Abstract
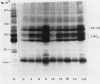
The photosynthetic performance of synchronously grown Chlamydomonas reinhardtii alternated rhythmically during the cell cycle. The activity of the “CO2 concentrating mechanism” including the ability to accumulate CO2 internally and the activity of carbonic anhydrase peaked after 6 to 9 hours of light and reached minimum after 6 to 9 hours of dark. Consequently, the apparent photosynthetic affinity to extracellular CO2 alternated rhythmically. At the end of the dark period the cells behaved as if they were adapted to high CO2 even though they were continuously aerated with air. Results from experiments in which the light or dark periods were extended bear on the interaction between the internal (cell cycle or biological clock) and the external (light) signal. The observed rhythmical alterations in photosynthetic Vmax may result from changes in PSII activity. The latter may be partly explained by the capacity for phosphorylation of thylakoid proteins, which reached maximum after 9 hours of light and decreased toward the dark period.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badger M. R., Kaplan A., Berry J. A. Internal Inorganic Carbon Pool of Chlamydomonas reinhardtii: EVIDENCE FOR A CARBON DIOXIDE-CONCENTRATING MECHANISM. Plant Physiol. 1980 Sep;66(3):407–413. doi: 10.1104/pp.66.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett J., Steinback K. E., Arntzen C. J. Chloroplast phosphoproteins: regulation of excitation energy transfer by phosphorylation of thylakoid membrane polypeptides. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5253–5257. doi: 10.1073/pnas.77.9.5253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyle D. J., Ohad I., Arntzen C. J. Membrane protein damage and repair: Selective loss of a quinone-protein function in chloroplast membranes. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4070–4074. doi: 10.1073/pnas.81.13.4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus Y., Harel E., Kaplan A. Adaptation of the Cyanobacterium Anabaena variabilis to Low CO(2) Concentration in Their Environment. Plant Physiol. 1983 Jan;71(1):208–210. doi: 10.1104/pp.71.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus Y., Zenvirth D., Harel E., Kaplan A. Induction of HCO(3) Transporting Capability and High Photosynthetic Affinity to Inorganic Carbon by Low Concentration of CO(2) in Anabaena variabilis. Plant Physiol. 1982 May;69(5):1008–1012. doi: 10.1104/pp.69.5.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohad I., Siekevitz P., Palade G. E. Biogenesis of chloroplast membranes. I. Plastid dedifferentiation in a dark-grown algal mutant (Chlamydomonas reinhardi). J Cell Biol. 1967 Dec;35(3):521–552. doi: 10.1083/jcb.35.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens G. C., Ohad I. Phosphorylation of chlamydomonas reinhardi chloroplast membrane proteins in vivo and in vitro. J Cell Biol. 1982 Jun;93(3):712–718. doi: 10.1083/jcb.93.3.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson G., Sweeney B. M., Matlick H. A., Prézelin B. B. Changes in Photosystem II Account for the Circadian Rhythm in Photosynthesis in Gonyaulax polyedra. Plant Physiol. 1983 Oct;73(2):329–331. doi: 10.1104/pp.73.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich J. L., Sager R. Regulation of the Chlamydomonas cell cycle by light and dark. J Cell Biol. 1980 Apr;85(1):136–145. doi: 10.1083/jcb.85.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staehelin L. A., Arntzen C. J. Regulation of chloroplast membrane function: protein phosphorylation changes the spatial organization of membrane components. J Cell Biol. 1983 Nov;97(5 Pt 1):1327–1337. doi: 10.1083/jcb.97.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]