Abstract
Background and Objectives:
Group A Rotavirus (RVA) is the most important causative agent of acute diarrheal disease in pediatrics 5 years and below. This study aimed to determine the distribution of circulating RVA in Mashhad, Iran to develop health improvement strategies and vaccine decision making.
Materials and Methods:
A total of 106 fecal specimens were collected from children admitted to Akbar and Dr. Sheikh referral pediatric hospitals of Mashhad City during the December 2020 to March 2021 and December 2021 to March 2022. All specimens were tested for specific bacterial, parasitic, and amoebic infections. Negative samples were analyzed for RVA infections using the RT-PCR method.
Results:
RVA was detected in 31.3% of the specimens, indicating no statistical significance in gender distribution or between fall and winter positivity rates. The number of RVA–positive specimens increased following age increasing in the range of 1 to 60 months.
Conclusion:
Today, acute diarrheal disease (ADD) is still caused mostly by Rotavirus infections in pediatrics in Mashhad. Comprehensive studies are needed to determine the genetic diversity of circulating Rotavirus strains in this era.
Keywords: Rotavirus, Children, Prevalence, Epidemiology, Diarrhea
INTRODUCTION
One of the important causes of acute diarrheal disease (ADD) is Rotavirus with its major appearance in watery or loose stools. Acute gastroenteritis is the most common disease all over the world and the main cause of death among children aged under five years old. Rotaviruses affect primarily children living in low- and middle-income countries with higher incidence rates due to poor quality of drinking water, contaminated food and water sources, and malnutrition risk factors (1, 2).
Worldwide published data on total child mortality are reported yearly, and the estimated number of deaths due to ADD in children aged under 5 years was about 525000. The Integrated Global Action Plan for the Prevention and Control of Pneumonia and Diarrhea (GAPPD), by WHO/UNICEF, goes to the heart of the challenge by recognizing that the only way to combat these two preventable diseases is not to address them separately, but to fight them together in an integrated approach. The goal is to see a drop in deaths from pneumonia to fewer than 3 children in 1000 live births, and from diarrhea to less than 1 in 1000 by 2025. This goal can be achieved by appealing to a wide range of activities through national decision-maker hard work (3). Gastroenteritis in children is caused by a broad range of viral infections, and ADD is more often associated with Rotaviruses, which is recognized as the main cause of morbidity and mortality from diarrhea (4). Rotaviruses, members of the Reoviridae family, have a double-stranded segmented RNA genome that encodes six structural and six non-structural proteins that are surrounded by a triple-layered capsid (5, 6). Based on VP6 (the major intermediate layer protein), these viruses have been identified in seven serogroups named A–J (7). Rotaviruses, specifically group A, are the etiological pathogens and the major cause of severe diarrhea worldwide among infants and children, however, infection with some strains of group B and group C of these viruses may also result in diarrhea (8). Also, gene rearrangement in RNA viruses with the segmented forms of the genome like Rotaviruses results in more severe conditions following virus evolution (9). Rotavirus gastroenteritis (RVGE) results in vomiting, fever, dehydration, imbalanced electrolytes, and finally, high rates of mortality and morbidity especially, in developing countries (10). VP4 and VP7 are two outer capsid proteins used to classify Rotaviruses into G types and P types respectively (11). The fecal-oral route, particularly contaminated drinking water and food, is the main transmission way of this viral infection (4). Also, it should be noted that in the cold seasons of the year (fall and winter), RVGE is more prevalent (12). In this study, among many other detection procedures, RT-PCR was selected, as it was available and had acceptable sensitivity. Today, there is little epidemiological data about this viral infection in different geographical regions of Iran. Therefore, the present study aims to present an overview of the incidence and clinical aspects of Rotavirus ADD in a selected population in Mashhad, Iran. It can provide updated information on Rotaviruses prevalence, behind a little other study. Many other studies in the epidemiology of gastroenteritis by Rotaviruses are needed to investigate the genetic diversity of circulating strains in this era. Hence in the present research, we attempted to determine the distribution of Rotaviruses in children under 5 years old in Mashhad, admitted to Dr. Sheikh and Akbar Pediatric Hospital of Mashhad, Iran, with RVGE. Due to the critical situation of Mashhad in terms of access to underground water sources with sewage overflows, it is better to periodically conduct epidemiological studies to investigate Rotavirus infections in this city. Also, as a result of a large number of Afghan immigrants in this city because of the proximity of Mashhad to Afghanistan (a country with a low level of hygiene and a high prevalence of gastrointestinal disorders), the current study was conducted to attract the attention of community health systems and decision-makers regarding the use of Rotavirus vaccines. Thereupon, Iran is a vast country with different climates in each region and the relative frequency (RF) of Rotavirus infections is extremely divergent in different parts of Iran (13). Therefore, different studies should be done for being updated about the distribution of Rotaviruses in each geographical area as the Rotaviruses are the main cause of viral gastroenteritis.
MATERIALS AND METHODS
For the two-year surveillance, 106 feces samples were collected from children under 5 years old who were confirmed with gastroenteritis symptoms including abdominal pain, diarrhea, and vomiting, and referred to the Dr. Sheikh and Akbar Pediatric Hospital of Mashhad City (Khorasan Razavi, Iran) from December 2020 to March 2021 and December 2021 to March 2022. It should be noted that the samples used in this study were collected from hospitals where it was possible to admit the general public and a specific group of people with a specific socio-economic level was not targeted.
Because of the prevalence of Rotavirus infection in cold seasons, all stool samples for this study were collected in fall and winter. All suspected specimens that were negative for parasite and amoeba infections were selected and included in our experiment. Also, the samples were examined for the presence of white blood cells, Salmonella spp, and Shigella spp. Next, samples with the specific bacterial infection were excluded. Ultimately, to do Rotavirus detection, all of the specimens were stored at −70°C.
Nucleic acid extraction and cDNA preparation.
To extract the RNA from fecal specimens, RNX-Plus Sinacolon Kit (CinnaGen Co., Tehran, Iran) was used. Following RNA extraction, cDNA was synthesized using Parstous Easy-TM cDNA synthesis kit in accordance with the instruction of the producing company.
RT-PCR for VP6 gene.
Following cDNA synthesis, RT-PCR was done for the detection of Rotaviruses group A using forward and reverse primers that were designed for the VP6 gene of the Rotaviruses group A (Table 1). To design the primers, 800 sequences were derived from National Center for Biotechnology Information (NCBI) and aligned using the Clustal method. A good coverage of group A Rotaviruses has been considered in these sequences. Primer design was conducted using Mega software for maximum conserved sites.
Table 1.
Sequences of forward and reverse primers used in this study for Rotavirus VP6 segment of genome detection
| Oligonucleotide | Sequences | Length of the products |
|---|---|---|
| VP6 forward primer | TGTACTCCTTRTCAAARACTCT | 22 |
| VP6 reverse primer | CCATTCATRGTAAYTATCATTTG | 23 |
| Target | CCTTCRACAATRTTGTCTCTAGCAT | 118 |
For the RT-PCR reaction, 10 μl of master mix, 0.5 μl of each primer, 2 μl of template, and 7 μl of PCR-grade water were used. The PCR was performed using GeneAtlas, ASTEC CO., LTD (G02, Japan) for 30 cycles.
RT-PCR was performed using cycling conditions consisting of 94°C for 5 min; 30 cycles at 94°C for 45 s, 53°C for 40 s, 72°C for 45 s, and a final elongation at 72°C for 8 min. The PCR product was expected to be 118 bp. Henceforward, a 1.5% agarose gel, and green viewer were subjected to electrophoresis to observe the RT-PCR product under ultraviolet radiation.
Statistical analysis.
Statistical analysis was performed using graphpad prism version 8. Chi-square (χ2) test was used to analyze the differences between the groups according to the variables. Statistically, significance was considered at p values ≤0.05. Logistic regression was used to determine the nominal variable, odds ratio, and sensitivity.
Ethical consideration.
This project (Registration: IR.MODARES.REC.1400.018) was approved by the ethics committee of Tarbiat Modares University (Tehran, Iran). All collected stool samples were disposable.
RESULTS
Fecal samples were collected from 106 patients with acute gastroenteritis (AGE) aged below 60 and the results showed positivity of Rotavirus infection in 31.3% (33/106) of the specimens. As demonstrated in Table 2, all of the Rotavirus-infected samples were collected during the cold months of the year (December to March) of 2020 (12 positive samples or 36.3%) and 2021 (15 positive samples or 45.4%). Fisher’s exact test was conducted to analyze the incidence of Rotavirus infection with regard to seasonality. The data represent that there is no significant difference in the distribution of Rotavirus infections in fall and winter with P value >0.9999 and confidence level (CL)= 95%. As well, the relative risk was 1.01 which indicates that there is no difference in the infection rate between fall and winter.
Table 2.
Demographic information of the patients
| Characteristics | 33 patients Positive for Rotavirus infection | 73 patients negative for Rotavirus infection | P value | |
|---|---|---|---|---|
| Sex | Male | 18 | 40 | >0.9999 |
| Female | 15 | 33 | ||
| Age | 1–36 Month | 7 | 17 | >0.9999 |
| 37–60 Month | 26 | 56 | ||
| Season of hospitalization | Fall | 6 | 13 | >0.9999 |
| Winter | 27 | 60 | ||
Among 106 patient’s collected specimens, 58 samples were from males (54.7%) and 48 samples were from females (45.2%) (Table 2).
The Rotavirus-positive rate in pediatrics with respect to gender was assessed by the Chi-square test. Fig. 1 shows that the results were not statistically considered meaningful.
Fig. 1.
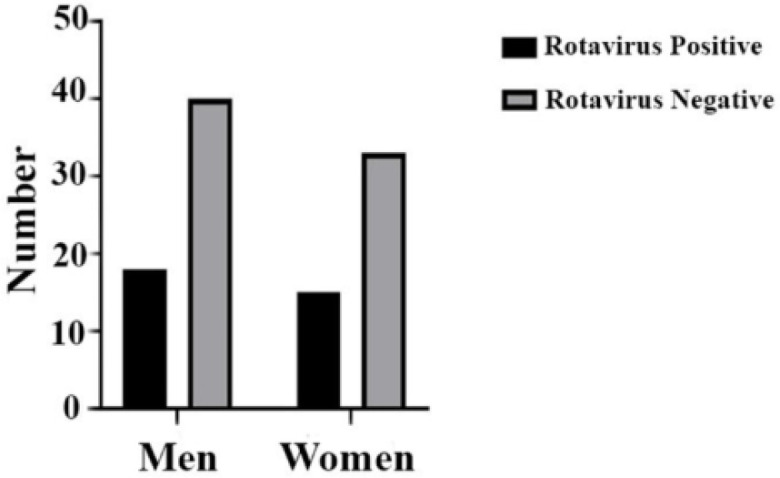
Chi-square analysis for prevalence of Rotavirus postive samples in pediatrics based on gender with P value >0.9999.
Table 2 shows that among 33 infected specimens, 7 positive samples were related to the age of 1 to 36 months (21.2%) and 26 samples were related to the age of 37 to 60 months (78.7%). Fisher analysis with P value >0.9999 and relative risk of 0.009 represents that in the mentioned age groups in Table 2, there is no significant difference in Rotavirus prevalence.
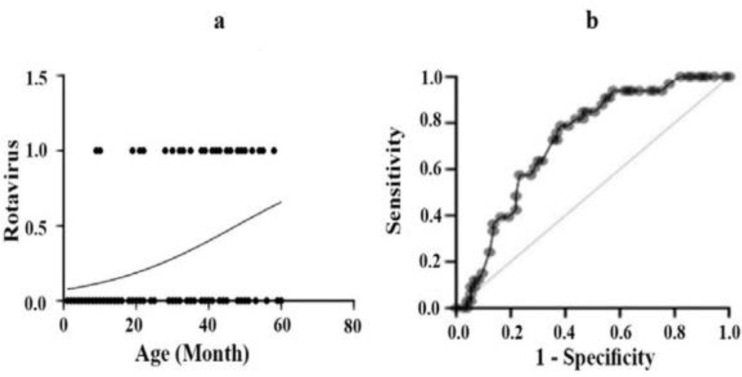
To perform a statistically significant evaluation for age factor in Rotavirus infection among pediatrics under 3 years of age, logistic regression analysis was conducted. Based on the results, in this age range, there is a strong relation between the rate of Rotavirus infection and age increase (Fig. 2a).
Fig. 2.
(a) Logistic regression analysis for distribution of age risk factor for Rotavirus positive samples in pediatric patients in first 3 years of life with P value= 0.0001 (b) Proportion of Rotavirus positive samples in pediatric patients according to age based on ROC curve P value= 0.0002.
The receiver operating characteristic (ROC) curve was also carried out to analyze fecal specimens. Based on the result, with a probability of 72% and P value=0.0002, by age increase, positive samples for Rotavirus infections increase (Fig. 2b).
Furthermore, to present the difference in the prevalence of Rotavirus infection between the age groups shown in Table 2, Fisher analysis was performed. No significant difference was demonstrated between the age groups with a P value >0.9999 and a relative risk of 0.009. As well, the relative risk was 0.999 which indicates that there is no remarkable difference in the infection rate between fall and winter.
DISCUSSION
Rotaviruses are known as the main cause of ADD with severe diarrhea in infants and young children under five years of age. Approximately 125000 - 200000 children die annually due to this viral infection (14), and most of these deaths (more than 85%) occur in low-income countries of Africa and Asia (15). There is a rare frequency of infections with Rotaviruses in adults due to their previous infections which resulted in their relative immunity (16).
Prevalence investigations of Rotaviruse infections in different geographical areas are happening globally. Therefore, it is possible to evaluate the environmental health situation in each region based on these surveys. This evaluation helps to improve the patient’s clinical management, demonstrates the necessity of including Rotavirus vaccines in comprehensive vaccination programs in different communities (17), and is essential for proactive infection control as well. The surveillance of common strains must also be determined to start an effective vaccination program (13). Two main Rotavirus vaccines approved for infants in the United States, RotaTeq® and Rotarix®, are used in different countries. These vaccines have shown significant efficacy in protecting against Rotavirus diarrhea and reducing mortality among children (18). Also, researchers endeavor to develop new efficient Rotavirus vaccines (19).
Based on the estimations in Iran, among hospitalized children under 5 years of age due to AGE, 40.04% were positive for Rotavirus infections. It should be noted that epidemiologic studies for the prevalence of this infection in the Eastern Mediterranean region were similar to Iran which was about 42.77% in 2015–2016 (20). Although over 40% of detection rates showed the necessity of incorporating Rotavirus vaccines in an expanded program on immunization, the authorities has not launched these vaccines into vaccination programs for Iranian children to date (21–23). The prevalence of Rotaviruses in Iran has been estimated at about 36.5% based on 19 different studies, which is similar to the epidemiological pattern of this viral infection in Mediterranean countries (24). As well, based on a meta-analysis done in 2017, Rotavirus infection has been detected in 50% of cities in Iran and the distribution of this viral infection is estimated at about 39.9% (13). Providing precise data about Rotavirus prevalence may be beneficial for proposing proper policies for Rotavirus vaccination in Iran.
In our study, Rotavirus was detected in 33 of 106 (31.3%) fecal samples which were collected from Dr. Sheikh and Akbar Pediatric Hospital of Mashhad, Iran. RT-PCR method was used to detect this viral infection in collected clinical stool specimens since this method was available, has acceptable sensitivity in detecting Rotaviruses (25) and can distinguish this infection from several other viral infections resulting in AGE including Noroviruses, enteric Adenoviruses serotypes 40 and 41, Human Parechoviruses, Picobirnaviruses, Astroviruses, and infrequently, Coronaviruses, and Toroviruses (26, 27). In 2010, another study was done to investigate the prevalence of Rotavirus infection in Mashhad by Sadeghian et al. Based on the results, the incidence of this viral infection among children suffering from AGE was 28.8% (28). The increase in positive Rotavirus samples in our study could be due to the lack of timely referral to the hospital for diagnosis and treatment during the COVID pandemic, and as a result, more shedding of the virus. Also, using more advanced techniques with high sensitivity and selectivity for Rotavirus detection and expert technicians in clinical laboratories to collect appropriate samples and perform the tests could be other reasons for an increase in positive cases in our investigation (24). In other epidemiological studies of Rotavirus infection in Ahwaz and Shiraz, the prevalence of the infection has been reported at 32% and 19.4% respectively (4, 29). This variation in number of the Rotavirus positive samples may be the result of differences in regions and populations studied in different time periods and also, the dissimilarity in number of the examined specimens (24). Based on our research results, there is a direct relationship between increasing age and the risk of Rotavirus infections till 60 months of age. This increase in the number of infected infants can be due to their use of milk bottles instead of breast milk. This result could be due to the protective effect of the IgA which is transferred to the infant through breast milk. The crucial protective effect of this antibody has been established in a study on Rotavirus infection in mice (30). Also, the consumption of contaminated materials, food, and water sources by children after infancy, when the child’s source of food is something other than the mother’s milk is another reason for our observations. Also, children try to put everything in their mouth to identify subjects and the surrounding environment at this age and this could be another source of Rotavirus infection (31). Furthermore, as the age of children increases, the probability of their presence in communities such as kindergartens increases, which could be followed by more transmission of Rotavirus infection among children. Of course, there are some other studies that show that with age increasing, the risk of Rotavirus infections in children decreases, which could be due to the development of anti-Rotavirus antibodies (32–35). Another statistical analysis indicates that there was no significant association between gender and the possibility of Rotavirus infections. This is similar to the results of another study done by Aminu et al. that shows an insignificant divergence in the probability of Rotavirus infection among males and females (36). Most of the ADDs attributed to Rotavirus infections occur in cold seasons of the year, with a peak in January (31). According to this knowledge, samples for this study were collected in December, January, February, and March. The peak season for the positivity of Rotavirus infection in fecal samples was February 2020 and 2021. Based on the results obtained from this study, no meaningful variation was observed between fall and winter in the number of Rotavirus-infected fecal samples. Among the limitations of this study we can point out the lack of access to a larger number of samples in other hospitals, which was due to limited budget and time. Also, the present study only shows the patients who have been referred to specialized pediatric hospitals in Mashhad with severe symptoms of gastroenteritis. In many cases, gastroenteritis is treated with home treatments and the patient does not go to medical centers to check the type of microbial agent causing the disease. As a result, accurate statistics of these patients are not available. The prevalence of Rotavirus infections among children over 5 years old, especially in the cold seasons of the year, is also prevalent, which was not possible to investigate in this study.
The challenge of increasing Rotavirus infection needs further investigations pre-vaccine period. In addition, monitoring different genotypes of this viral infection can give a better view of their epidemiological distribution and helps in making better decisions for community health and vaccination program. Besides, more research with a larger number of specimens from various geographical regions of Iran is needed to force the policymakers to include Rotavirus vaccination in national vaccine programs.
Hence, this study was conducted to update the epidemiological investigations of Rotavirus infections in children under 5 years of age in Mashhad, Iran to recommend appropriate policies in this context.
ACKNOWLEDGEMENTS
The authors thank Faculty of Medical Sciences, Tarbiat Modares University of Tehran and the deputy of research, Mashhad University of Medical Science for their kind assistance and support.
REFERENCES
- 1.Black RE, Cousens S, Johnson HL, Lawn JE, Rudan I, Bassani DG, et al. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet 2010; 375: 1969–1987. [DOI] [PubMed] [Google Scholar]
- 2.Estes MK, Kapikian A. (2007). Rotaviruses. In: Fields Virology. Knipe D, Griffin D, Lamb R, Martin M, Roizman B, Straus S, Eds. Wolters Kluwer Health; Lippincott Williams and Wilkins, Philadelphia, PA, USA, pp. 1917–1974. [Google Scholar]
- 3.Organization WH. Ending preventable deaths from pneumonia and diarrhoea by 2025: WHO; 2013. [Available from: https://www.who.int/news/item/10-04-2013-ending-preventable-deaths-from-pneumonia-and-diarrhoea-by-2025. [Google Scholar]
- 4.Azaran A, Makvandi M, Teimoori A, Ebrahimi S, Heydari F, Nikfar R. Distribution of rotavirus genotypes circulating in Ahvaz, Iran in 2016. Iran Biomed J 2018; 22: 107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greenberg HB, Estes MK. Rotaviruses: from pathogenesis to vaccination. Gastroenterology 2009; 136: 1939–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asensio-Cob D, Rodríguez JM, Luque D. Rotavirus particle disassembly and assembly in vivo and in vitro. Viruses 2023; 15: 1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johne R, Schilling-Loeffler K, Ulrich RG, Tausch SH. Whole genome sequence analysis of a prototype strain of the novel putative rotavirus species L. Viruses 2022; 14: 462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dung TT, Phat VV, Nga TV, My PV, Duy PT, Campbell JI, et al. The validation and utility of a quantitative one-step multiplex RT real-time PCR targeting rotavirus A and norovirus. J Virol Methods 2013; 187: 138–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahmadi E, Soleimanjahi H, Sadeghizadeh M, Teimoori A. Development of poly (A)-tailed universal reverse transcription PCR method for sequence-independent amplification of rearranged rotavirus. Arch Iran Med 2016; 19: 625–630. [PubMed] [Google Scholar]
- 10.Crawford SE, Ramani S, Tate JE, Parashar UD, Svensson L, Hagbom M, et al. Rotavirus infection. Nat Rev Dis Primers 2017; 3: 17083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kudesia G, Wreghitt T. Clinical and diagnostic virology: Cambridge university press; 2009. [Google Scholar]
- 12.van Gaalen RD, van de Kassteele J, Hahné SJM, Bruijning-Verhagen P, Wallinga J. Determinants of rotavirus transmission: a lag nonlinear time Series analysis. Epidemiology 2017; 28: 503–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monavari SHR, Hadifar S, Mostafaei S, Miri A, Keshavarz M, Babaei F, et al. Epidemiology of rotavirus in the Iranian children: A systematic review and meta-analysis. J Glob Infect Dis 2017; 9: 66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Troeger C, Khalil IA, Rao PC, Cao S, Blacker BF, Ahmed T, et al. Rotavirus vaccination and the global burden of rotavirus diarrhea among children younger than 5 years. JAMA Pediatr 2018; 172: 958–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention (CDC). Rotavirus surveillance--worldwide, 2001–2008. MMWR Morb Mortal Wkly Rep 2008; 57: 1255–1257. [PubMed] [Google Scholar]
- 16.Niendorf S, Ebner W, Marques AM, Bierbaum S, Babikir R, Huzly D, et al. Rotavirus outbreak among adults in a university hospital in Germany. J Clin Virol 2020; 129: 104532. [DOI] [PubMed] [Google Scholar]
- 17.Azaran A, Makvandi M, Samarbafzadeh A, Neisi N, Hoseinzadeh M, Rasti M, et al. Study on Rotavirus infection and its genotyping in children below 5 years in south west iran. Iran J Pediatr 2016; 26(2): e2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teimoori A, Soleimanjahi H, Makvandi M. Characterization and transferring of human rotavirus double-layered particles in MA104 cells. Jundishapur J Microbiol 2014; 7(6): e10375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jalilian S, Teimoori A, Makvandi M. In silico characterization of epitopes from human rotavirus VP7 genotype G9 design for vaccine development. Iran J Allergy Asthma Immunol 2019; 18: 664–670. [DOI] [PubMed] [Google Scholar]
- 20.Jalilvand S, Roohvand F, Arashkia A, Shoja Z. Update on epidemiology and circulating genotypes of rotavirus in Iranian children with severe diarrhea: 1986–2015. Int J Travel Med Glob Health 2018; 6: 7–10. [Google Scholar]
- 21.Mousavi Jarrahi Y, Zahraei SM, Sadigh N, Esmaeelpoor Langeroudy K, Khodadost M, Ranjbaran M, et al. The cost effectiveness of rotavirus vaccination in Iran. Hum Vaccin Immunother 2016; 12: 794–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Javanbakht M, Moradi-Lakeh M, Yaghoubi M, Esteghamati A, Ghanaie RM, Mahmoudi S, et al. Cost-effectiveness analysis of the introduction of rotavirus vaccine in Iran. Vaccine 2015; 33 Suppl 1: A192–200. [DOI] [PubMed] [Google Scholar]
- 23.Shakerian S, Moradi Lakeh M, Esteghamati A, Zahraei M, Yaghoubi M. Cost-effectiveness of rotavirus vaccination for under-five children in Iran. Iran J Pediatr 2015; 25(4): e2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shoja Z, Jalilvand S, Mokhtari-Azad T, Nategh R. Epidemiology of cocirculating human rotaviruses in Iran. Pediatr Infect Dis J 2013; 32(4): e178–181. [DOI] [PubMed] [Google Scholar]
- 25.El-Ageery SM, Ali R, Abou El-Khier NT, Rakha SA, Zeid MS. Comparison of enzyme immunoassay, latex agglutination and polyacrylamide gel electrophoresis for diagnosis of rotavirus in children. Egypt J Basic Appl Sci 2020; 7: 47–52. [Google Scholar]
- 26.Xu C, Fu J, Zhu Y. A narrative review of norovirus gastroenteritis: more global attention is needed. Int J Travel Med Glob Health 2016; 4: 101–106. [Google Scholar]
- 27.Tan EM, Cawcutt KA, Zomok CD, Go RS, Sia IG. Activity of nitazoxanide against viral gastroenteritis: a systematic review. Int J Travel Med Glob Health 2017; 5: 107–112. [Google Scholar]
- 28.Sadeghian A, Hamedi A, Sadeghian M, Sadeghian H. Incidence of rotavirus diarrhea in children under 6 years referred to the Pediatric Emergency and Clinic of Ghaem Hospital, Mashhad, Iran. Acta Med Iran 2010; 48: 263–265. [PubMed] [Google Scholar]
- 29.Phan TG, Khamrin P, Quang TD, Dey SK, Takanashi S, Okitsu S, et al. Detection and genetic characterization of group A Rotavirus strains circulating among children with acute gastroenteritis in Japan. J Virol 2007; 81: 4645–4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blutt SE, Miller AD, Salmon SL, Metzger DW, Conner ME. IgA is important for clearance and critical for protection from rotavirus infection. Mucosal Immunol 2012; 5: 712–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ojobor CD, Olovo CV, Onah LO, Ike AC. Prevalence and associated factors to rotavirus infection in children less than 5 years in Enugu State, Nigeria. Virusdisease 2020; 31: 316–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bonkoungou IJ, Sanou I, Bon F, Benon B, Coulibaly SO, Haukka K, et al. Epidemiology of Rotavirus infection among young children with acute diarrhoea in Burkina Faso. BMC Pediatr 2010; 10: 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cardoso Dd, Soares CM, Dias e Souza MB, de Azevedo Mda S, Martins RM, Queiróz DA, et al. Epidemiological features of Rotavirus infection in Goiânia, Goiás, Brazil, from 1986 to 2000. Mem Inst Oswaldo Cruz 2003; 98: 25–29. [DOI] [PubMed] [Google Scholar]
- 34.Ndze VN, Akum AE, Kamga GH, Enjema LE, Esona MD, Banyai K, et al. Epidemiology of rotavirus diarrhea in children under 5 years in Northern Cameroon. Pan Afr Med J 2012; 11: 73. [PMC free article] [PubMed] [Google Scholar]
- 35.Tagbo B, Chukwubike C, Mwenda J, Seheri M, Armah G, Mphahlele J, et al. Molecular characterization of rotavirus strains circulating in Enugu Nigeria: 2011 to 2016. World J Vaccines 2019; 9: 22–36. [Google Scholar]
- 36.Aminu M, Esona MD, Geyer A, Steele AD. Epidemiology of rotavirus and astrovirus infections in children in Northwestern Nigeria. Ann Afr Med 2008; 7: 168–174. [DOI] [PubMed] [Google Scholar]