
-
7.Prepare a suitable volume of 1% agar dissolved in deionized water. Also see the note under the key resources table.
-
a.Using either a microwave or hot plate, dissolve the agar with frequent stirring. Minimize evaporation loss by loosely covering the container with plastic wrap.
-
b.Cool to ∼ 55°C.
-
c.Dispense 10 mL of 1% agar in each vial.
-
d.Cap all vials and allow the agar to solidify. Should excess moisture be present on the side of the vial, follow step 5 above.
-
e.Store refrigerated in sealed plastic bags up to two weeks.
-
a.
Compound testing set up
Timing: 120–180 min
Note: ideal for examination throughout long time courses, compounds can be mixed with food and fed to flies. Alternatively, test molecules may be administered through “self-service” feeding out of a capillary containing a drug-laced solution.20 The latter method lends itself best to short-term studies.
-
8.Food-added compound.
-
a.Using a micropipette, deposit 20 μL (small vial) or 50–200 μL (large vial) of compound solution at the center surface of the food. Gently swirl the vial to spread the liquid around. For each experimental set, dispense the same volume in each vial.Note: If testing different drug concentrations, prepare appropriate dilutions to dispense equal volume and yield the desired drug concentration in food, after diffusion. Also see the note for point 8b.
-
b.Prepare identical vehicle control vials containing equal volume of the solvent in which the compound was diluted.Note: In these conditions, solvent concentrations are constant and drug concentration may vary. Also see notes after point 8f.
-
c.Allow compound diffusion for 2 h to 12–16 h.
-
i.For this step, vials can be placed at 4°C but should be equilibrated to room temperature prior to use.
-
i.
-
d.Add flies of the desired genotype. Large vials can contain up to 50 flies, while small vials can house up to 25. See the critical notes below.
-
e.Incubate at the desired temperature and lighting conditions.
-
f.Transfer the flies into identical fresh vials every two or three days to maintain consistent food quality and drug dosage. Frequency may be varied considering compound availability and larval activity. See the critical notes below.Note: Drug solvent. Closely related to the natural Drosophila food and life cycle, water or ethanol (<= 2% final in food) are choice solvents for the tested compounds (e.g., melatonin,2 rapamycin3). Low-solubility drugs are often dissolved in dimethylsulfoxide (DMSO). DMSO can melt the food surface, making it sticky enough to trap flies and interfere with survival assays. When DMSO must be used, we limit the added volume to 10 μL in large vials (final 0.1%) and dispense it inside a small puncture at the center of the food instead of its surface. Heat-resistant compounds may be added to molten food prior to dispensing into vials.Note: Effective drug dosage must be determined empirically through dose response experiments. To determine an initial effective range in Drosophila, one may consider that oral administration may require a ten times higher dose than injection to compensate for compromised drug stability in the gastrointestinal tract.21 However, this has not been extensively tested and can only be considered a prudent guideline. We have discussed previously the use of translating effective drug doses from Drosophila to other species.22 Guidelines to convert doses from mammalian pharmacological models have been produced to inform human trials.23,24 A potential limitation of this approach is that drug response is multifactorial, depending e.g., on drug mechanism of action, stability and pharmacokinetics, expression and molecular affinity of the implicated receptor(s) and their polymorphisms. These parameters vary in different species, as well as among individuals within the same species. In humans, drug response often differs in men and women and within the population. Active research to determine optimal, individualized drug dosage through direct monitoring has been recently reviewed.25,26
 CRITICAL: Solvents may have biological effects independent of the compounds tested, that has been documented well for DMSO.27 Thus, proper vehicle controls carried out identically and synchronously are crucial to interpret results correctly.
CRITICAL: Solvents may have biological effects independent of the compounds tested, that has been documented well for DMSO.27 Thus, proper vehicle controls carried out identically and synchronously are crucial to interpret results correctly. CRITICAL: Larval activity changes food texture and can create a sticky surface trapping adults and subtracting them from the assayed population. When using fertile genotypes, pre-empt this occurrence by transferring the adults into fresh vials frequently, typically every two or three days. In efforts to economize compound consumption (e.g., when using custom- synthesized molecules in analytical scale) we have tried to consolidate the vial surface through several methods, e.g., depositing a disk of either filter paper to absorb excess moisture or of nylon mesh to provide extra grip. However, none of these strategies produced satisfactory results with the OregonR (OreR) wild types. Therefore, frequent replacement of the drug-laced culture vials remains necessary.Note: When transferring adult flies to fresh vials, it is important to minimize accidental losses due to escape or injury. Besides optimal food moisture, to prevent flies getting stuck in the food, it is best to gently tap the vial on the side, rather than on the end, and twist (rather than pull) the plug off. Gentle handling is especially important when using mutants with reduced motility. Because CO2 stresses flies,28 it is better to avoid using anesthesia.In summary, the optimal drug testing strategy can balance availability of the tested molecule with food amounts, testing time, and size of the assayed population(s).Note: To obtain survival curves in longevity studies, we typically set up three to five replicates of the large culture vials containing 50 flies each. Miniaturized assays in the small vials, each housing up to 25 flies, are used for phenotypic assays (e.g., cystic index in micro-dissected Malpighian tubules1,2). Using analytical amounts of compounds, the latter setup is best suited to study the structure activity relationships of new compounds and for drug discovery.
CRITICAL: Larval activity changes food texture and can create a sticky surface trapping adults and subtracting them from the assayed population. When using fertile genotypes, pre-empt this occurrence by transferring the adults into fresh vials frequently, typically every two or three days. In efforts to economize compound consumption (e.g., when using custom- synthesized molecules in analytical scale) we have tried to consolidate the vial surface through several methods, e.g., depositing a disk of either filter paper to absorb excess moisture or of nylon mesh to provide extra grip. However, none of these strategies produced satisfactory results with the OregonR (OreR) wild types. Therefore, frequent replacement of the drug-laced culture vials remains necessary.Note: When transferring adult flies to fresh vials, it is important to minimize accidental losses due to escape or injury. Besides optimal food moisture, to prevent flies getting stuck in the food, it is best to gently tap the vial on the side, rather than on the end, and twist (rather than pull) the plug off. Gentle handling is especially important when using mutants with reduced motility. Because CO2 stresses flies,28 it is better to avoid using anesthesia.In summary, the optimal drug testing strategy can balance availability of the tested molecule with food amounts, testing time, and size of the assayed population(s).Note: To obtain survival curves in longevity studies, we typically set up three to five replicates of the large culture vials containing 50 flies each. Miniaturized assays in the small vials, each housing up to 25 flies, are used for phenotypic assays (e.g., cystic index in micro-dissected Malpighian tubules1,2). Using analytical amounts of compounds, the latter setup is best suited to study the structure activity relationships of new compounds and for drug discovery.
-
a.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, peptides, and recombinant proteins | ||
| Sucrose | Sigma-Aldrich | S0389 |
| Tegosept/nipagen/methylparaben | Apex Bioresearch | CAT#20-259 |
| Propionic acid | Apex Bioresearch | CAT#20-271 |
| Melatonin | Sigma-Aldrich | M5250 |
| Rapamycin | Tocris Bioscience | CAT#1292 |
| Phosphate-buffered saline (PBS) tablets | Sigma-Aldrich | P4417-100TAB |
| Tween 20 | Fisher Scientific | BP337 |
| Experimental models: Organisms/strains | ||
| D. melanogaster: Df(2L)RA5 | Bloomington Stock Center 6905 | |
| D. melanogaster: BicC[YC33] | In house | |
| D. melanogaster: BicC[IIF34] | In house | |
| Other | ||
| Agar (also see Alternatives further) | Flystuff Nutri-Fly Drosophila agar, gelidium, 100 mesh | CAT#66-103 |
| Corn flour | Great River Organic Milling, specialty flour, stone ground cornmeal (Amazon) | https://www.amazon.com/dp/B004ET1534/?coliid=I4LJOCSLN1FZU&colid=W17Z08POBWXE&ref_=lv_ov_lig_dp_it&th=1 |
| Soy flour | Naturevibe Botanicals organic soy flour (Amazon) | https://www.amazon.com/dp/B07NS8H1YQ/?coliid=I1IQJ24WPF53HZ&colid=W17Z08POBWXE&psc=1&ref_=lv_ov_lig_dp_it |
| Malt | Anthony’s premium dry malt powder (Amazon) | https://www.amazon.com/dp/B00WGUYX96/?coliid=INKWRUVEC2DNN&colid=W17Z08POBWXE&psc=1&ref_=lv_ov_lig_dp_it |
| Yeast | Fleischmann’s Yeast, active dry yeast | CAT#2192, available in grocery stores. |
| Corn syrup/molasses | Golden Barrel unsulfured blackstrap molasses (Amazon) | https://www.amazon.com/dp/B00M1ZYPXA/?coliid=I5E8MP1AP5Y1P&colid=W17Z08POBWXE&psc=1&ref_=lv_ov_lig_dp_it |
| Drosophila vial, wide, diameter: 28.5 mm, height: 95 mm, polypropylene | VWR | CAT#75813-164 |
| Drosophila container, non-sterile, diameter: 27 mm, height: 64 mm, polystyrene | Greiner Bio-One | CAT#205101 |
| Capillary | Kimble Chase Life Science and Research Products | CAT#41A2502 |
| Green food color | Kissan | https://www.kissan.ca/products/kissan-green-food-colour |
Alternatives: Agar quality is key to obtaining proper fly food texture and is often the cause of inconsistencies in both homemade and commercial food batches. Besides the agar listed in the table above, we have also successfully used bacteriological grade agar from Wisent Bioproducts (CAT#800-010-IK). Food dye should be tested to be inconsequential to the phenotypes studied and for potentially eliciting food aversion.
Materials and equipment
Cornmeal fly food
| Reagent | Final concentration | Amount |
|---|---|---|
| diH2O | 5 L | |
| Agar | 1% (w/v) | 40 g |
| Corn flour | 8% (w/v) | 320 g |
| Soy flour | 1% | 40 g |
| Malt | 8% | 320 g |
| Yeast | 1.8% | 72 g |
| Corn syrup/molasses | 2.2% | 88 g |
| Nipagen, Tegosept, or methylparaben | 0.24% | 9.6 g |
| Propionic acid | 0.0031% | 12.4 mL |
Vials are optimal for two weeks when stored in plastic bags at 4°C.
5% sucrose aqueous solution
-
•
In a 50 mL conical centrifuge tube, dissolve 2.5 g of sucrose in 50 mL distilled H2O. Filter through a 0.45 μM filter and store at 25°C up to two months preventing contamination or freeze into aliquots for short-term use.
CRITICAL: Propionic acid is caustic and should be handled carefully and using protective equipment.
Alternatives: Although the recipe presented here has been optimized for our assays, other formulations of fly food are available commercially as mixes or ready-to-use food vials.
Step-by-step method details
Generating BicC mutant flies by genetic crossing and culturing of control wild types
Timing: 2–3 weeks, excluding stock amplification
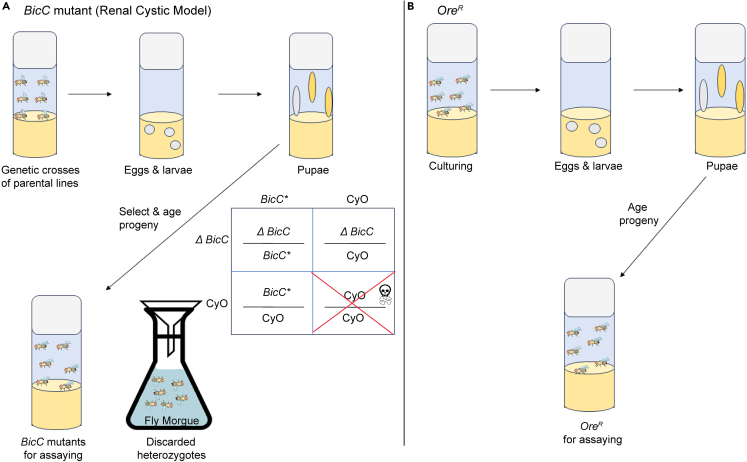
Note: Like control OreR flies,1,2,3 stable mutant lines can be maintained and amplified in culture vials or bottles.29 Also see note below. In contrast, sterile mutants, e.g., the BicC mutants, must be constantly generated by genetic crossing. To minimize interference from possible second side mutations, use trans-heterozygote flies whenever possible. For BicC, unmated females harboring chromosomal deletion Df (2L) RA5 (Δ) encompassing the BicC gene, in trans to the Curly of Oyster (CyO) balancer are crossed to males from two hypomorphic alleles, also in trans to CyO: BicCYC33/CyO and BicCIIF34/CyO, to obtain BicCΔ/YC33 and BicCΔ/IIF34 progenies.3 Unmated maternal Df (2L) RA5/CyO flies and paternal BicCYC33/CyO and BicCIIF34/CyO flies are collected from amplified stocks. Note, the BicC flies grow best in wide culture vials.
CRITICAL: In drug and survival assays, genetic crosses must be set up in culture vials with empirically titrated numbers of females and males to obtain reliably healthy progeny from each genotypic combination. Typically, 12 BicCΔ/CyO females and 7 BicCYC33/CyO or BicCIIF34/CyO males are crossed in each vial, with yeast sprinkled on top. Especially helpful for sickly mutants, yeast supplementation supports fly health and fertility. To obtain similar numbers of progeny BicCΔ/YC33 and BicCΔ/IIF34, crosses are established in a 40/60 ratio respectively. To obtain equivalent amounts and quality of control OreR flies, simultaneous and parallel cultures in identical yeasted vials are prepared with five females and three males and handled identically to the BicC crosses.
Note: All genotypes tested should be cultured and handled simultaneously and in identical conditions.
Note: About 30% of the wild type stocks and lines in the Bloomington Stock Center collection harbor the intracellular bacterium Wolbachia pipientis.30Wolbachia alters the reproductive fitness, male survival, fly immunity, behavior, activity levels, longevity, several metabolic pathways, and may have dramatic effects on mutant phenotypes.31,32,33,34,35,36,37,38Wolbachia can be cured by treating flies with tetracycline (250 μg/mL in food, for two generations), taking into account that this treatment may have long-lasting effects.39
-
1.Stock Amplification.
-
a.3–4 weeks prior to the planned experiment, amplify stocks.
-
i.Transfer adults every 3–4 days to build up enough collection vials.
-
i.
-
a.
Note: For BicC mutants, 50 vials of the maternal line BicCΔ/CyO and 20 vials of the paternal lines BicCYC33/CyO and BicCIIF34/CyO yield enough flies to seed 120 crosses in 3–5 days. Staggered cultures allow continued collection to maintain a constant pipeline of genetic crosses.
Note: working with large cultures such as those described here increases the risk of contamination and may amplify the results of events of genetic drift and reversion. Ensure proper handling to prevent the former and regularly monitor to spot the latter. Organizing the amplified stock vials in sets of identical racks with “lineage tracing” allows to effectively purge all vials related to such events while minimizing consequences for the yield of the pipeline.
-
2.Genetic Crossing (Figure 2). The described conditions yield comparable amounts of BicC mutants and OreR wild type flies.
-
a.From the amplified Df(2L)RA5/CyO maternal line, collect 1200 unmated females.
-
b.From the amplified BicCYC33/CyO paternal line, collect 200–280 males (enough for 40 crosses, see note below).
-
c.From the amplified BicC IIF34/CyO paternal line, collect 300–420 males (enough for 60 crosses, see note below).
-
d.For each yeasted vial, add 12 Df(2L)RA5/CyO unmated females and 5–7 male flies from the respective paternal lines.
-
e.Set up control vials with 5 OreR females and 3 males.
-
f.Incubate at the desired temperature and lighting conditions for ten days, or the developmental period of the specific genotypes used.
-
g.Transfer adults to fresh yeasted vials on day four, day seven, day ten and then discard.
-
a.
-
3.Fly collection and sorting.
-
a.Two days after the progeny flies begin to eclose, recover the desired phenotypes.Note: For BicC, straight winged individuals are sorted and housed into food vials. This yields populations of 0–2 days old individuals that are optimal for most assays.22
-
b.Continue collecting eclosed flies from the vials every 2 days.
-
i.To prevent recovery of second-generation individuals, do not harvest from the same vial beyond 9 days.Note: Staggered cultures and proper scaling yield a sustainable culturing pipeline producing adults for a medium throughput drug and phenotypic testing to be used by multiple people. Adoption of standard labeling and operating procedures for all laboratory personnel minimizes confusion and the potential consequences of incorrect population aging or inconsistent culturing conditions.
-
i.
-
a.
Figure 2.
Producing test fly populations
(A) Genetic crossing and phenotypic selection to isolate BicC transheterozygote mutants. The Punnett square depicts the progeny genotypes and viability from crossing maternal Df(2L)RA5/CyO (ΔBicC, deletion, curly wings) and either one of paternal lines BicCYC33/CyO and BicCIIF34/CyO (BicC ∗, hypomorphs, curly wings). The BicC progeny from this cross (ΔBicC/BicC∗) is straight winged.
(B) Continuous culturing to yield OreR flies or stable mutants.
Aging
-
4.
When aging the progeny flies, maintain them in batches of up to 50 flies per large vial and transfer to fresh food every 2–3 days to keep them well fed until the desired age for testing is reached.
-
5.
Supplementary yeast (3-4 2 mm diameter pellets) sprinkled on top of the food vial may support health of aging mutant flies.
Note: Excess yeast may affect lifespan and egg laying.40
Note: Considerations about population size and potentially compromised survival of sickly mutants are vital to plan a reliable yield of the fly pipeline to sustain the throughput of the planned drug testing assays.
Compound administration
Timing: up to 1 month
Depending on the experimental conditions, tested compounds can be administered mixed with food or through a self-feeding protocol using capillaries. Here we discuss typical set ups and possible modifications.
-
6.Compound added food 30 min set up, 3 h to overnight (12–16 h) for compound diffusion. If using capillary feeding, skip directly to 7.
-
a.Small (2 mL) vials.
-
i.Add up to 20 μL of compound or vehicle to each vial and let diffuse into the food for at least 3 h.
-
ii.Add up to 25 testing flies (here, 25 BicC or OreR control females plus 5–7 males of the same genotype to simulate normal conditions).
-
iii.Every 3 days, transfer the flies into identical new vials until either testing time or, for survival assays, until all flies are dead.
-
iv.Using populations of ∼20 flies per each time point and drug concentration, and a time course format, establish effective drug dosage and treatment length by performing the desired readout assay, e.g., the cystic index described in the phenotypic assays section.
-
v.Perform the drug assay with large population size for statistical confidence (n = 150-200).
-
i.
-
b.Large (10 mL) vials.
-
i.Add up to 200 μL of compound or vehicle to each vial and allow diffusion for at least 3 h.
-
ii.Add up to 50 testing flies and proceed as in 6a iii-v.
-
i.
-
c.Perform a drug consumption control assay.
-
i.Use colored food vials as in Figure 1 and culture as in 6a i-ii.
-
ii.Monitor the fly abdomen every day using a stereomicroscope.
-
iii.Record consumption. See the four notes below.
-
i.
-
a.
Note: Ingested dye will be visible through the semi-transparent cuticle and can be photographed.
Note: Depending on the food consumption and dye, stain intensity and contrast change over time. Daily monitoring for three days typically allows to find the clearest pattern. Once ingested by live flies, food coloring eventually diffuses in the entire body and reduces overall contrast. Flies can also be frozen and photographed later.
Note: Although this is a qualitative assay, it could be made semi-quantitative using dyes the consumption and excretion of which can be measured spectrophotometrically (e.g., FD&C Blue 1,41 Orange 4,42 Orange G and Yellow 1043). Alternatively, food could be spiked with radioisotopes, in which case the labeled flies must be strictly contained.44
Note: a comparative review of food intake assays45 and a table of timespan and temporal resolution of food intake assays46 have been published recently.
-
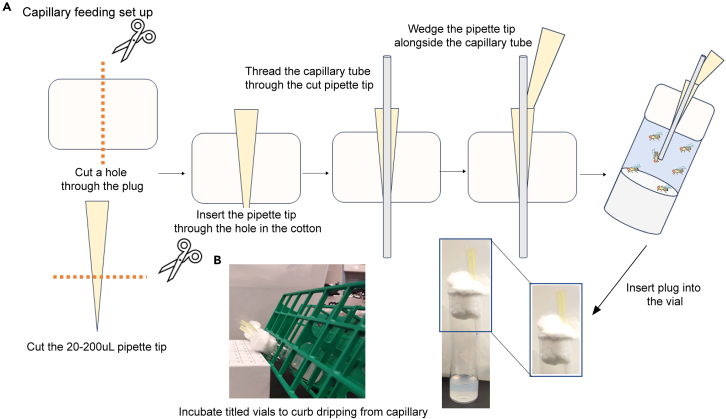
7.Capillary feeding 60–90 min. Capillary feeding (CAFÉ)47 assay set up, based on Stuivenberg et al. (2022)20 with modifications.
-
a.Prepare the vial caps:
-
i.Use a pair of scissors to cut a hole through the center of a vial plug, either cotton or foam.
-
ii.Cut off the terminal third of a 20-200 μL micropipette tip and insert it through the holed vial plug.
-
iii.Wedge an uncut yellow tip to secure the capillary inside the cut pipette housing (Figure 3).
-
iv.Place the plug on a large agar vial.
-
i.
-
b.Capillary filling.
-
i.Use capillaries of the following specifications: 75 mm, I.D: 1.1–1.2 mm, Wall: 0.2 ± 0.02 mm.
-
ii.Prepare aliquots of compound solutions at the desired concentration(s) and vehicle control in 5% sucrose.
-
iii.Touch one end of the capillary tube to the surface of the solution and tilt to stream mixture into the capillary until full.
-
iv.Carefully blotting the tip on a paper towel, remove solution until approximately 40 mm of solution is left in the capillary. Using a ruler, measure the exact length of the liquid column in each loaded capillary and record it.
-
v.Slide the loaded capillary tube through the cut pipette tip till its end hits halfway down the vial.
-
i.
-
c.Add 25 test flies. Here, 25 flies (0–2 day old) of each sex are used in sextuplicate to yield test populations of 150 flies of each sex and genotypes.
-
d.Prevent leakage from the capillary end and approximate the natural feeding stance, by tilting the vials 45 degrees (Figure 3B).
-
e.Incubate at the desired temperatures and light conditions.
-
f.Evaporation control. Set up an identical vial with no flies and a capillary loaded with the vehicle control solution. Incubate alongside the test vials in identical conditions.
-
g.Every 24 h, record the liquid left in the capillary and refill it to reconstitute a 40 mm liquid column.
-
i.For each vial, determine the 24-h consumption by subtracting the remaining volume in the capillary of the test vial from the remaining volume in the capillary of the evaporation control.
-
i.
-
a.
Note: The agar vials are normally replaced every 3–4 days to ensure optimal moisture, or more often should there be larval interference.
Note: Each trial is minimally done at least in triplicate. Flies are typically 0–2 days old at the start but can be aged as described in the next section.
Optional: To facilitate data collection in survival assays, making a preset spreadsheet file allows to monitor the experiment in real time.
Figure 3.
Capillary feeding setup
(A) Preparation and setting of the capillary feeding.
(B) Tilting the vials in racks prevents capillary leakage and approximates the natural feeding stance.
Phenotypic assays
Timing: up to 2 months
Several drug treatment readouts are possible, which will depend on the studied mechanisms and phenotypes. Here we discuss three common assays with the relative statistical analyses: cystic index, survival, and negative geotaxis.
Note: Microdissection enables direct monitoring of the organs of interest for phenotypic analysis. Here we describe the cystic index, as in Millet-Boureima et al., Millet-Boureima et al., and Gamberi et al.1,2,3 based on the study of the Malpighian tubules, which are analogous to the tubular portion of the human nephron.48 If not performed, skip directly to step 9.
-
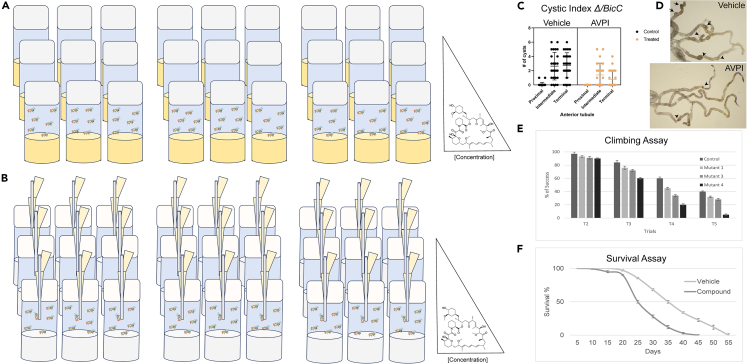
8.Microdissection and cystic index (Figure 4).
-
a.Set up parallel populations of flies for all tested conditions, food and supplementation and incubate until the testing age is reached (Figures 4A and 4B).
-
b.Micro-dissect the Malpighian tubule pairs in a concave well containing PBT (1× phosphate buffer saline (PBS) 0.01% Tween-20).
-
c.Place a ∼50 μL drop of PBT on a microscope slide and align the tubules.
-
d.Photograph the tubules ex vivo through a stereomicroscope equipped with cold lights and a camera. We find that double illumination from the top and bottom yields optimal contrast (Figure 4D). Other types of illumination can be used.
-
e.Score and record the cysts and their location from triplicate or quadruplicate sets of 50 females in large vials for both control and drug treatments (n = 150-200, Figures 4C and 4D).
-
i.Assign cysts to the anterior and posterior tubule pair.
- ii.
-
i.
-
f.Perform statistical analysis (see quantification and statistical analysis section).Note: Relying on a natural Drosophila reflex for which flies climb on the wall of a container after mechanical stimulation, the assays below entail tapping the flies to the bottom of a graduated container and measuring the distance they climb upwards during a set time.49,50 The resulting performance value is used as a proxy for neuromuscular fitness in different conditions, age, and genotypes. If not performed, skip directly to step 10.
-
a.
-
9.Negative geotaxis (climbing) assays (Figure 4E).
-
a.The day before testing, place sets of five to ten appropriately aged flies each into one culture vial and incubate as usual. Prepare separate test populations of female and males.For regular assays we use triplicate vials with fifty flies. Pilot experiments can be carried out with 25 individuals.
-
b.Mark ten centimeters from the cap on the side of a graded 15 mL conical tube.
-
c.Use a funnel to transfer the flies from the culture vial to the graded conical tube without using fly anesthesia to minimize stress triggers.
-
d.Place the tube on the bench, cap side down.
-
e.Acclimate the flies for 1 min.
-
f.Gently tap the vial on a rubber fly mat three times to collect all flies on the cap, which acts as a starting line.
-
g.Start a timer and record the number of flies reaching a 10 cm threshold in 10 s.Note: This can be done manually with small batches of 5-10 flies, or it could be video-recorded and analyzed.50
- h.
-
i.Analyze and plot the data as described in the quantification and statistical analysis section.Note: Certain genotypes may show fatigue (fewer flies cross the line) or learning (more flies cross the line) during the assay replicas. It is therefore important to examine the trend of the repeats and evaluate the suitability of averaging all results or compare the plotted results separately from each replica.Note: Survival assays are used to monitor longevity in populations of different genotypes, conditions, and/or drug treatment, compared to control.40 If not performed, skip directly to the quantification and statistical analysis section.
-
a.
-
10.Survival assays (Figure 4F).
-
a.Set up parallel populations of 50 flies each in large culture vials with the chosen food and supplementation.
- b.
-
c.From now on, manage the populations in parallel, identically, and simultaneously. Males and females can be mixed in known amounts, aware that mating can affect egg laying and longevity.40
-
d.Every three days record the number of surviving flies, ideally at the same time of the day. Record males and females separately. Also note any occurrence that may affect the results and help data interpretation (e.g., accidental deaths of flies trapped in food).Note: For conditions reducing survival to 24 h (e.g., desiccation) monitor every 1–2 h.
-
e.Calculate the percentage survival for each vial.
-
f.Continue monitoring as in 10d-e. every three days until all flies in the testing populations have died.
-
g.Perform statistical analysis as discussed in the quantification and statistical analysis section below.Note: Wet vials (from improper food preparation or larval activity) may trap adults on the food surface and increase casualty deaths. Use quality food and transfer flies avoiding tapping too harshly. For lines with high egg-laying, consider transferring adults more frequently (e.g., every 2 days), recording this change to aid result interpretation.
-
a.
Figure 4.
Typical experimental design to test the biological activity of a molecule (e.g., rapamycin)
(A and B) For both food-added (A) and capillary-fed compounds (B), at least triplicate vials are prepared for each time point and condition (e.g., drug concentration in dose response assays, right). All genotypes, conditions and controls are assayed synchronously and in identical conditions. Three exemplary readout assays are shown.
(C and D) Cystic index of Malpighian tubules of BicC flies treated with AVPI as in Millet-Boureima et al. (2019)1 or vehicle. (C) Nested plot quantification and (D) micro photographs showed reduced cysts (arrows) upon treatment.
(E) Example of negative geotaxis (or climbing) assay to compare the fitness of mutant and control genotypes over time, with bar graph representation.
(F) Survival assays compare survival of drug- and vehicle-treated populations over time.
Table 1.
Example of raw data collected for cystic index
| Control flies | Anterior terminal | Anterior intermediate | Anterior proximal | Posterior terminal | Posterior intermediate | Posterior proximal |
|---|---|---|---|---|---|---|
| 1 | 1 | 5 | 3 | 3 | 3 | 1 |
| 2 | 8 | 11 | 0 | 6 | 7 | 2 |
| 3 | 6 | 3 | 0 | 5 | 5 | 3 |
| 4 | 4 | 13 | 2 | 4 | 7 | 4 |
| 5 | 5 | 7 | 1 | 3 | 8 | 1 |
| Total cysts | 24 | 39 | 6 | 21 | 30 | 11 |
Expected outcomes
An example of data collected for the cystic index is shown in Table 1, with associated nested plots (Figure 4C). The vehicle-treated control flies exhibit more cysts than drug-treated flies. Moreover, diverse tubular regions respond differentially to the drug.1,2
Quantification and statistical analysis
Note: Cystic index analysis specific to our studies aims at determining whether drug treatments can reduce cysts and ameliorate or rescue the BicC mutant cystic phenotype as assessed in parallel vehicle- and drug-treated populations. Treatments that affect longevity change the slope of the survival curve and/or the time at which the population is halved.
-
1.Cystic index.
-
a.Using GraphPad Prism or equivalent software, plot the individual values as nested distributions with associated standard deviations. Also see note below.
-
b.When the measured parameter has a normal distribution, perform unpaired t-tests to compare the experimental groups and determine if their averages differ more likely than expected based on random chance.
-
c.Introduce the Welch’s correction because the populations may not have equal standard deviations.
-
d.Compare the distributions and standard deviations of the vehicle-treated vs. compound-treated populations and calculate the probability (p) value for statistical confidence. Also see note below.
-
a.
Note: Column or bar graphs could be used instead of nested plots. Nested plots are preferable because they represent the natural variation of the scored character in the natural population. For example, the cystic index as in Millet-Boureima et al.1,2 indicates the number of cysts occurring in the entire renal tubule and its proximal, intermediate, and distal regions. p-values of 0.02 and 0.05 are often regarded as significance thresholds, representing respectively a 2% and 5% chance that the recorded data may be due by random occurrence. Detailed discussions of the p-value for biological studies can be found.54,55
Note: When comparing two conditions, e.g., vehicle control versus compound assays, a t-test is suitable. When several conditions or groups are compared, Analysis of Variance test (ANOVA) can determine whether the means of multiple independent population assays are statistically different from one another.
-
2.Negative geotaxis.
-
a.For each tested set (5-10 flies) and repetition, calculate the average and standard deviation.
-
b.Calculate and plot an overall average and standard deviation for each repetition.
-
c.Check the trends obtained in the assay repetitions for signs of fatigue (over time, fewer flies climb to the target line) or learning (over time, more flies climb to the target line).
-
d.If applicable, average all repetitions for each tested genotype and condition.
-
e.Perform unpaired t-test and Welch correction (or ANOVA) and calculate the p-value as described for the cystic index in 1. Similarly, bar graphs could be generated, at the expense of the original data set representation.
-
a.
-
3.Survival curves.
-
a.Each data point is calculated as percentage of survivors at a single time.
-
i.Turn the raw data (number of survivors) into a percentage using the calculation: percentage = (live flies number/population size)∗100.
-
ii.For each replicate and time point, calculate average and standard deviations as percentages.
-
iii.Plot all survival over time values and standard deviations with Excel or equivalent software. An exemplary result is displayed in Figure 4F in which the survival of a toxin-treated population is reduced compared to that of a control vehicle-treated population.
-
iv.Calculate the intercepts at 50% survival, which is often used as a reference comparison.
-
v.Perform a t-test with Welch correction, if applicable (or ANOVA) and calculate the p-value.
-
i.
-
a.
Note: Additional comparative tools for survival assays can be found in Piper and Partridge (2018).40
Limitations
When working with live animals such as Drosophila, understanding their biology and physiological responses to environmental stimuli is paramount. Steady environmental conditions positively affect fitness and reduce data fluctuation. Likewise, proper food preparation, careful handling, temperature and light cycle regulation are all variables to be aware of and control to obtain consistent results.
Troubleshooting
Problem 1
Poor culturing and inconsistent food quality.
Potential solution
-
•
Ensure all ingredients for food preparation are added in the correct order. See culture media preparation.
-
•
Properly hydrate all dry ingredients and mix thoroughly while cooking.
-
•
Ensure that food is cooled to approximately 55 degrees before pouring.
-
•
While pouring, frequently stir the food to prevent settling of the particulates.
-
•
Use clean or even sterile equipment to avoid introducing environmental contaminants.
Problem 2
Improper handling.
Potential solution
-
•
Clearly label and cap all vials and keep track of vial “lineage” to minimize loss, contamination, and genetic drift.
-
•
Be mindful of where the flies are being placed when in use to avoid disruptions (e.g., dropping or knocking over vials e.g., wearing shirts with loose fitting sleeves).
-
•
Minimize the flies CO2 exposure, especially for climbing and behavioral assays.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Chiara Gamberi (cgamberi@coastal.edu).
Materials availability
This study did not generate unique reagents. BicC flies are available through the Bloomington Drosophila collection or by reasonable request.
Data and code availability
Not applicable.
Acknowledgments
We want to thank Gerrit Stuivenberg and Dr. Jeremy Burton, Lawson Health Research Institute, for introducing the capillary feeding method to us; Dr. William D. Lubell, Université de Montréal and Dr. Trudy Mackay, Clemson University for discussion; and members of the Gamberi laboratory for comments on the manuscript. This research was funded by the National Institutes of Health (NIH) South Carolina IDeA Networks of Biomedical Research Excellence (SC-INBRE) Developmental Research Program grant 5P20GM103499-22-235114, the TANGO2 Research Foundation, the Horry County Higher Education Commission, and startup funds to C.G. C.G. is a member of INBRE, the Center of Excellence in Research on Orphan Diseases – Fondation Courtois, and the Disease Modeling Research Center at Coastal Carolina University.
Author contributions
J.D. and C.M.-B. performed the experiments, co-wrote the first draft, made figures, and edited the manuscript. C.G. planned the experiments, co-wrote the first draft, edited the figures and manuscript, and supervised the projects.
Declaration of interests
The authors declare no competing interests.
References
- 1.Millet-Boureima C., Chingle R., Lubell W.D., Gamberi C. Cyst reduction in a polycystic kidney disease Drosophila model using Smac mimics. Biomedicines. 2019;7:82. doi: 10.3390/biomedicines7040082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Millet-Boureima C., Rozencwaig R., Polyak F., Gamberi C. Cyst reduction by melatonin in a novel Drosophila model of polycystic kidney disease. Molecules. 2020;25:5477. doi: 10.3390/molecules25225477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gamberi C., Hipfner D.R., Trudel M., Lubell W.D. Bicaudal C mutation causes myc and TOR pathway upregulation and polycystic kidney disease-like phenotypes in Drosophila. PLoS Genet. 2017;13 doi: 10.1371/journal.pgen.1006694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lang K.W., Steinberg M.P. Calculation of moisture content of a formulated food system to any given water activity. J. Food Sci. 1980;45:1228–1230. [Google Scholar]
- 5.Isengard H.-D. Rapid water determination in foodstuffs. Trends Food Sci. Technol. 1995;6:155–162. [Google Scholar]
- 6.Isengard H.-D. Water content, one of the most important properties of food. Food Control. 2001;12:395–400. [Google Scholar]
- 7.Bass T.M., Grandison R.C., Wong R., Martinez P., Partridge L., Piper M.D.W. Optimization of dietary restriction protocols in Drosophila. J. Gerontol. A Biol. Sci. Med. Sci. 2007;62:1071–1081. doi: 10.1093/gerona/62.10.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhandari P., Jones M.A., Martin I., Grotewiel M.S. Dietary restriction alters demographic but not behavioral aging in Drosophila. Aging Cell. 2007;6:631–637. doi: 10.1111/j.1474-9726.2007.00320.x. [DOI] [PubMed] [Google Scholar]
- 9.Grandison R.C., Piper M.D.W., Partridge L. Amino-acid imbalance explains extension of lifespan by dietary restriction in Drosophila. Nature. 2009;462:1061–1064. doi: 10.1038/nature08619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holmbeck M.A., Rand D.M. Dietary fatty acids and temperature modulate mitochondrial function and longevity in Drosophila. J. Gerontol. A Biol. Sci. Med. Sci. 2015;70:1343–1354. doi: 10.1093/gerona/glv044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schriner S.E., Coskun V., Hogan S.P., Nguyen C.T., Lopez T.E., Jafari M. Extension of Drosophila lifespan by Rhodiola rosea depends on dietary carbohydrate and caloric content in a simplified diet. J. Med. Food. 2016;19:318–323. doi: 10.1089/jmf.2015.0105. [DOI] [PubMed] [Google Scholar]
- 12.Xia B., de Belle J.S. Transgenerational programming of longevity and reproduction by post-eclosion dietary manipulation in Drosophila. Aging. 2016;8:1115–1134. doi: 10.18632/aging.100932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Piper M.D.W., Blanc E., Leitão-Gonçalves R., Yang M., He X., Linford N.J., Hoddinott M.P., Hopfen C., Soultoukis G.A., Niemeyer C., et al. A holidic medium for Drosophila melanogaster. Nat. Methods. 2014;11:100–105. doi: 10.1038/nmeth.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Birse R.T., Choi J., Reardon K., Rodriguez J., Graham S., Diop S., Ocorr K., Bodmer R., Oldham S. High-fat-diet-induced obesity and heart dysfunction are regulated by the TOR pathway in Drosophila. Cell Metab. 2010;12:533–544. doi: 10.1016/j.cmet.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morris S.N.S., Coogan C., Chamseddin K., Fernandez-Kim S.O., Kolli S., Keller J.N., Bauer J.H. Development of diet-induced insulin resistance in adult Drosophila melanogaster. Biochim. Biophys. Acta. 2012;1822:1230–1237. doi: 10.1016/j.bbadis.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Regalado J.M., Cortez M.B., Grubbs J., Link J.A., van der Linden A., Zhang Y. Increased food intake after starvation enhances sleep in Drosophila melanogaster. J. Genet. Genomics. 2017;44:319–326. doi: 10.1016/j.jgg.2017.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Unckless R.L., Rottschaefer S.M., Lazzaro B.P. The complex contributions of genetics and nutrition to immunity in Drosophila melanogaster. PLoS Genet. 2015;11 doi: 10.1371/journal.pgen.1005030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bayliak M.M., Lylyk M.P., Shmihel H.V., Sorochynska O.M., Manyukh O.V., Pierzynowski S.G., Lushchak V.I. Dietary alpha-ketoglutarate increases cold tolerance in Drosophila melanogaster and enhances protein pool and antioxidant defense in sex-specific manner. J. Therm. Biol. 2016;60:1–11. doi: 10.1016/j.jtherbio.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 19.Katzenberger R.J., Ganetzky B., Wassarman D.A. Age and diet affect genetically separable secondary injuries that cause acute mortality following traumatic brain injury in Drosophila. G3 (Bethesda) 2016;6:4151–4166. doi: 10.1534/g3.116.036194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stuivenberg G.A., Chmiel J.A., Akouris P.P., Burton J.P., Reid G. Probiotic Bifidobacteria mitigate the deleterious effects of para-Cresol in a Drosophila melanogaster toxicity model. mSphere. 2022;7 doi: 10.1128/msphere.00446-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pandey U.B., Nichols C.D. Human disease models in Drosophila melanogaster and the role of the fly in therapeutic drug discovery. Pharmacol. Rev. 2011;63:411–436. doi: 10.1124/pr.110.003293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Millet-Boureima C., Selber-Hnatiw S., Gamberi C. Drug discovery and chemical probing in Drosophila. Genome. 2021;64:147–159. doi: 10.1139/gen-2020-0037. [DOI] [PubMed] [Google Scholar]
- 23.Nair A.B., Jacob S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016;7:27–31. doi: 10.4103/0976-0105.177703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nair A., Morsy M.A., Jacob S. Dose translation between laboratory animals and human in preclinical and clinical phases of drug development. Drug Dev. Res. 2018;79:373–382. doi: 10.1002/ddr.21461. [DOI] [PubMed] [Google Scholar]
- 25.Kantasiripitak W., Van Daele R., Gijsen M., Ferrante M., Spriet I., Dreesen E. Software tools for model-informed precision dosing: how well do they satisfy the needs? Front. Pharmacol. 2020;11:620. doi: 10.3389/fphar.2020.00620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Del Valle-Moreno P., Suarez-Casillas P., Mejías-Trueba M., Ciudad-Gutiérrez P., Guisado-Gil A.B., Gil-Navarro M.V., Herrera-Hidalgo L. Model-informed precision dosing software tools for dosage regimen individualization: a scoping review. Pharmaceutics. 2023;15:1859. doi: 10.3390/pharmaceutics15071859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Verheijen M., Lienhard M., Schrooders Y., Clayton O., Nudischer R., Boerno S., Timmermann B., Selevsek N., Schlapbach R., Gmuender H., et al. DMSO induces drastic changes in human cellular processes and epigenetic landscape in vitro. Sci. Rep. 2019;9:4641. doi: 10.1038/s41598-019-40660-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suh G.S.B., Wong A.M., Hergarden A.C., Wang J.W., Simon A.F., Benzer S., Axel R., Anderson D.J. A single population of olfactory sensory neurons mediates an innate avoidance behaviour in Drosophila. Nature. 2004;431:854–859. doi: 10.1038/nature02980. [DOI] [PubMed] [Google Scholar]
- 29.Asadi P., Milev M.P., Saint-Dic D., Gamberi C., Sacher M. Vitamin B5, a coenzyme A precursor, rescues TANGO2 deficiency disease-associated defects in Drosophila and human cells. J. Inherit. Metab. Dis. 2023;46:358–368. doi: 10.1002/jimd.12579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clark M.E., Anderson C.L., Cande J., Karr T.L. Widespread prevalence of wolbachia in laboratory stocks and the implications for Drosophila research. Genetics. 2005;170:1667–1675. doi: 10.1534/genetics.104.038901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laven H. Speciation by cytoplasmic isolation in the Culex pipiens-complex. Cold Spring Harb. Symp. Quant. Biol. 1959;24:166–173. doi: 10.1101/sqb.1959.024.01.017. [DOI] [PubMed] [Google Scholar]
- 32.Yen J.H., Barr A.R. The etiological agent of cytoplasmic incompatibility in Culex pipiens. J. Invertebr. Pathol. 1973;22:242–250. doi: 10.1016/0022-2011(73)90141-9. [DOI] [PubMed] [Google Scholar]
- 33.Starr D.J., Cline T.W. A host parasite interaction rescues Drosophila oogenesis defects. Nature. 2002;418:76–79. doi: 10.1038/nature00843. [DOI] [PubMed] [Google Scholar]
- 34.Fry A.J., Rand D.M. Wolbachia interactions that determine Drosophila melanogaster survival. Evolution. 2002;56:1976–1981. doi: 10.1111/j.0014-3820.2002.tb00123.x. [DOI] [PubMed] [Google Scholar]
- 35.Wong A.C.N., Wang Q.P., Morimoto J., Senior A.M., Lihoreau M., Neely G.G., Simpson S.J., Ponton F. Gut microbiota modifies olfactory-guided microbial preferences and foraging decisions in Drosophila. Curr. Biol. 2017;27:2397–2404.e4. doi: 10.1016/j.cub.2017.07.022. [DOI] [PubMed] [Google Scholar]
- 36.Qiao H., Keesey I.W., Hansson B.S., Knaden M. Gut microbiota affects development and olfactory behavior in Drosophila melanogaster. J. Exp. Biol. 2019;222:jeb192500. doi: 10.1242/jeb.192500. [DOI] [PubMed] [Google Scholar]
- 37.Ote M., Yamamoto D. Impact of Wolbachia infection on Drosophila female germline stem cells. Curr. Opin. Insect Sci. 2020;37:8–15. doi: 10.1016/j.cois.2019.10.001. [DOI] [PubMed] [Google Scholar]
- 38.Burdina E.V., Gruntenko N.E. Physiological aspects of Wolbachia pipentis-Drosophila melanogaster relationship. J. Evol. Biochem. Physiol. 2022;58:303–317. [Google Scholar]
- 39.O'Shea K.L., Singh N.D. Tetracycline-exposed Drosophila melanogaster males produce fewer offspring but a relative excess of sons. Ecol. Evol. 2015;5:3130–3319. doi: 10.1002/ece3.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Piper M.D.W., Partridge L. Drosophila as a model for ageing. Biochim. Biophys. Acta, Mol. Basis Dis. 2018;1864:2707–2717. doi: 10.1016/j.bbadis.2017.09.016. [DOI] [PubMed] [Google Scholar]
- 41.Shell B.C., Schmitt R.E., Lee K.M., Johnson J.C., Chung B.Y., Pletcher S.D., Grotewiel M. Measurement of solid food intake in Drosophila via consumption-excretion of a dye tracer. Sci. Rep. 2018;8 doi: 10.1038/s41598-018-29813-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shell B.C., Luo Y., Pletcher S., Grotewiel M. Expansion and application of dye tracers for measuring solid food intake and food preference in Drosophila. Sci. Rep. 2021;11 doi: 10.1038/s41598-021-99483-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shell B.C., Grotewiel M. Identification of additional dye tracers for measuring solid food intake and food preference via consumption-excretion in Drosophila. Sci. Rep. 2022;12:6201. doi: 10.1038/s41598-022-10252-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carvalho G.B., Kapahi P., Anderson D.J., Benzer S. Allocrine modulation of feeding behavior by the Sex Peptide of Drosophila. Curr. Biol. 2006;16:692–696. doi: 10.1016/j.cub.2006.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Deshpande S.A., Carvalho G.B., Amador A., Phillips A.M., Hoxha S., Lizotte K.J., Ja W.W. Quantifying Drosophila food intake: comparative analysis of current methodology. Nat. Methods. 2014;11:535–540. doi: 10.1038/nmeth.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mahishi D., Huetteroth W. The prandial process in flies. Curr. Opin. Insect Sci. 2019;36:157–166. doi: 10.1016/j.cois.2019.09.004. [DOI] [PubMed] [Google Scholar]
- 47.Ja W.W., Carvalho G.B., Mak E.M., de la Rosa N.N., Fang A.Y., Liong J.C., Brummel T., Benzer S. Prandiology of Drosophila and the CAFE assay. Proc. Natl. Acad. Sci. USA. 2007;104:8253–8256. doi: 10.1073/pnas.0702726104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Millet-Boureima C., Porras Marroquin J., Gamberi C. Modeling renal disease “on the fly”. BioMed Res. Int. 2018;2018 doi: 10.1155/2018/5697436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ganetzky B., Flanagan J.R. On the relationship between senescence and age-related changes in two wild-type strains of Drosophila melanogaster. Exp. Gerontol. 1978;13:189–196. doi: 10.1016/0531-5565(78)90012-8. [DOI] [PubMed] [Google Scholar]
- 50.Gargano J.W., Martin I., Bhandari P., Grotewiel M.S. Rapid iterative negative geotaxis (RING): a new method for assessing age-related locomotor decline in Drosophila. Exp. Gerontol. 2005;40:386–395. doi: 10.1016/j.exger.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 51.Jones M.A., Grotewiel M. Drosophila as a model for age-related impairment in locomotor and other behaviors. Exp. Gerontol. 2011;46:320–325. doi: 10.1016/j.exger.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Piper M.D.W., Partridge L. Protocols to study aging in Drosophila. Methods Mol. Biol. 2016;1478:291–302. doi: 10.1007/978-1-4939-6371-3_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Linford N.J., Chan T.P., Pletcher S.D. Re-patterning sleep architecture in Drosophila through gustatory perception and nutritional quality. PLoS Genet. 2012;8 doi: 10.1371/journal.pgen.1002668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Andrade C. The P value and statistical significance: Misunderstandings, explanations, challenges, and alternatives. Indian J. Psychol. Med. 2019;41:210–215. doi: 10.4103/IJPSYM.IJPSYM_193_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pollard D.A., Pollard T.D., Pollard K.S. Empowering statistical methods for cellular and molecular biologists. Mol. Biol. Cell. 2019;30:1359–1368. doi: 10.1091/mbc.E15-02-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.