Abstract
Background
The studies evaluating patients’ characteristics and lipid-lowering therapy for patients with homozygous familial hypercholesterolemia (HoFH) are scarce.
Objectives
This study aims to evaluate the characteristics of and treatments for patients with HoFH.
Methods
This study included 201 patients who were diagnosed with definite or probable HoFH from the National Database of the Japanese Ministry of Health, Labour, and Welfare.
Results
The patients’ median age at diagnosis was 27 years and exhibited a bimodal distribution. Approximately 70% of patients had coronary artery disease. Regarding genetic backgrounds, mutations in the low-density lipoprotein (LDL) receptor (LDLR) were identified in most of the patients, followed by proprotein convertase subtilisin/kexin type 9 (PCSK9) and double heterozygotes of LDLR. High-intensity statins were introduced to 74% of the patients, lipoprotein apheresis was performed in 21%, and PCSK9 inhibitors were administered to 50%. The mean of LDL cholesterol before and after treatment were 10.1 mmol/L and 3.9 mmol/L, respectively. Patients with coronary artery disease had significantly decreased LDL cholesterol. A quarter of the patients (n = 49, 24%) exhibited valvular diseases, particularly aortic valvular disease (n = 34, 61%).
Conclusions
The national epidemiological study of patients with HoFH showed patient’s clinical and genetic characteristics and LDL-lowering therapy in Japan. There was considerable diversity in the severity of phenotypes, including LDL cholesterol levels, among patients with HoFH. In Japan, the management of LDL cholesterol in HoFH is still inadequate despite the availability of intensive lipid-lowering therapies.
Key Words: genetics, homozygous familial hypercholesterolemia, low-density lipoprotein cholesterol, low-density lipoprotein receptor, proprotein convertase subtilisin/kexin type 9
Central Illustration
Mutations in genes connected to the low-density lipoprotein (LDL) receptor (LDLR) system cause an inherited condition known as familial hypercholesterolemia (FH), including apolipoprotein B (APOB), low-density lipoprotein receptor adapter protein 1 (LDLRAP1), proprotein convertase subtilisin/kexin type 9 (PCSK9), and LDL receptor (LDLR).1 Particularly, patients with homozygous familial hypercholesterolemia (HoFH) possessing homozygotes of identical mutations or compound or double heterozygotes of mutations in the related genes have an extremely high risk of premature atherosclerotic cardiovascular disease and a poor prognosis without early intervention with lipid-lowering therapy.1, 2, 3, 4, 5 HoFH is a very rare disease, and clinical data on patients’ characteristics and on lipid-lowering therapy in a real-world clinical setting are insufficient.6, 7, 8, 9 In Japan, HoFH is registered as one of the designated intractable diseases. For these diseases, the government pays for the patients’ medical costs, and such cases are registered in the National Database (operated by the Ministry of Health, Labour, and Welfare of Japan). Considering this situation, this study aimed to analyze the characteristics of and lipid-lowering therapies for patients with HoFH using a national epidemiological survey by the Japanese Ministry of Health, Labour, and Welfare.
Methods
Study population
Based on the clinical personal records of HoFH patients in the National Database of Rare and Intractable Disease of the Ministry of Health, Labour, and Welfare of Japan, this cross-sectional study was conducted using the national epidemiological survey about HoFH in 2019. From 230 patients, 201 were identified as diagnosed with definite or probable HoFH.
The study protocol was approved by the Institutional Review Board of Kanazawa University (approval no. 2021-058). Because of the retrospective nature of the study, written informed consent was waived in both registries; however, patients who refused to participate in the study when reached out during follow-up were excluded. This approach complies with the recommendations from the Japanese Ministry of Health, Labour, and Welfare.
Definitions and diagnosis of HoFH
Patients with definite HoFH were those in whom LDLR pathway gene mutations were found or LDLR activity was used to identify HoFH. Patients with probable HoFH were those having total cholesterol levels >450 mg/dL (11.64 mmol/L) or LDL cholesterol levels >370 mg/dL (9.57 mmol/L) in the fasting steady state or the presence of clinical manifestations suggesting severe hypercholesterolemia, such as the presence of percutaneous xanthoma from their childhood and being refractory to medications.10
Data collection
The attending physician obtained patients’ medical records by reviewing hospital charts or conducting interviews. Generally, medical records are submitted to the government to register patients for designated intractable diseases. The collected variables described in this study were age, sex, body mass index, heart rate, systolic blood pressure, diastolic blood pressure, family history, age at diagnosis, cutaneous xanthoma, tendon xanthoma, Achilles tendon thickness, valvular disease, intervention for valvular disease, prior coronary artery bypass grafting (CABG), coronary artery disease, prior percutaneous coronary intervention (PCI), aortic aneurysm, arteriosclerosis obliterans, carotid arteriosclerosis, arcus corneae, total cholesterol before medication, triglycerides before medication, high-density lipoprotein cholesterol before medication, LDL cholesterol before medication, genetic testing, lipoprotein apheresis, and lipid-lowering medication (statin, resin, probucol, ezetimibe, and PCSK9 inhibitors).
Statistical analysis
Continuous variables were expressed as mean ± SD and median (IQR). These variables were analyzed using the Student’s t-test or the Wilcoxon rank-sum test based on their distributions. On the other hand, categorical variables were expressed as frequencies and percentages and evaluated using the chi square test. We conducted a subgroup analysis on the patient features stratified by age, definite or probable HoFH, and the presence or absence of coronary artery disease (CAD).
Statistical analyses were conducted using R version 3.6.1 (R Foundation for Statistical Computing). P values <0.05 were considered as statistically significant, and all the stated P values were 2-tailed.
Results
Patients’ characteristics
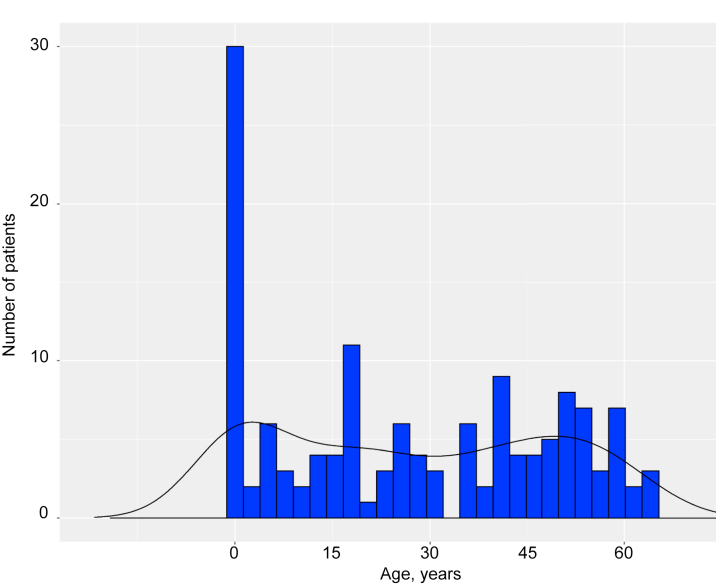
The mean age of the patients was 54 years, and 43% of them were men (Table 1). The median age at diagnosis was 27 years and exhibited a bimodal distribution (Figure 1). Cutaneous xanthoma was observed in 56% of the patients, tendon xanthoma in 80%, and Achilles tendon thickening in majority of them (Table 1). Regarding concomitant valvular disease, 24% of the patients had valvular disease, the majority of whom had aortic valvular disease (Table 1). Furthermore, 70% of the patients had CAD; according to the data, 28% had a previous CABG and 57% had a previous PCI (Table 1).
Table 1.
Characteristics of Patients With Definite or Probable HoFH
| Definite or Probable HoFH (N = 201) |
No. of Patients Evaluated | |
|---|---|---|
| Male | 86 (43) | 201 |
| Age at assessments, y | 54 ± 15 | 201 |
| Body mass index, kg/m2 | 24.4 ± 4.5 | 193 |
| Systolic blood pressure, mm Hg | 125 ± 16 | 192 |
| Diastolic blood pressure, mm Hg | 70 ± 13 | 192 |
| Heart rate, beats/min | 72 ± 11 | 177 |
| Family history | 163 (93) | 175 |
| Age at diagnosis, y | 27 (4.5-47.5) | 140 |
| Cutaneous xanthoma | 112 (56) | 199 |
| Tendon xanthoma | 159 (80) | 200 |
| Achilles tendon thickness, mm | 17.53 ± 9.72 | 144 |
| Achilles tendon thickness ≥9 mm | 140 (97) | 144 |
| Valvular disease | 49 (24) | 201 |
| Aortic valvular disease | 34 (69) | |
| Other valvular disease | 21 (43) | |
| Intervention for valvular disease | 19 (27) | 70 |
| Coronary artery disease | 139 (70) | 198 |
| Prior PCI | 85 (57) | 149 |
| Prior CABG | 39 (28) | 139 |
| Aortic aneurysm | 12 (6.0) | 201 |
| Arteriosclerosis obliterans | 12 (6.0) | 199 |
| Carotid arteriosclerosis | 105 (55) | 192 |
| Arcus corneae | 60 (32) | 190 |
| Total cholesterol before medication, mmol/L | 12.36 ± 2.52 | 137 |
| Triglycerides before medication, mmol/L | 1.92 ± 1.35 | 133 |
| HDL cholesterol before medication, mmol/L | 1.40 ± 1.02 | 131 |
| LDL cholesterol before medication, mmol/L | 10.15 ± 2.32 | 139 |
| Genetic testing | 65 (33) | 198 |
| Diagnosis | 201 | |
| Definite | 58 (29) | |
| Probable | 143 (71) | |
| Lipoprotein apheresis | 42 (21) | 198 |
| Statin | 192 (96) | 200 |
| High-intensity statina | 148 (74) | 200 |
| Resin | 18 (9.7) | 185 |
| Probucol | 24 (12) | 193 |
| Ezetimibe | 139 (70) | 199 |
| PCSK9 inhibitors | 100 (50) | 201 |
Values are n (%), mean ± SD, or median (IQR) unless otherwise indicated.
CABG = coronary artery bypass grafting; HDL = high-density lipoprotein; HoFH = homozygous familial hypercholesterolemia; LDL = low-density lipoprotein; PCI = percutaneous coronary intervention; PCSK9 = proprotein convertase subtilisin kexin 9.
In this study, statin doses ≥20 mg atorvastatin, 4 mg pitavastatin, or 10 mg rosuvastatin were considered as high-intensity statin therapy.
Figure 1.
Age Distribution at Diagnosis
Histogram showing distribution of age at diagnosis as homozygous familial hypercholesterolemia.
Mutations in genes
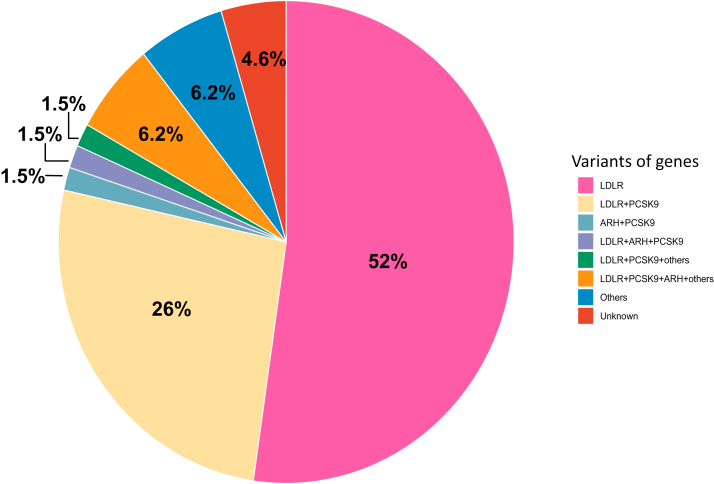
Genetic testing was conducted on 65 (32.3%) patients with HoFH. Regarding genes, mutations in LDLR were identified in 52% of the patients and mutations in LDLR and PCSK9 in 26%. Other types of double heterozygotes, such as LDLR+LDLRAP1+PCSK9, LDLR+PCSK9+Others, and LDLR+PCSK9+LDLRAP1+Others, were identified in 1.5%, 1.5%, and 6.2% of the patients, respectively. Moreover, 1.5% of patients had mutations in LDLRAP1 and PCSK9 (Figure 2).
Figure 2.
Variants of Mutated Genes in Patients With HoFH
Pie chart showing the frequency of mutated gene variants in patients with homozygous familial hypercholesterolemia (HoFH).
ARH = autosomal recessive hypercholesterolemia; LDLR = low-density lipoprotein receptor; PCSK9 = proprotein convertase subtilisin kexin 9.
Among patients with 143 probable HoFH, 15 patients (10.5%) of patients were conducted genetic testing. Among these 15 patients, mutations in LDLR were identified in 8 of 15 (53%) of patients, other than LDLR, PCSK9, and LDLRAP1 mutations were identified in 3 of15 (20%) of patients, and others were identified in 1 of 15 (6.7%) patients.
LDL measurements and lipid-lowering therapies
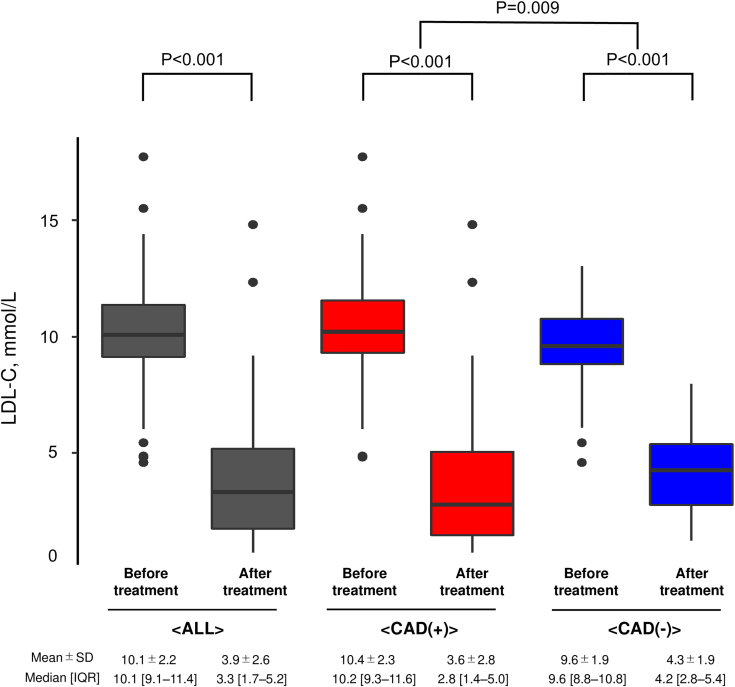
In terms of lipid-lowering therapies, 96% of the patients took statins, 70% took ezetimibe, and 50% used PCSK9 inhibitors. High-intensity statins were used by 74% of the patients. Furthermore, lipoprotein apheresis was performed in 21% of the patients (Table 1). The mean of LDL cholesterol before and after treatment were 10.1 mmol/L and 3.9 mmol/L, respectively, indicating a significant reduction (P < 0.001) (Figure 3). Stratifying according to the presence of CAD, a more significant reduction in LDL cholesterol was achieved in patients with CAD than in those without CAD (P = 0.009) (Figure 3).
Figure 3.
LDL-C Change Before/After Treatment in Patients With and Without CAD
Boxplots showing low-density lipoprotein cholesterol (LDL-C) levels. Gray indicates all patients. Red indicates patients with coronary artery disease (CAD). Blue indicates patients without CAD.
Subgroup analysis
In the subgroup whose age at diagnosis was below 15 years, most of the patients were diagnosed at 0 years old. Tendon xanthoma was more frequently observed in patients <15 years of age (at diagnosis) than in those ≥15 years of age (at diagnosis). The prevalence of CAD was higher in patients ≥15 years of age (at diagnosis), but the difference was not statistically significant; also, prior PCI was more frequently observed in patients ≥15 years of age (at diagnosis) than in those <15 years of age (at diagnosis).
LDL cholesterol before treatment was not different between patients <15 years of age (at diagnosis) and those ≥15 years of age (at diagnosis). Genetic testing was more frequently conducted in patients <15 years of age (at diagnosis) than in those ≥15 years of age (at diagnosis) (61% and 20%; P < 0.001); of those <15 years of age (at diagnosis), 51% were diagnosed with definite HoFH.
In terms of lipid-lowering therapies, there was no significant difference between the 2 groups regarding the intake of statins, ezetimibe, and PCSK9 inhibitors. However, a higher percentage of patients <15 years of age (at diagnosis) received lipoprotein apheresis than those ≥15 years of age (at diagnosis) (40% and 13%, respectively, P = 0.001) (Table 2).
Table 2.
Characteristics of Patients With HoFH Dichotomized According to Age at Diagnosis
| Age <15 y (n = 47) |
Age ≥15 y (n = 92) |
P Value | |
|---|---|---|---|
| Male | 18 (38) | 41 (45) | 0.60 |
| Age at assessments, y | 46 ± 17 | 57 ± 13 | <0.001 |
| Body mass index, kg/m2 | 23.8 ± 4.9 | 24.5 ± 4.1 | 0.37 |
| Systolic blood pressure, mm Hg | 119 ± 15 | 127 ± 16 | 0.009 |
| Diastolic blood pressure, mm Hg | 66 ± 13 | 71 ± 12 | 0.03 |
| Heart rate, beats/min | 70 ± 9 | 71 ± 10 | 0.94 |
| Family history | 44 (98) | 75 (93) | 0.42 |
| Age at diagnosis, y | 0.0 (0.0-4.5) | 40.5 (25.0-52.0) | <0.001 |
| Cutaneous xanthoma | 32 (68) | 48 (53) | 0.14 |
| Tendon xanthoma | 43 (92) | 66 (73) | 0.02 |
| Achilles tendon thickness, mm | 19.05 (14.26) | 16.81 (7.67) | 0.31 |
| Achilles tendon thickness ≥9 mm | 38 (100) | 58 (95) | 0.43 |
| Valvular disease | 16 (34) | 24 (26) | 0.43 |
| Aortic valvular disease | 17 (71) | 11 (69) | |
| Coronary artery disease | 28 (61) | 66 (73) | 0.23 |
| Prior PCI | 12 (38) | 44 (62) | 0.04 |
| Prior CABG | 11 (36) | 15 (23) | 0.28 |
| Carotid arteriosclerosis | 30 (65) | 47 (53) | 0.26 |
| Arcus corneae | 19 (44) | 28 (32) | 0.23 |
| Total cholesterol before medication, mmol/L | 12.84 ± 3.27 | 12.16 ± 2.57 | 0.28 |
| Triglycerides before medication, mmol/L | 1.28 ± 0.60 | 1.97 ± 1.34 | 0.01 |
| HDL cholesterol before medication, mmol/L | 1.22 ± 0.41 | 1.53 ± 1.35 | 0.25 |
| LDL cholesterol before medication, mmol/L | 10.56 ± 2.84 | 9.89 ± 2.14 | 0.20 |
| Genetic testing | 28 (61) | 18 (20) | <0.001 |
| Diagnosis | 0.001 | ||
| Definite | 24 (51) | 19 (21) | |
| Probable | 23 (49) | 73 (79) | |
| Lipoprotein apheresis | 19 (40) | 12 (13) | 0.001 |
| Statin | 44 (94) | 90 (99) | 0.22 |
| High-intensity statina | 37 (79) | 69 (75) | 0.78 |
| Ezetimibe | 36 (78) | 62 (68) | 0.30 |
| PCSK9 inhibitors | 21 (45) | 44 (48) | 0.86 |
Values are n (%), mean ± SD, or median (IQR) unless otherwise indicated.
Abbreviations as in Table 1.
In this study, statin doses ≥20 mg atorvastatin, 4 mg pitavastatin, or 10 mg rosuvastatin were considered as high-intensity statin therapy.
Similar baseline characteristics were obtained between definite HoFH and probable HoFH, except for age at diagnosis, valvular disease, carotid arteriosclerosis, arcus corneae, genetic testing, and lipoprotein apheresis. Age at diagnosis was younger in definite HoFH, and genetic testing and lipoprotein apheresis were more frequent in definite HoFH than in probable HoFH (Table 3).
Table 3.
Characteristics of Patients Stratified by Definite or Probable HoFH
| Definite (n = 58) |
Probable (n = 143) |
P Value | |
|---|---|---|---|
| Male | 24 (41) | 62 ( 43) | 0.92 |
| Age at assessments, y | 52 ± 15 | 55 ± 15 | 0.21 |
| Body mass index, kg/m2 | 23.8 ± 5.1 | 24.6 ± 4.2 | 0.24 |
| Systolic blood pressure, mm Hg | 120 ± 14 | 127 ± 17 | 0.01 |
| Diastolic blood pressure, mm Hg | 66 ± 12 | 72 ± 13 | 0.007 |
| Heart rate, beats/min | 71 ± 11 | 72 ± 12 | 0.46 |
| Family history | 50 (94) | 113 (93) | 0.93 |
| Age at diagnosis, y | 7.0 (0-35.5) | 33.5 (15-51) | <0.001 |
| Cutaneous xanthoma | 35 (60) | 77 (55) | 0.56 |
| Tendon xanthoma | 51 (88) | 108 (76) | 0.09 |
| Achilles tendon thickness, mm | 19.3 ± 13.5 | 16.8 ± 7.5 | 0.15 |
| Achilles tendon thickness ≥9 mm | 42 (98) | 98 (97) | 1.00 |
| Valvular disease | 23 (40) | 26 (18) | 0.002 |
| Intervention for valvular disease | 11 (39) | 8 (19) | 0.11 |
| Coronary artery disease | 44 (77) | 95 (67) | 0.23 |
| Prior PCI | 23 (49) | 62 (61) | 0.24 |
| Prior CABG | 11 (25) | 28 (30) | 0.73 |
| Carotid arteriosclerosis | 41 (72) | 64 (47) | 0.003 |
| Arcus corneae | 27 (48) | 33 (25) | 0.003 |
| Total cholesterol before medication, mmol/L | 12.28 ± 2.99 | 12.40 ± 2.30 | 0.79 |
| Triglycerides before medication, mmol/L | 1.67 ± 1.05 | 2.01 ± 1.45 | 0.19 |
| HDL cholesterol before medication mmol/L | 1.20 ± 0.32 | 1.47 ± 1.17 | 0.19 |
| LDL cholesterol before medication, mmol/L | 10.41 ± 2.68 | 10.06 ± 2.17 | 0.43 |
| Genetic testing | 50 (88) | 15 (11) | <0.001 |
| Lipoprotein apheresis | 18 (31) | 24 (17) | 0.047 |
| Statin | 55 (95) | 137 (97) | 0.89 |
| High-intensity statina | 45 (78) | 103 (72) | 0.53 |
| Ezetimibe | 41 (71) | 98 (70) | 1.00 |
| PCSK9 inhibitors | 32 (55) | 68 (48) | 0.41 |
Values are n (%), mean ± SD, or median (IQR) unless otherwise indicated.
Abbreviations as in Table 1.
In this study, statin doses ≥20 mg atorvastatin, 4 mg pitavastatin, or 10 mg rosuvastatin were considered as high-intensity statin therapy.
In the subgroup analysis stratified based on the presence or absence of CAD, patients without CAD were predominantly female, younger in age, and diagnosed at a younger age compared to those with CAD (Table 4). They also exhibited a higher prevalence of family history, lower occurrence of cutaneous xanthoma, and reduced incidence of carotid arteriosclerosis. However, there was no significant difference in LDL cholesterol levels before medication between the 2 groups.
Table 4.
Characteristics of Patients With HoFH Stratified Based on the Presence or Absence of CAD
| With CAD (n = 139) |
Without CAD (n = 59) |
P Value | |
|---|---|---|---|
| Male | 71 (51) | 15 (25) | 0.002 |
| Age at assessments, y | 58 ± 13 | 46 ± 15 | <0.001 |
| Body mass index, kg/m2 | 24.5 ± 4.2 | 24.1 ± 5.2 | 0.62 |
| Systolic blood pressure, mm Hg | 126 ± 16 | 122 ± 16 | 0.19 |
| Diastolic blood pressure, mm Hg | 71 ± 13 | 69 ± 11 | 0.23 |
| Heart rate, beats/min | 71 ± 12 | 73 ± 10 | 0.40 |
| Family history | 107 (90) | 54 (100) | 0.04 |
| Age at diagnosis, y | 32.0 (5.8-49.0) | 18.0 (4.5-37.0) | 0.19 |
| Cutaneous xanthoma | 86 (62) | 25 (44) | 0.03 |
| Tendon xanthoma | 113 (81) | 45 (78) | 0.69 |
| Achilles tendon thickness, mm | 17.7 ± 7.7 | 17.4 ± 13.7 | 0.86 |
| Achilles tendon thickness ≥9 mm | 99 (99) | 39 (93) | 0.14 |
| Valvular disease | 40 (29) | 9 (15) | 0.07 |
| Aortic valvular disease | 29 (73) | 5 (56) | |
| Prior PCI | 84 (62) | 0 (0) | <0.001 |
| Prior CABG | 39 (31) | 0 (0) | 0.07 |
| Carotid arteriosclerosis | 80 (61) | 25 (43) | 0.03 |
| Arcus corneae | 46 (36) | 13 (22) | 0.08 |
| Total cholesterol before medication, mmol/L | 12.51 ± 2.49 | 12.03 ± 2.60 | 0.31 |
| Triglycerides before medication, mmol/L | 2.14 ± 1.49 | 1.45 ± 0.78 | 0.008 |
| HDL cholesterol before medication, mmol/L | 1.25 ± 0.30 | 1.72 ± 1.77 | 0.02 |
| LDL cholesterol before medication, mmol/L | 10.34 ± 2.50 | 9.73 ± 1.86 | 0.16 |
| Genetic testing | 52 (38) | 12 (21) | 0.03 |
| Diagnosis | 0.23 | ||
| Definite | 44 (32) | 13 (22) | |
| Probable | 95 (68) | 46 (78) | |
| Lipoprotein apheresis | 31 (23) | 11 (19) | 0.71 |
| Statin | 132 (96) | 57 (97) | 1.00 |
| High-intensity statina | 101 (73) | 44 (75) | 0.92 |
| Ezetimibe | 95 (68) | 42 (74) | 0.57 |
| PCSK9 inhibitors | 74 (53) | 24 (41) | 0.14 |
Values are n (%), mean ± SD, or mean (IQR) unless otherwise indicated.
Abbreviations as in Table 1.
In this study, statin doses ≥20 mg atorvastatin, 4 mg pitavastatin, or 10 mg rosuvastatin were considered as high-intensity statin therapy.
Discussion
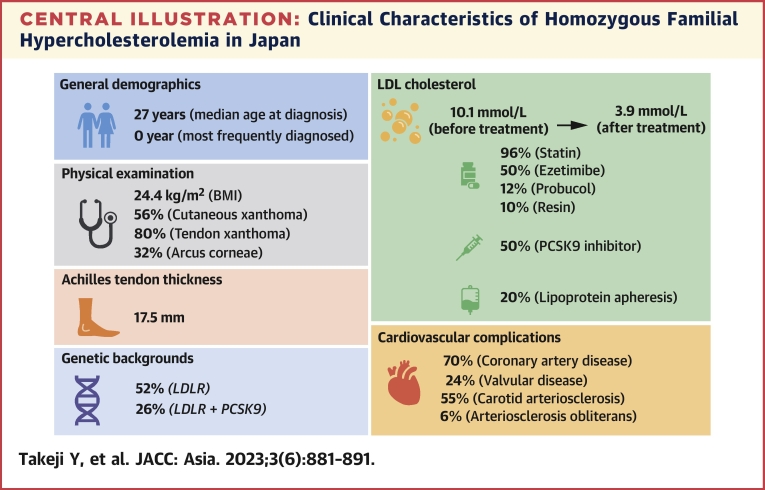
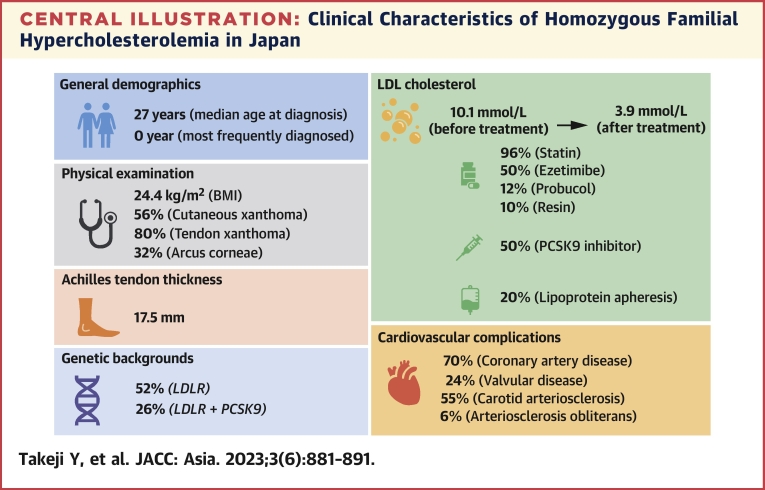
The major findings of this study evaluating the characteristics of patients and the treatment of HoFH from a national epidemiological survey were as follows (Central Illustration): 1) The median age at diagnosis was 27 years and exhibited a bimodal distribution; 2) approximately 70% of the patients had CAD; 3) the primary cause of this situation was LDLR, followed by double heterozygotes of LDLR and PCSK9; 4) the mean of LDL cholesterol before and after treatment were 10.1 mmol/L and 3.9 mmol/L, respectively, indicating a significant reduction due to the use of intensive lipid-lowering therapies; and 5) patients with CAD experienced a more notable decrease in LDL cholesterol than those without CAD.
Central Illustration.
Clinical Characteristics of Homozygous Familial Hypercholesterolemia in Japan
General demographics, physical examination, Achilles tendon thickness, genetic backgrounds, low-density lipoprotein (LDL) cholesterol, and cardiovascular complications of homozygous familial hypercholesterolemia in Japan are summarized. BMI = body mass index; LDLR = low-density lipoprotein receptor; PCSK9 = proprotein convertase subtilisin kexin 9.
HoFH is an inherited disease caused by mutations in genes connected to the LDLR pathway, and unless treatment is provided, atherosclerotic cardiovascular disease events start to occur even in the first decade.1, 2, 3, 4, 5 Given that HoFH is a rare disease with prevalence estimated at 1:175,000 to 1:300,000, large-scale studies evaluating patient characteristics and management for patients with HoFH are scarce.6, 7, 8, 9,11, 12, 13, 14, 15 Therefore, this study evaluated the characteristics of and management for patients with genetically or clinically diagnosed HoFH using a national epidemiological survey from the Ministry of Health, Labour, and Welfare of Japan.
In this study, the median age of diagnosis exhibited a bimodal distribution. Most patients were diagnosed at 0 years old, and we presumed that these patients had manifestations specific to HoFH, such as cutaneous xanthomas from their childhood. Contrarily, some patients were diagnosed at approximately 40 years of age. We presumed that these patients: 1) were overlooked in their childhood and were diagnosed later in their medical examinations; or 2) were categorized as having severe heterogeneous FH whose phenotype was similar to HoFH because of additional factors, such as elevated Lp(a) and hypertension.
Previous studies from Europe had shown that more than 90% of patients with HoFH have pathogenic variants in both LDLR alleles.6,8,16 However, as much as a quarter of the HoFH cases were caused by double heterozygous mutations in LDLR and PCSK9. This was probably because a particular missense mutation in PCSK9 ((NM_174936.4) : c.94G>A (p.Glu32Lys)) was common in Japan.17, 18, 19 We have previously shown that a double heterozygous mutation in LDLR and this particular missense mutation in PCSK9 led to the phenotype of typical HoFH.10 However, there was no patient with HoFH caused by mutations in APOB in this database. A particular pathogenic mutation (c.10580 G>A: p.[Arg3527Gln] in APOB) has been shown as one of the most common pathogenic mutations as FH in some European countries, probably because of the founder effect.20 However, it has been shown that the proportion of FH patients with this variant in APOB is low in Asian countries, including Japan. 21 In fact, the first FH case with this variant was identified very recently in Japan. 22
In this study, LDL cholesterol before treatment was not different between patients with definite HoFH (molecularly defined) and those with probable HoFH (clinically defined). This fact adds justification to the criteria of probable FH (clinically defined) in terms of diagnostic criteria and risk stratification for this disease.
Regarding comorbidity, as many as 70% of the patients had a history of CAD, although their mean age was only 54 years. Importantly, more intensive lipid-lowering therapies had already been introduced for patients in secondary prevention than for those in primary prevention. However, LDL cholesterol after medication in each group was inadequate considering the targets (<2.6 mmol/L [100 mg/dL] for primary prevention and <1.8 mml/L [70 mg/dL] for secondary prevention).5 More intensive lipid-lowering therapies are warranted for patients with high risks of arteriosclerotic events to reduce cardiovascular events and increase patients’ life expectancy. Tromp et al23 reported that use of more numbers of lipid-lowering therapies were associated with less LDL cholesterol level on treatment among patients with HoFH. In Japan, HoFH is registered as one of the designated intractable diseases where medical costs are fully covered by the government. So, several emerging new therapies, such as lomitapide and evinacumab that are rather expensive can be introduced for Japanese HoFH patients when indicated.24,25 Alves et al26 reported that phenotype was variable even among patients with HoFH. We also observed that the severity of their phenotypes, including LDL cholesterol were diverse, partly due to their genetic backgrounds (true homozygous or compound heterozygous).
As for the differential diagnosis of HoFH, to register HoFH patients in this Japanese National database, sitosterolemia, cerebrotendinous xanthomatosis, hypothyroidism and nephrotic syndrome should be ruled out as differential diagnoses of HoFH. Therefore, these conditions, including sitosterolemia were presumed to have been denied as potential differential diagnoses. Moreover, sitosterolemia is also defined as one of the designated intractable diseases in Japan by the Ministry of Health, Labour, and Welfare of Japan.
Study limitations
First, a cross-sectional survey was used in this study, and we could not evaluate clinical outcomes or estimate risk. The results of this study were different from the ones publicly announced by the Japanese Ministry of Health, Labour, and Welfare. Second, we did not have information about the accurate timing of LDL cholesterol measurement before and after treatment. Third, we did not have details about gene mutations in LDLR (true homozygous or compound heterozygous). Fourth, although this is the study with the largest National Database of HoFH in Japan, we acknowledge that this cohort does not include all of the patients with HoFH in Japan. However, when we consider the prevalence of patients with HoFH among general population (1 in 360,000) based on the assumption of the prevalence of HoFH (1 in 300), it is estimated that there are ∼300 heterozygous FH patients in Japan (total population is 120 M).27 Accordingly, as much as two-thirds of patients were included in this study; therefore, the current study can represent a landscape of the clinical features in Japanese HoFH patients. Fifth, only one-third of the patients with HoFH underwent genetic testing for FH, which can lead to an underestimation of the prevalence of definite HoFH. However, the cost of genetic testing for FH has been covered by national health insurance since 2022 in Japan. Accordingly, we anticipate that more patients will undergo genetic testing for FH, which would lead to the identification of more patients with definite HoFH in the near future. Sixth, information about causal gene(s) are available although there is no specific information on genetic variants in this database. So, it is rather unclear how pathogenicity of genetic variants was determined. However, it is common to use American College of Medical Genetics and/or Clinvar criteria in Japan as well.28 Seventh, information about the supravalvular aortic stenosis, which is specific to HoFH, is lacking.29 Eighth, Lp(a) level was not available in this database, although it has been described that Lp(a) levels in HoFH were higher than that in heterozygous FH, which should affect the phenotype of HoFH.30 Ninth, we could not provide the information of when CAD occurred in this population. Finally, the phenotypes included in this database were limited; thus, some of their important clinical phenotypes might be missed. In this regard, another multicenter registry, the Committee on Primary Dyslipidemia of the Research Program on Rare and Intractable Disease of the Ministry of Health, Labour, and Welfare of Japan, conducted a study on HoFH to thoroughly evaluate its genotypes and phenotypes. 31 This study will provide insights into the clinical management of HoFH in the future.
Conclusions
The national epidemiological study of patients with HoFH showed patient’s clinical and genetic characteristics and LDL-lowering therapy in Japan. There was considerable diversity in the severity of phenotypes, including LDL cholesterol levels, among patients with HoFH. In Japan, the management of LDL cholesterol in HoFH is still inadequate despite the availability of intensive lipid-lowering therapies.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: This study has shown that at least a part of the patients with HoFH were undiagnosed during their childhood and that management of their LDL cholesterol level remains inadequate despite the availability of intensive lipid-lowering therapies.
TRANSLATIONAL OUTLOOK: Future studies are warranted to determine whether early diagnosis and treatment for patients with HoFH would lead to a better prognosis and to know how low their LDL cholesterol should be.
Funding Support and Author Disclosures
This work is supported by a grant from the Labor and Welfare Sciences Research Grant for Research on Rare and Intractable Diseases (21FC0201). The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The authors thank Ms Kazuko Honda and Mr Sachio Yamamoto for their outstanding technical assistance. The Committee on Primary Dyslipidemia under the Research Program on Rare and Intractable Disease of the Ministry of Health, Labour and Welfare of Japan includes: Mariko Harada-Shiba (Cardiovascular Center, Osaka Medical and Pharmaceutical University, Takatsuki, Japan), Shun Ishibashi (Division of Endocrinology and Metabolism, Department of Internal Medicine, School of Medicine, Jichi Medical University, Tochigi, Japan), Shinji Yokoyama (Institute for Biological Functions, Chubu University, Aichi, Japan), Hitoshi Shimano (Department of Internal Medicine (Endocrinology and Metabolism), Faculty of Medicine University of Tsukuba, Tsukuba, Japan), Koutaro Yokote (Department of Endocrinology, Hematology and Gerontology, Chiba University Graduate School of Medicine, Chiba, Japan), Hideaki Bujo (Department of Clinical-Laboratory and Experimental-Research Medicine, Toho University Sakura Medical Center, Chiba, Japan), Shizuya Yamashita (Rinku General Medical Center, Osaka, Japan), Kazuhisa Tsukamoto (Department of Internal Medicine, Teikyo University, Tokyo, Japan), Katsunori Ikewaki (Division of Neurology, Anti-Aging, and Vascular Medicine, Department of Internal Medicine, National Defense Medical College, Tokorozawa, Japan), Takanari Gotoda (Department of Metabolic Biochemistry, Faculty of Medicine, Kyorin University, Tokyo, Japan), Kazushige Dobashi (Department of Pediatrics, School of Medicine, University of Yamanashi, Yamanashi, Japan), Misa Takegami (Department of Preventive Medicine and Epidemiology, National Cerebral and Cardiovascular Center, Osaka, Japan), Yoshiki Sekijima (Department of Medicine (Neurology & Rheumatology), Shinshu University School of Medicine, Matsumoto, Japan), Yasushi Ishigaki (Division of Diabetes, Metabolism and Endocrinology, Department of Internal Medicine, Iwate Medical University, Iwate, Japan), Hiroaki Okazaki (Division of Endocrinology and Metabolism, Department of Internal Medicine, School of Medicine, Jichi Medical University, Tochigi, Japan), Atsushi Nohara (Ishikawa Prefectural Central Hospital, Kanazawa, Japan), Shingo Koyama (Division of Neurology and Clinical Neuroscience, Department of Internal Medicine III, Yamagata University Faculty of Medicine, Yamagata, Japan), Kyoko Inagaki (Division of Diabetes, Endocrinology, and Metabolism, Department of Medicine, Nippon Medical School, Tokyo, Japan), Koh Ono (Department of Cardiovascular Medicine, Kyoto University Graduate School of Medicine, Kyoto, Japan), Masahiro Koseki (Division of Cardiovascular Medicine, Department of Medicine, Osaka University Graduate School of Medicine, Osaka, Japan), Hiroyuki Daida (Faculty of Health Science, Juntendo University, Juntendo University Graduate School of Medicine, Tokyo, Japan), Manabu Takahashi (Division of Endocrinology and Metabolism, Department of Internal Medicine, Jichi Medical University, Tochigi, Japan), Kimitoshi Nakamura (Department of Pediatrics, Kumamoto University Graduate School of Medical Sciences, Kumamoto, Japan), Takashi Miida (Department of Clinical Laboratory Medicine, Juntendo University Graduate School of Medicine, Tokyo, Japan), Masa-aki Kawashiri (Department of Internal Medicine, Kaga Medical Center, Kaga, Japan), Tetsuo Minamino (Department of Cardiorenal and Cerebrovascular Medicine, Faculty of Medicine, Kagawa University, Kagawa, Japan), Sachiko Okazaki (Division for Health Service Promotion, The University of Tokyo, Tokyo, Japan), Hayato Tada (Department of Cardiovascular Medicine, Kanazawa University Graduate School of Medical Sciences, Kanazawa, Japan), Jun Wada (Department of Nephrology, Rheumatology, Endocrinology and Metabolism, Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences, Okayama, Japan), Masatsune Ogura (Department of Medical Laboratory Technology, Faculty of Medical Science, Juntendo University, Urayasu, Japan), Hirotoshi Ohmura (Department of Cardiovascular Medicine, School of Medicine, Juntendo University, Tokyo, Japan), Mika Hori (Department of Endocrinology, Research Institute of Environmental Medicine, Nagoya University, Nagoya, Japan), Kota Matsuki (Department of Endocrinology and Metabolism, Hirosaki University Graduate School of Medicine, Hirosaki, Japan), Masashi Yamamoto (Department of Endocrinology, Hematology and Gerontology, Chiba University Graduate School of Medicine, Chiba, Japan), Yasuo Takeuchi (Division of Nephrology, Kitasato University School of Medicine, Kanagawa, Japan), Atsuko Nakatsuka (Department of Nephrology, Rheumatology, Endocrinology and Metabolism, Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences, Okayama, Japan), Daisaku Masuda (Department of Cardiology, Health Care Center, Rinku Innovation Center for Wellness Care and Activities (RICWA), Rinku General Medical Center, Osaka, Japan), Satoshi Hirayama (Department of Clinical Laboratory Medicine, Juntendo University Graduate School of Medicine, Tokyo, Japan), Masayuki Kuroda (Center for Advanced Medicine, Chiba University Hospital, Chiba University, Chiba, Japan), Takashi Yamaguchi (Center of Diabetes, Endocrinology and Metabolism, Toho University Sakura Medical Center, Chiba, Japan).
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Contributor Information
Hayato Tada, Email: ht240z@sa3.so-net.ne.jp.
Committee on Primary Dyslipidemia under the Research Program on Rare and Intractable Disease of the Ministry of Health, Labor, and Welfare of Japan:
Mariko Harada-Shiba, Shun Ishibashi, Shinji Yokoyama, Hitoshi Shimano, Koutaro Yokote, Hideaki Bujo, Shizuya Yamashita, Kazuhisa Tsukamoto, Katsunori Ikewaki, Takanari Gotoda, Kazushige Dobashi, Misa Takegami, Yoshiki Sekijima, Yasushi Ishigaki, Hiroaki Okazaki, Atsushi Nohara, Shingo Koyama, Kyoko Inagaki, Koh Ono, Masahiro Koseki, Hiroyuki Daida, Manabu Takahashi, Kimitoshi Nakamura, Takashi Miida, Masa-aki Kawashiri, Tetsuo Minamino, Sachiko Okazaki, Hayato Tada, Jun Wada, Masatsune Ogura, Hirotoshi Ohmura, Mika Hori, Kota Matsuki, Masashi Yamamoto, Yasuo Takeuchi, Atsuko Nakatsuka, Daisaku Masuda, Satoshi Hirayama, Masayuki Kuroda, and Takashi Yamaguchi
References
- 1.Goldstein J.L., Hobbs H.H., Brown M.S. In: Familial Hypercholesterolemia: The Metabolic and Molecular Bases of Inherited Disease. Eighth edition. Scriver C.R., Beaudet A.L., Sly W.S., et al., editors. McGraw-Hill; 2001. [Google Scholar]
- 2.Soutar A.K., Naoumova R.P. Mechanisms of disease: genetic causes of familial hypercholesterolemia. Nat Clin Pract Cardiovasc Med. 2007;4:214–225. doi: 10.1038/ncpcardio0836. [DOI] [PubMed] [Google Scholar]
- 3.Raal F.J., Santos R.D. Homozygous familial hypercholesterolemia: current perspectives on diagnosis and treatment. Atherosclerosis. 2012;223:262–268. doi: 10.1016/j.atherosclerosis.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 4.Defesche J.C., Gidding S.S., Harada-Shiba M., Hegele R.A., Santos R.D., Wierzbicki A.S. Familial hypercholesterolaemia. Nat Rev Dis Prim. 2017;3 doi: 10.1038/nrdp.2017.93. [DOI] [PubMed] [Google Scholar]
- 5.Harada-Shiba M., Arai H., Ishigaki Y., et al. Guidelines for diagnosis and treatment of familial hypercholesterolemia 2017. J Atheroscler Thromb. 2018;25:751–770. doi: 10.5551/jat.CR003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bertolini S., Calandra S., Arca M., et al. Homozygous familial hypercholesterolemia in Italy: clinical and molecular features. Atherosclerosis. 2020;312:72–78. doi: 10.1016/j.atherosclerosis.2020.08.027. [DOI] [PubMed] [Google Scholar]
- 7.Sánchez-Hernández R.M., Civeira F., Stef M., et al. Homozygous familial hypercholesterolemia in Spain: prevalence and phenotype-genotype relationship. Circ Cardiovasc Genet. 2016;9:504–510. doi: 10.1161/CIRCGENETICS.116.001545. [DOI] [PubMed] [Google Scholar]
- 8.Sjouke B., Kusters D.M., Kindt I., et al. Homozygous autosomal dominant hypercholesterolaemia in The Netherlands: prevalence, genotype-phenotype relationship, and clinical outcome. Eur Heart J. 2015;36:560–565. doi: 10.1093/eurheartj/ehu058. [DOI] [PubMed] [Google Scholar]
- 9.Stefanutti C., Pang J., Di Giacomo S., et al. A cross-national investigation of cardiovascular survival in homozygous familial hypercholesterolemia: the Sino-Roman Study. J Clin Lipidol. 2019;13(4):608–617. doi: 10.1016/j.jacl.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 10.Nohara A., Tada H., Ogura M., et al. Homozygous familial hypercholesterolemia. J Atheroscler Thromb. 2021;28:665–678. doi: 10.5551/jat.RV17050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mabuchi H., Nohara A., Noguchi T., et al. Molecular genetic epidemiology of homozygous familial hypercholesterolemia in the Hokuriku district of Japan. Atherosclerosis. 2011;214:404–407. doi: 10.1016/j.atherosclerosis.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 12.Benn M., Watts G.F., Tybjærg-Hansen A., Nordestgaard B.G. Mutations causative of familial hypercholesterolaemia: screening of 98 098 individuals from the Copenhagen General Population Study estimated a prevalence of 1 in 217. Eur Heart J. 2016;37:1384–1394. doi: 10.1093/eurheartj/ehw028. [DOI] [PubMed] [Google Scholar]
- 13.de Ferranti S.D., Rodday A.M., Mendelson M.M., Wong J.B., Leslie L.K., Sheldrick R.C. Prevalence of familial hypercholesterolemia in the 1999 to 2012 United States National Health and Nutrition Examination Surveys (NHANES) Circulation. 2016;133:1067–1072. doi: 10.1161/CIRCULATIONAHA.115.018791. [DOI] [PubMed] [Google Scholar]
- 14.Beheshti S.O., Madsen C.M., Varbo A., Nordestgaard B.G. Worldwide prevalence of familial hypercholesterolemia: meta-analyses of 11 million subjects. J Am Coll Cardiol. 2020;75:2553–2566. doi: 10.1016/j.jacc.2020.03.057. [DOI] [PubMed] [Google Scholar]
- 15.Hu P., Dharmayat K.I., Stevens C.A.T., et al. Prevalence of familial hypercholesterolemia among the general population and patients with atherosclerotic cardiovascular disease: a systematic review and meta-analysis. Circulation. 2020;141:1742–1759. doi: 10.1161/CIRCULATIONAHA.119.044795. [DOI] [PubMed] [Google Scholar]
- 16.Bertolini S., Pisciotta L., Rabacchi C., et al. Spectrum of mutations and phenotypic expression in patients with autosomal dominant hypercholesterolemia identified in Italy. Atherosclerosis. 2013;227:342–348. doi: 10.1016/j.atherosclerosis.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 17.Noguchi T., Katsuda S., Kawashiri M.-A., et al. The E32K variant of PCSK9 exacerbates the phenotype of familial hypercholesterolaemia by increasing PCSK9 function and concentration in the circulation. Atherosclerosis. 2010;210:166–172. doi: 10.1016/j.atherosclerosis.2009.11.018. [DOI] [PubMed] [Google Scholar]
- 18.Tada H., Kawashiri M.-A., Nohara A., Inazu A., Mabuchi H., Yamagishi M. Impact of clinical signs and genetic diagnosis of familial hypercholesterolaemia on the prevalence of coronary artery disease in patients with severe hypercholesterolaemia. Eur Heart J. 2017;38:1573–1579. doi: 10.1093/eurheartj/ehx004. [DOI] [PubMed] [Google Scholar]
- 19.Tada H., Hori M., Nomura A., et al. A catalog of the pathogenic mutations of LDL receptor gene in Japanese familial hypercholesterolemia. J Clin Lipidol. 2020;14(3):346–351.e9. doi: 10.1016/j.jacl.2020.03.002. [DOI] [PubMed] [Google Scholar]
- 20.Futema M., Ramaswami U., Tichy L., et al. Comparison of the mutation spectrum and association with pre and post treatment lipid measures of children with heterozygous familial hypercholesterolaemia (FH) from eight European countries. Atherosclerosis. 2021;319:108–117. doi: 10.1016/j.atherosclerosis.2021.01.008. [DOI] [PubMed] [Google Scholar]
- 21.Nohara A., Yagi K., Inazu A., Kajinami K., Koizumi J., Mabuchi H. Absence of familial defective apolipoprotein B-100 in Japanese patients with familial hypercholesterolaemia. Lancet. 1995;345(8962):1438. doi: 10.1016/s0140-6736(95)92627-5. [DOI] [PubMed] [Google Scholar]
- 22.Hori M., Takahashi A., Son C., Ogura M., Harada-Shiba M. The first Japanese cases of familial hypercholesterolemia due to a known pathogenic APOB gene variant, c.10580 G>A: p.(Arg3527Gln) J Clin Lipidol. 2020;14(4):482–486. doi: 10.1016/j.jacl.2020.05.007. [DOI] [PubMed] [Google Scholar]
- 23.Tromp T.R., Hartgers M.L., Hovingh G.K., et al. Worldwide experience of homozygous familial hypercholesterolaemia: retrospective cohort study. Lancet. 2022;399:719–728. doi: 10.1016/S0140-6736(21)02001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harada-Shiba M., Koezuka R., Makino H., Ogura M. Gradual dose titration of lomitapide may prevent therapeutic delays in patients with homozygous familial hypercholesterolemia. J Atheroscler Thromb. 2023;30:203–205. doi: 10.5551/jat.LE003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raal F.J., Rosenson R.S., Reeskamp L.F., et al. Evinacumab for homozygous familial hypercholesterolemia. N Engl J Med. 2020;383:711–720. doi: 10.1056/NEJMoa2004215. [DOI] [PubMed] [Google Scholar]
- 26.Alves A.C., Alonso R., Diaz-Diaz J.L., et al. Phenotypical, Clinical, and Molecular Aspects of Adults and Children With Homozygous Familial Hypercholesterolemia in Ibero-America. Arterioscler Thromb Vasc Biol. 2020;40(10):2508–2515. doi: 10.1161/ATVBAHA.120.313722. [DOI] [PubMed] [Google Scholar]
- 27.Harada-Shiba M., Ohtake A., Sugiyama D., et al. Guidelines for the diagnosis and treatment of pediatric familial hypercholesterolemia 2022. J Atheroscler Thromb. 2023;30(5):531–557. doi: 10.5551/jat.CR006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tada H., Kojima N., Yamagami K., et al. Impact of healthy lifestyle in patients with familial hypercholesterolemia. JACC Asia. 2023;3(1):152–160. doi: 10.1016/j.jacasi.2022.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okada H., Tada H., Nomura A., et al. Whole exome sequencing insufficient for a definitive diagnosis of a patient with compound heterozygous familial hypercholesterolemia. Intern Med. 2022;61(19):2883–2889. doi: 10.2169/internalmedicine.8989-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Boer L.M., Reijman M.D., Hutten B.A., Wiegman A. Lipoprotein(a) levels in children with homozygous familial hypercholesterolaemia: a cross-sectional study. J Clin Lipidol. 2023;17(3):415–419. doi: 10.1016/j.jacl.2023.03.010. [DOI] [PubMed] [Google Scholar]
- 31.Tada H., Kurashina T., Ogura M., et al. Prospective Registry Study of Primary Dyslipidemia (PROLIPID): rationale and study design. J Atheroscler Thromb. 2022;29:953–969. doi: 10.5551/jat.63222. [DOI] [PMC free article] [PubMed] [Google Scholar]