Summary
Effective delivery of viruses into required brain regions is critical to the success of optogenetic or chemogenetic experiments. However, in monkeys, due to the large size and heterogeneity of their brain, precise injections in deep brain regions have been challenging. Here, we present a protocol for virus injection in monkey deep brain regions under the guidance of MRI. We describe the steps for installing the guiding grid, MRI scanning, MRI-based localization, and virus injection.
For complete details on the use and execution of this protocol, please refer to Chen et al. (2023).1
Subject areas: Cognitive Neuroscience, Behavior
Graphical abstract

Highlights
-
•
Step-by-step guide to virus injection in macaque deep brain regions
-
•
MRI-guided localization technology brings accurate targeting
-
•
In vivo validation to demonstrate efficiency and accuracy
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
Effective delivery of viruses into required brain regions is critical to the success of optogenetic or chemogenetic experiments. However, in monkeys, due to the large size and heterogeneity of their brain, precise injections in deep brain regions have been challenging. Here, we present a protocol for virus injection in monkey deep brain regions under the guidance of MRI. We describe the steps for installing the guiding grid, MRI scanning, MRI-based localization, and virus injection.
Before you begin
Optogenetics and chemogenetics are widely used in neuroscience research using experimental animals like mice and rats.2,3,4 In recent years, more and more research groups studying nonhuman primates (NHP) are getting interested in using these advanced technologies.5,6,7 One key step of these technologies is to use viral vectors to deliver opsin or chemogenetical effectors to targeted brain regions. Here we provide the step-by-step protocol we developed for accurate virus injection in macaque monkeys, which we have validated in many experiments.8,9,10,11,12 Unlike mice, the brain of monkeys is much larger and heterogeneous. Therefore, it is normally unreliable to reach regions in the deep brain using coordinates obtained from a monkey atlas. To improve the injection precision, we first scan the experimental monkey using magnetic resonance imaging and obtain the detailed brain structure individually. The proposed protocol critically relies on MRI guiding. In particular, to translate the coordinates in the MRI images to real-world coordinates, we install a grid on the skull to work as fiducial markers to guide offline localization. Of note, although we only take the commonly used virus–Adeno-Associated Virus (AAV) as an example in the following, this protocol can also be expanded to injecting other viruses or liquids. Before you begin, the following steps should be checked or ready prior to the surgery.
Institutional permissions
All experimental animals were purchased in accordance with the standard procedure stipulated by the Regulations of Guangdong Province on the Administration of Experimental Animals. All experimental animals were housed in an AAALAC-accredited facility managed by the Nonhuman Primate Research Center at the Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences. The ambient temperature of the facility was 24 ± 1°C, and the relative humidity was 50% ± 5%. All animals received sufficient food, water, and professional veterinary care. All experimental procedures and the use of laboratory animals were reviewed and approved by the Institutional Animal Care and Use Committee at Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences (approved case ID: SIAT-IACUC-20230828-NS-NZNZX-DJ-DJ-A1387) following the guidelines stated in the Guide for Care and Use of Laboratory Animals (eighth edition, 2011).
Preoperative examination for experimental animals
Timing: 1–3 days
This section introduces the necessary examination and preparation for experimental monkeys before the surgery, which is crucial to the success of virus injection.
-
1.
Check the state of the subject monkeys.
The examination needs to be done 1–2 weeks before the surgery.-
a.Verify the general health and activity state of the subject monkeys including locomotor activity, reaction capacity, nutritional status, mental state, and diet state are suitable for the experiment.Note: A sick monkey or a monkey recovering from a previous operation should be excluded.
-
b.Check the monkey’s record to ensure the monkey does not have allergy to drugs or resistance to anesthetic.
-
c.Perform basic physiological test and biochemical examination (e.g., blood routine test) to obtain the animal’s body weight and information regarding blood sugar, anemia, leukocytes, etc.
-
d.Make sure no metal component in the monkey’s brain or body may influence the MRI scan.
-
a.
-
2.
AAV antibody test.
Some monkeys naturally express one or more AAV antibodies, which can lower the efficiency of virus expression if the same serotype is used as the viral vector. To ensure the successful expression of a specific serotype of AAV, we suggest performing an antibody test to confirm the subject monkey does not possess the antibody of the planned serotype.
Preparation before surgery operation
Timing: 1–2 days
-
3.Surgical instruments.
- a.
- b.
-
c.Check the operating table, shadowless lamp, physiological monitor, and respirator are working properly.
-
4.
Microinjection syringe.
Since the syringe cannot be effectively sterilized by a gas sterilization method due to the small size of the needle, we use the following procedures to sterilize the microinjection syringe.-
a.Prepare ∼500 mL disinfectant (e.g., 10% Bleach disinfectant).
-
b.Fill the syringe with disinfectant, and then soak the syringe plunger and syringe in the disinfectant for 30 min.
-
c.Flush the syringe including the needle and plunger with sterile water 3–5 times.
-
d.Flush the syringe with 75% ethanol for 3-5 times (ethanol is easier to volatilize).
-
e.Place the syringe inside a sterilized container with gauze on the bottom, and then put it in a dryer to dry (recommended temperature: 55°C).
-
a.
-
5.
Guiding grid.
We use a custom-made grid (Chinese patent: ZL202021548817.X) filled with contrast agent as a fiducial marker during the MRI scan and virus injection.8,9 One sample of the grid and grid holder we often use is illustrated in Figure 3.-
a.As shown in Figure 3, the grid has multiple holes (diameter 0.6 mm, spaced by 1 mm, depth 12 mm) that can be filled with contrast agents, which can be seen in the MRI images.
-
b.The grid mounts on a grid holder, which can be rigidly installed on the skull with ceramic screws and dental cement (see Part 1 of Step-by-step section). Both components are made of polyether-ether-ketone (PEEK) and might be generated using 3D printing.Note: The curvature at the bottom of the holder can be modified to best fit the skull.
-
a.
-
6.
Experimental animals.
Figure 1.
Examples of surgical instruments
(A) Basic surgical instruments that can be autoclaved.
(B) Common devices for gas sterilization.
Figure 2.
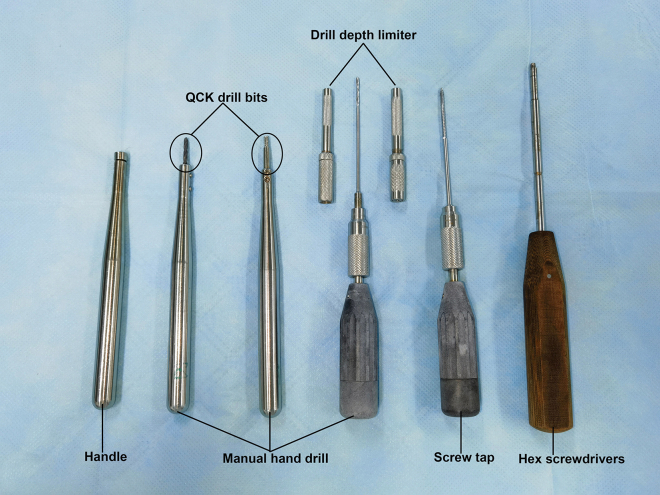
Two sets of hand drills for screw installation
Figure 3.
The picture and diagrams of the guiding grid
Check the health status of the experimental monkey. Fast the monkey for 8 h prior to the surgery.
-
7.
Operating room (OR).
Sterilize the OR with UV for 30 min.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Bacterial and virus strains | ||
| AAV2/9-mCaMKIIα-hM3Dq-mCherry | Shanghai Taitool Bioscience Co., Ltd. | Titer 1.86×1013 |
| AAV2/8-mCaMKIIα-hM3Dq-mCherry-WPRE | OBiO Technology (Shanghai) Corp., Ltd. | Titer 1.89×1013 |
| Chemicals, peptides, and recombinant proteins | ||
| Atropine | Controlled substance – contact animal facility veterinarian | N/A |
| Ketamine | Controlled substance – contact animal facility veterinarian | N/A |
| Isoflurane | Controlled substance – contact animal facility veterinarian | N/A |
| Zoletil | Controlled substance – contact animal facility veterinarian | N/A |
| Propofol | Controlled substance – contact animal facility veterinarian | N/A |
| Saline | Jiangxi Kelun Pharmaceutical Co., Ltd. | 250 mL/25 g |
| Glucose injection | Jiangxi Kelun Pharmaceutical Co., Ltd. | 100 mL/5 g |
| Bactroban | GSK China | 10 g |
| Humigel | Virbac | 10 g |
| Lincomycin hydrochloride and lidocaine hydrochloride gel | Jiangxi Decheng Pharmaceutical Co., Ltd. | 22 g |
| Denture base resin | Shanghai New Century Dental Materials | Type II, class I |
| Acrylic acid | Shanghai New Century Dental Materials | 500 mL |
| Disinfectant | Jiangsu Aitefu Group | 500 mL |
| Critical commercial assays | ||
| Stereotaxic frame | Kopf Instruments | Model 1430 |
| 3D manipulator | Kopf Instruments | Model 1460 |
| MRI-compatible stereotaxic frame | Kopf Instruments | Model 1430M |
| Experimental models: Organisms/strains | ||
| Rhesus macaque (Macaca mulatta) Cynomolgus monkeys (Macaca fascicularis) |
Institutional Animal Facility | Genotype: wild type Sex: male/female Age: >1 year |
| Software and algorithms | ||
| 3D Slicer | www.slicer.org | N/A |
| Other | ||
| Microinjection syringe pump | World Precision Instruments | UMP3T-1 |
| A Combined MRI and Histology Atlas of the Rhesus Monkey Brain in Stereotaxic Coordinates | Kadharbatcha Saleem, Nikos Logothetis | ISBN: 9780123850881 |
| Large animal stereotaxic instruments | RWD | 68921 |
| Inoculating instrument sterilizer | GAT | JZ-2 |
| Hex screwdrivers | Veterinary Orthopedic Implants (VOI) | T6-T25 |
| QCK drill bits | VOI | N/A |
| Drill depth limiter | VOI | N/A |
| Screw tap | VOI | N/A |
| Hand-held electrical cranial drill | Shenzhen Zhongshi Scientific Instrument Co., Ltd. | 50060 |
| Grid and holder | Dai Lab | Patent: ZL202021548817.X |
| Ceramic screw | Thomas RECORDING | SA06 |
| Pet clipper | Codos | CP-9200 |
| Microinjection needle | Hamilton | 701N |
| Regular surgical tools | N/A | N/A |
Step-by-step method details
Part 1: Grid installation
Timing: 1–2 h
This section introduces the procedure of grid installation. The grid is used as a fiducial marker to identify the injection target. The purpose of this step is to install the grid holder in the proper position of the skull.
-
1.Anesthetize the experimental monkey.
-
a.Administer the subject monkey with atropine (0.02–0.05 mL/kg) to decrease bronchial secretions before anesthetization.
-
b.Anesthetized by ketamine (10 mg/kg) or Zoletil (5 mg/kg), followed by isoflurane to maintain anesthesia
-
c.Keep monitoring the physiological indicators including SpO2, heart rate, respiration rate, blood pressure, and temperature during anesthesia.
-
a.
Note: This procedure is performed by veterinarians.
-
2.
Fix the monkey on the stereotaxic frame (Figure 4).
Note: It’s better to use ophthalmic ointment to protect the eyes from drying and abrasion.
CRITICAL: Ensure the head of the monkey is located in the middle of the stereotaxic frame by checking the scales of the ear bar on the left and right sides are equal.
-
3.Disinfect the head skin of the monkey and the stereotaxic frame.
-
a.Shave all hairs off the monkey’s head.
-
b.Use iodophor and ethanol (70% or 95%) alternately to scrub the head skin and the stereotaxic frame multiple times (>3) to ensure effective disinfection.
-
a.
-
4.
Clean your hands with a surgical scrub brush, and then put on a sterile surgical gown and surgical gloves.
-
5.
Place a sterile drape over the monkey and the operating table, and then have the required instruments ready in the proper position.
-
6.Install the 3D manipulator.
-
a.Install the 3D manipulator on one side of the stereotaxic frame according to the position of the target area.
-
b.Put a locating rod on the manipulator, which helps to read the coordinates by pointing the tip at a given place (Figure 4).
-
a.
-
7.
Identify the appropriate location of the injection site on the skull using the manipulator and the locating rod according to the coordinates of target injection site on 3D manipulator.
Note: Refer to a monkey atlas to get the appropriate coordinates.
-
8.
Identify the appropriate install position for the grid holder.
Figure 4.
The stereotaxic frame and the 3D manipulator
Adjust the angle and orientation of grid holder to find the position where the curvature fits the holder best; meanwhile, keep the grid on the appropriate location.
Note: It does not need to be accurate at this step, because the final position will be determined at step 10 after the skull is exposed.
-
9.
Expose the skull surface at the site for grid holder installation using scalpels.
-
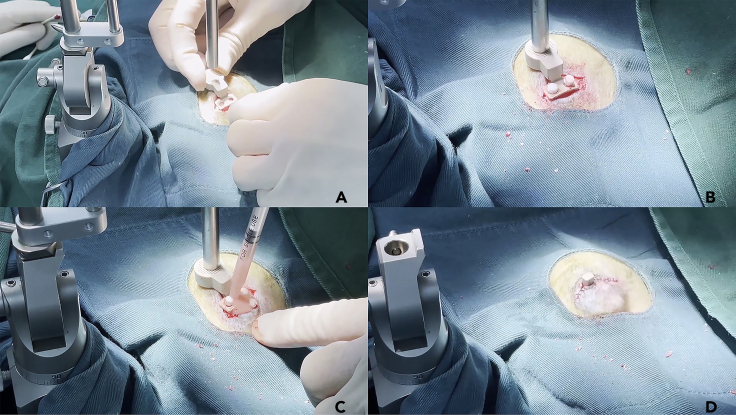
10.Place the grid holder (with the grid on) on the skull.
-
a.While ensuring the grid sits right above the target area, adjust the angle and orientation of the holder to find the best location for installation (Figure 5A).
-
b.Use the flat end of the locating rod to ensure the top surface of the grid is horizontal.
-
a.
-
11.
Secure the grid holder with ceramic screws (Figure 5B).
Figure 5.
Install the grid holder on the skull
(A) Localize the best location for installing the grid.
(B) Secure the grid holder with ceramic screws.
(C) Fix the grid holder and screws with dental cement.
(D) Remove the grid and sew up the skin.
Drill three holes using drills and taps of proper size and install the screws firmly.
Note: We normally use three screws to fix the grid holder. Two for the screw holes on the grid holder, and the third installed adjacent to it, working as an anchor to reinforce the holder after coating it with dental cement.
-
12.Fix the grid holder with dental cement (Figure 5C).
-
a.Adjust the tightness of the screws to make sure the grid is horizontal (with the assistance of the locating rod).
-
b.Use a syringe to deliver dental cement to cover all ceramic screws and the base of the grid holder.
-
a.
CRITICAL: Be cautious to keep the bottom of the grid free of cement.
-
13.
Remove the grid from the holder and sew up the scalp to cover the cement base, only exposing the top of the grid holder (Figure 5D).
Part 2: MRI scan
Timing: ∼1 h
Following the installation of the grid, the next step is to have the monkey undergo an MRI scan with the guiding grid, which aims to identify the precise target and trajectory for virus injection. We recommend performing the scan right after the grid installation on the same day.
-
14.
Prepare the experimental monkey.
The monkey can be transferred to the MRI scan right after the grid installation keeping anesthetization.
Note: In case extra recovery time is required, to prepare for an MRI scan, the monkeys must undergo the anesthetizing procedure again following the standard protocol.
-
15.
Fill the grid with a contrast agent.
The grid works as a fiducial marker in the MRI images. Therefore, a contrast agent must be filled in the holes in the grid to make them visible in the MRI images. Commonly used contrast agents under T1 structural images include Vitamin E, Vitamin D, and fish oil.-
a.Ensure the holes in the grid are not stuck.
-
b.Use a piece of tape to cover the bottom to prevent leaking of contrast agent.Note: Since the holes in the grid we use are interpenetrated, we use a piece of tape to cover the bottom of the grid to prevent the leaking of the contrast agent. One may also design a grid with a blocked bottom to hold fluid.
-
c.Use a syringe to inject the contrast agent into the holes. Alternatively, fill the contrast agent in glass tubes that fit the holes in advance, and then insert the tubes into the holes.Note: It is technically not required to fill in all the holes, but the holes at the fringe and the center of the grid should be filled.
-
a.
-
16.MRI scan.
-
a.Fix the monkey on the MRI-compatible stereotaxic frame, and then install the grid filled with contrast agent in advance on the pre-implanted holder.
-
b.Put on the head coil. Some types of head coil may touch the grid or may be unable to sit on the usual place and lose some quality of signal. Try to adjust the placement of the head coil to generate the best imaging quality.
-
c.Ensure the monkey is in good status for the MRI scan by checking the heart rate, SpO2, etc.
-
d.Start the scan using a sequence for brain structure. A 0.5 mm isotropic resolution T1-weighted imaging is recommended.
-
e.Check the status of the monkey immediately after the scan is completed.
-
a.
CRITICAL: Ensure that the monkey cannot make any movement during the scan. Otherwise, the scan quality may be affected. Usually, this should not be an issue if proper anesthesia procedures are applied. In case motion is detected, scan the animal again after adjusting anesthesia states.
Part 3: Pre-injection localization
Timing: 1–2 h
After obtaining the structural images, pre-operation localization must be performed before the injection. This step can be done any time between the MRI scan and the virus injection. A monkey atlas is strongly recommended while doing this planning. This step aims to identify the precise target and the optimal injection trajectory based on the MRI results.
-
17.Load the MRI images into a viewing software. We recommend using “3D slicer” as it is open-access and free of charge (www.slicer.org).
-
a.Adjust the image to ensure the grid can be seen clearly in all dimensions.
-
b.Align the coronal axis and the sagittal axis with the holes in the grid (Figure 6).
-
a.
-
18.Calculate the coordinates of the target injection site.
-
a.Use the coordinates in the atlas to locate the appropriate position of the target. Try to avoid blood vessels while planning injection paths.
-
b.After the exact location is identified, use a specific grid hole as a reference to calculate the coordinates of the target. For example, in Figure 6, the hole indicated by the crossline serves as the origin. If the target site is right below the origin, then the coordinates for x- and y-axes are (0,0). For a target 1 mm to the right of the origin, its coordinates are (1,0).
-
c.As to the depth, we recommend setting the point at the surface of the skull as zero when calculating the depth (Figure 6). These coordinates will then be used for virus injection.
-
a.
Note: The atlas we refer to is “A Combined MRI and Histology Atlas of the Rhesus Monkey Brain in Stereotaxic Coordinates,” which is a classic textbook in the field of primate neuroscience.13
Figure 6.
Offline identification of the coordinates of the target region
The horizontal coordinates can be obtained from the horizontal view of the grid (left), and the depth of the target can be obtained by measuring the distance between the target and the skull surface (right).
Part 4: Virus injection
Timing: 3–6 h
This section introduces the detailed process for virus injection. We normally allow the monkey to recover for 2–3 days after MRI scan.
-
19.
Anesthetize the experimental monkey following the procedure described above (Step 1).
-
20.
Unfrozen the virus by placing it in ice.
-
21.
Fix the monkey on the stereotaxic frame (see Step 2).
-
22.
Disinfect the scalp of the experimental monkey and the stereotaxic frame following the steps described in Step 3.
-
23.
Clean your hands with a surgical scrub brush, and then put on a sterile surgical gown and surgical gloves. Place a sterile drape over the monkey and the operating table, and then have the required instruments ready in the proper position.
-
24.
Install the 3D manipulator. Mount the locating rod on the manipulator with its flat end pointing downward.
-
25.
Install the grid on the grid holder. Check if the surface of the grid is horizontal using the flat end of the locating rod.
Note: Clean and sterilize the grid before the installation on the grid holder. The grid used in this step needs to be clean and sterilized without any contrast agent.
-
26.
Remove the grid and expose the area underneath.
-
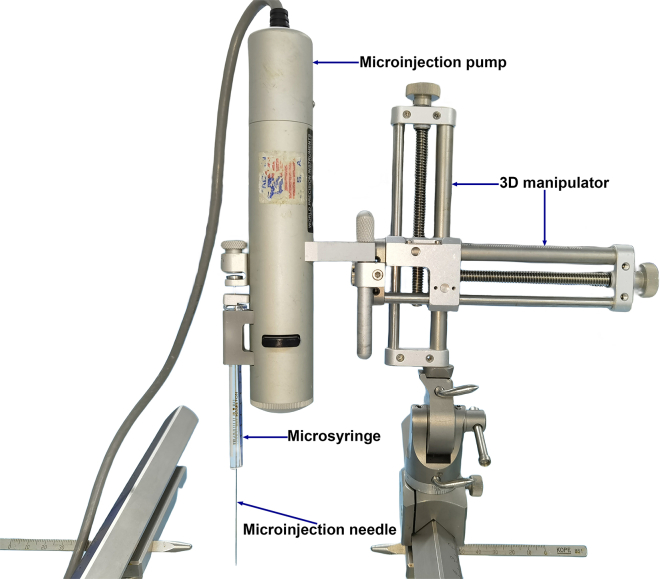
27.Assemble the microinjection pump (including the pump controller) and load the injection syringe (Figure 7). Locate the injection target using the grid-based coordinates.
-
a.Mount the injection pump on the 3D manipulator.
-
b.Install the syringe on the pump.
-
c.Adjust the 3D manipulator and use the needle tip of the syringe to locate the target position obtained from Part 3 (see also Figure 6). First, find the reference hole, and then move the needle to the desired coordinates (if the target position is not located at the reference hole).
-
d.Lower the needle until it touches the skull. Make a marker on the skull at that point.
-
a.
CRITICAL: Make sure the injection needle is not blocked if it touches blood.
-
28.Drill a hole at the marker point on the skull for the microinjection entry.
-
a.Remove the grid from the holder.
-
b.Use an electric drill to make one small hole through the skull at the marker point.
-
c.Verify the hole is drilled at the desired position by lowering the needle to penetrate through.
-
a.
CRITICAL: Please be careful when using the electric drill. Try your best not to damage the dura or brain tissue. It is recommended to punch the dura with an extra sharp needle.
-
29.
Use the withdraw mode of the microinjection pump to fill the microsyringe with the virus. The speed of withdrawal can be set at 1–4 μL/min depending on the size of the syringe and the total amount of virus needed.
CRITICAL: At the end of the process, ensure that the amount of virus in the microsyringe matches the anticipated quantity.
-
30.Start virus injection.
-
a.Lower the needle until the tip reaches the surface of the skull and set that point as the origin of the Z-axis. Keep lowering the needle until the tip reaches the target depth according to the coordinates obtained in Part 3.
-
b.After reaching the target position, keep the needle still and wait for 5–10 min to allow the tissue to rebound. While lowering the needle, the tissue may be deformed by pressure and may need extra time to recover.
-
c.Start to infuse virus. The infusion speed we normally use is 200–300 nL/min.
-
d.After the infusion is done, wait for another 5–10 min to allow virus diffusion before retracting the syringe.
-
e.Retract the needle or move to the next injection site. If multiple sites are required during one penetration, we normally start injecting from the deepest site.
-
a.
Note: It is necessary to wait for 5–10 min after the needle reaches the target position because the tissue may be deformed by pressure in the process of lowering the needle and may need extra time to recover.
-
31.
After finishing all injections, remove the grid holder from the skull after removing dental cement using an electric drill. We recommend using bone wax to fill the injection holes and screw holes in the skull.
-
32.
Clean the skull surface using diluted iodophor (10%) and saline before finally sewing up the scalp. Apply some lidocaine gel on the wound after the suture is recommended to relieve local pain.
Figure 7.
Assembled microinjection pump, microinjection syringe, and the 3D manipulator
Expected outcomes
After successful virus injection into the target region, gene expression should be expected. In mice, a histology experiment is typically needed to validate the efficiency of expression. In NHP, to reduce the usage of experimental animals, we might skip this step and jump to in vivo validation. For anatomical validation, a post-injection MRI scan is recommended. When a needle of ∼0.4 mm diameter is used to perform injection in deep brain regions, a needle track is commonly seen in the MRI images. Figure 8A shows an example of a needle tract that points directly to the target site and terminates right inside the target zone. In addition to imaging, in vivo recording is also a useful validation method. For optogenetic experiments, in vivo electrophysiological recording with optical stimulation is effective in verifying the expression of opsin.9,11,12 For chemogenetic experiments, electrophysiological recording in the target region in combination with ligand administration is also helpful to validate the DREADD expression.1 For example, neurons expressing excitatory receptors like HM3Dq should increase their firing rates following ligand (e.g., clozapine N-oxide (CNO)/deschloroclozapine (DCZ)) administration over a substantial period (Figure 8B). In addition, behavioral or physiological changes may be observed based on which areas are modulated.8,1
Figure 8.
Post-injection verification and in vivo recording
(A) A post-injection MRI scan verified the needle tract pointing at the target site, which is located right beneath the anterior commissure (labeled by a circle).
(B) A neuron recorded in the HM3Dq injection site showed an enhanced response after DCZ administration. The top row shows the spike traces, the blue line shows the firing rate, and the red dashed line indicates the DCZ administrate time.
Limitations
The localization of the injection target in this protocol strongly depends on the quality of the MRI images and the offline identification of the injection coordinates. For small regions in the deep brain, it may be difficult to identify the boundary of the target, especially if the clarity of the MRI images is low. On the other hand, since the diameter of the holes in the grid is only about 0.6 mm, it is required that the resolution of the MRI image is finer than 0.6 mm. There are cases in which not all filled holes can be clearly seen in the images, which may affect the re-orientation of the images and the readout of the coordinates. Sufficient experience with MRI image reading and familiarity with brain anatomy are helpful in breaking these limits.
Troubleshooting
Problem 1
The holes of the grid cannot be seen or can only be partially seen in the MRI images.
Potential solution
-
•
Check if sufficient contrast agent is injected into the desired holes.
-
•
Make sure the contrast agent does not leak away during the MRI scan.
-
•
Make sure the grid is placed within the head coil.
-
•
Make sure the resolution of the MRI scan is higher than the diameter of the holes.
Problem 2
-
•
The amount of injected virus is less than desired. This issue occurs when there is air inside the syringe, which causes the amount indicated by the pump controller to be inaccurate.
Potential solution
-
•
Try not to withdraw any air inside the syringe. Pre-fill the syringe with silicone oil is a solution to eliminate dead space inside the syringe.
-
•
If a small amount of air is unavoidable, read the volume of the virus from the syringe rather than from the pump controller.
-
•
Make sure a sufficient amount of virus has been delivered by double-checking the volume changes inside the syringe.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Ji Dai (ji.dai@siat.ac.cn).
Technical contact
The technical contact for this protocol is Ji Dai (ji.dai@siat.ac.cn).
Materials availability
This study did not generate new unique reagents.
Data and code availability
This study did not generate/analyze datasets.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (32371066), the Guangdong Basic and Applied Basic Research Foundation (2022A1515010134), the Youth Innovation Promotion Association of Chinese Academy of Sciences (2017120), the Shenzhen-Hong Kong Institute of Brain Science–Shenzhen Fundamental Research Institutions (NYKFKT2019009), and the Shenzhen Technological Research Center for Primate Translational Medicine (F-2021-Z99-504979).
Author contributions
Z.Z., P.Y., and Y.W. performed the protocols. P.Y. and J.D. wrote the manuscript. J.D. designed the experiments and supervised the study.
Declaration of interests
J.D. is a co-inventor of a Chinese patent, ZL202021548817.X, which claims the rights to the usage and manufacture of the grid system reported in this article.
References
- 1.Chen Y., Hong Z., Wang J., Liu K., Liu J., Lin J., Feng S., Zhang T., Shan L., Liu T., et al. Circuit-specific gene therapy reverses core symptoms in a primate Parkinson’s disease model. Cell. 2023;186:5394–5410.e18. doi: 10.1016/j.cell.2023.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Deisseroth K. Optogenetics: 10 years of microbial opsins in neuroscience. Nat. Neurosci. 2015;18:1213–1225. doi: 10.1038/nn.4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roth B.L. DREADDs for Neuroscientists. Neuron. 2016;89:683–694. doi: 10.1016/j.neuron.2016.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Y., Roy D.S., Zhu Y., Chen Y., Aida T., Hou Y., Shen C., Lea N.E., Schroeder M.E., Skaggs K.M., et al. Targeting thalamic circuits rescues motor and mood deficits in PD mice. Nature. 2022;607:321–329. doi: 10.1038/s41586-022-04806-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li L., Liu Z. Genetic Approaches for Neural Circuits Dissection in Non-human Primates. Neurosci. Bull. 2023;10 doi: 10.1007/s12264-023-01067-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raper J., Galvan A. Applications of chemogenetics in non-human primates. Curr. Opin. Pharmacol. 2022;64:102204. doi: 10.1016/j.coph.2022.102204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tremblay S., Acker L., Afraz A., Albaugh D.L., Amita H., Andrei A.R., Angelucci A., Aschner A., Balan P.F., Basso M.A., et al. An Open Resource for Non-human Primate Optogenetics. Neuron. 2020;108:1075–1090.e6. doi: 10.1016/j.neuron.2020.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Z., Shan L., Wang Y., Li W., Jiang M., Liang F., Feng S., Lu Z., Wang H., Dai J. Primate preoptic neurons drive hypothermia and cold defense. Innovation. 2023;4 doi: 10.1016/j.xinn.2022.100358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Y., Wang J., Liu J., Lin J., Lin Y., Nie J., Yue Q., Deng C., Qi X., Li Y., et al. A Novel Retrograde AAV Variant for Functional Manipulation of Cortical Projection Neurons in Mice and Monkeys. Neurosci. Bull. 2023 doi: 10.1007/s12264-023-01091-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deng C., Yuan H., Dai J. Behavioral Manipulation by Optogenetics in the Nonhuman Primate. Neuroscientist. 2018;24:526–539. doi: 10.1177/1073858417728459. [DOI] [PubMed] [Google Scholar]
- 11.Dai J., Ozden I., Brooks D.I., Wagner F., May T., Agha N.S., Brush B., Borton D., Nurmikko A.V., Sheinberg D.L. Modified toolbox for optogenetics in the nonhuman primate. Neurophotonics. 2015;2 doi: 10.1117/1.NPh.2.3.031202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dai J., Brooks D.I., Sheinberg D.L. Optogenetic and Electrical Microstimulation Systematically Bias Visuospatial Choice in Primates. Curr. Biol. 2014;24:63–69. doi: 10.1016/j.cub.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 13.Saleem K., Logothetis N. 2nd Edition. Academic Press; 2012. A Combined MRI and Histology Atlas of the Rhesus Monkey Brain in Stereotaxic Coordinates. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This study did not generate/analyze datasets.