Abstract
Following contact with a eucaryotic cell, Yersinia species pathogenic for humans (Y. pestis, Y. pseudotuberculosis, and Y. enterocolitica) export and translocate a distinct set of virulence proteins (YopE, YopH, YopJ, YopM, and YpkA) from the bacterium into the eucaryotic cell. During in vitro growth at 37°C in the presence of calcium, Yop secretion is blocked; however, in the absence of calcium, Yop secretion is triggered. Yop secretion occurs via a plasmid-encoded type III, or “contact-dependent,” secretion system. The secreted YopN (also known as LcrE), TyeA, and LcrG proteins are necessary to prevent Yop secretion in the presence of calcium and prior to contact with a eucaryotic cell. In this paper we characterize the role of the yscB gene product in the regulation of Yop secretion in Y. pestis. A yscB deletion mutant secreted YopM and V antigen both in the presence and in the absence of calcium; however, the export of YopN was specifically reduced in this strain. Complementation with a functional copy of yscB in trans completely restored the wild-type secretion phenotype for YopM, YopN, and V antigen. The YscB amino acid sequence showed significant similarities to those of SycE and SycH, the specific Yop chaperones for YopE and YopH, respectively. Protein cross-linking and immunoprecipitation studies demonstrated a specific interaction between YscB and YopN. In-frame deletions in yopN eliminating the coding region for amino acids 51 to 85 or 6 to 100 prevented the interaction of YopN with YscB. Taken together, these results indicate that YscB functions as a specific chaperone for YopN in Y. pestis.
Yersinia species pathogenic for humans (Y. pestis, Y. pseudotuberculosis, and Y. enterocolitica) interact directly with the surfaces of eucaryotic cells and translocate a distinct set of virulence proteins (YopE, YopH, YopJ, YopM, and YpkA) from the cytoplasm of the bacterium into the cytoplasm of the eucaryotic cell (11, 12). Once translocated into the eucaryotic cell, Yop proteins disrupt intracellular signaling pathways (YopH [8, 15, 40, 41] and YpkA [25]), prevent specific cytoskeletal rearrangements (YopE [50]), induce apoptotic events in macrophages (YopJ [35, 36, 52]), and eventually kill the target cell. This capability enables the yersiniae to avoid phagocytosis and ensures survival of the bacteria within host tissues (14, 18, 48, 49).
Yops are secreted by a type III, or “contact-dependent,” secretion mechanism (12, 33, 61). The secretion of Yops is not a constitutive process; instead, Yop secretion occurs in response to specific signals associated with contacting a eucaryotic target cell (18, 51). Several of the secreted proteins (YopB, YopD, YopK, LcrG, TyeA, and YopN) are not translocated into the eucaryotic cell but function either to regulate Yop export or to target Yops with direct antihost functions into the eucaryotic cell. The secreted LcrG, TyeA, and YopN proteins are thought to function in gating the Yop secretion channel and are required to prevent Yop secretion in the presence of calcium prior to contact with a eucaryotic target cell (19, 28, 56, 63). The secreted V antigen interacts directly with LcrG within the cell and may be required to counteract LcrG’s role in blocking Yop secretion (38). In addition, LcrV is specifically required for the secretion of YopB and YopD (53). YopB and YopD are required for translocation of Yops across the eucaryotic cell membrane (9, 26). YopB induces the formation of pores in eukaryotic cell membranes (26), while YopK has been shown to be involved in regulating the translocation of Yops through this channel (27). Recently, Iriarte et al. demonstrated that TyeA was specifically required for the translocation of YopE and YopH (28).
Yersinia species growing in vitro at 37°C in calcium-depleted medium express and secrete large amounts of Yops into the surrounding media. This process requires gene products from the yscBCDEFGHIJKL operon (4, 24, 32, 33, 44) and the yscNOPQRSTU operon (2, 7, 17, 68), a lipoprotein termed VirG (3), and the lcrD gene product (42, 43). In the presence of calcium, virulence plasmid-encoded operons are transcriptionally downregulated (23, 59, 60) and Yop secretion is blocked (11, 33, 61). The downregulation of virulence plasmid operon transcription that occurs in conjunction with the block in Yop secretion requires the participation of YopD (67), LcrH (also called SycD [45, 64]), and LcrQ (also called YscM [47, 58]). Together, these gene products control the production and delivery of Yop proteins in response to specific triggering signals associated either with growth at 37°C in the absence of calcium in vitro or with contact with a eucaryotic cell in vivo.
The sequence elements responsible for targeting YopE and YopH for either secretion into the medium in vitro or translocation into a eucaryotic cell in vivo have been localized to amino-terminal regions of these proteins (54, 57). Sequences encoding the amino-terminal 15 to 17 amino acids of YopE (54, 57, 69), YopN (5), and YopH (57, 69) were shown to be sufficient to direct the export of hybrid reporter proteins; however, translocation of either YopE or YopH hybrid proteins into a eucaryotic cell required a larger amino-terminal region that contained the binding site for a chaperone-like accessory protein termed a specific Yop chaperone (Syc) (65). Recently, Cheng et al. (10) demonstrated that YopE of Y. enterocolitica possesses two independent secretion mechanisms, each of which is sufficient but not required for the secretion of YopE hybrid proteins. One secretion signal is found within the sequence encoding the first 15 amino acids of YopE, and the second is located downstream, between residues 15 and 100. The function of the second secretion signal is dependent upon a functional SycE protein, the specific Yop chaperone for YopE (65). Most recently, Anderson and Schneewind (5) provided evidence that the secretion signal targeting YopE hybrids containing the coding region for only the amino-terminal 15 amino acids of YopE appears to be encoded in the mRNA sequence rather than the peptide sequence, thus suggesting a cotranslational mechanism for Yop secretion.
In addition to the SycE protein, specific Yop chaperones for YopH (SycH [64, 69]) and YopD (SycD [64]) have also been identified and characterized. Syc-like proteins have also been identified in other bacteria equipped with type III secretion pathways (20, 22, 64). Efficient secretion of the protein recognized by the Syc or Syc-like protein is dependent upon the chaperone and the binding site for the chaperone on the secreted protein. The binding site for SycE and SycH has been localized to within amino acids 15 to 70 of YopE and YopH (57, 69), respectively. The presence of SycE has been shown to protect YopE from proteolytic degradation within the bacterial cell; thus, the Syc chaperones may also have a protective function in addition to their specific role in Yop secretion (21).
The present report investigates the role of the yscB gene product (24, 32) in Yop secretion and in the regulation of Yop secretion in Y. pestis. We constructed and characterized a Y. pestis strain carrying a nonpolar deletion in yscB. Our data indicate that YscB, like YopN, TyeA, and LcrG, is required to prevent Yop secretion prior to reception of the proper secretion-triggering signal. Specifically, YscB appears to function as a specific chaperone for YopN in Y. pestis.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
Bacterial strains and plasmids are described in Table 1 and Fig. 1. Y. pestis strains were routinely grown in heart infusion broth or on tryptose blood agar base plates (Difco Laboratories, Detroit, Mich.). For growth curves, Y. pestis strains were cultured in the defined liquid medium TMH (23). In this medium, low-Ca2+ response (LCR) yersiniae require added Ca2+ for full growth yield at 37°C. Escherichia coli strains were grown in L broth or on L agar. Bacteria with resistance to antibiotics were grown in the presence of the appropriate antibiotic(s) at a final concentration of 25 μg/ml (chloramphenicol and kanamycin) or 50 μg/ml (ampicillin and streptomycin).
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Construction and propertiesb | Source or reference |
|---|---|---|
| E. coli strains | ||
| DH5αF′ | F′ endA1 hsdR17 (rK− mK+) supE44 thi-1 recA1 gyrA (Nalr) relA1 Δ(lacZYA-argF)U169 deoR [φ80dlacΔ(lacZ)M15] | GIBCO-BRL |
| SY327 (λpir) | Δ(lac-pro) argE(Am) rif nalA recA56 | 34 |
| XL1-Blue | endΔ1 hsdR17 (rK− mK+) supE44 thi-1 λ− recA1 gyrA96 (Nalr) relA1 (Δlac) [F′ proAB+ lacIqZΔM15::Tn10 (Tcr)] | Stratagene |
| Y. pestis strainsa | ||
| KIM8 | pCD1 pPCP1− (Pla−) pMT1 | 23 |
| KIM10 | pCD1− (Lcr−) pPCP1− (Pla−) pMT1 | 23 |
| KIM5-3001 | Smr pCD1 (Lcr+) pPCP1 pMT1 | 30 |
| KIM5-3001.5 | Smr pCD1 (ΔlcrG [aa 39–53]) pPCP1 pMT1 | 56 |
| KIM5-3001.6 | Smr pCD1 (ΔyopN [aa 48–197]) pPCP1 pMT1 | 44 |
| KIM5-3001.P1 | Smr pCD1 (ΔyscB [aa 61–125]) pPCP1 pMT1 | This study |
| KIM8-3002 | Smr pCD1 (Lcr+) pPCP1− (Pla−) pMT1 | S. C. Straley |
| KIM8-3002.P2 | Smr pCD1 (ΔyopN [aa 2–10]) pPCP1− (Pla−) pMT1 | This study |
| KIM8-3002.P3 | Smr pCD1 (ΔyopN [aa 6–25]) pPCP1− (Pla−) pMT1 | This study |
| KIM8-3002.P4 | Smr pCD1 (ΔyopN [aa 21–40]) pPCP1− (Pla−) pMT1 | This study |
| KIM8-3002.P5 | Smr pCD1 (ΔyopN [aa 51–85]) pPCP1− (Pla−) pMT1 | This study |
| KIM8-3002.P6 | Smr pCD1 (ΔyopN [aa 6–100]) pPCP1− (Pla−) pMT1 | This study |
| KIM8-3002.P7 | Smr pCD1 (ΔyopN [aa 48–197]) pPCP1− (Pla−) pMT1 | This study |
| Plasmids | ||
| pBluescript II SK(−) | Cloning vector; Apr | Stratagene |
| pBCKS− | Cloning vector; Cmr | Stratagene |
| pUK4134 | Suicide vector; Apr | 55 |
| pTRC99a | Expression vector; Apr | Pharmacia |
| pYSCB1 | 1.7-kb XhoI-BamHI fragment of pPH11 (25) cloned into pBluescriptII SK(−)c; Apr | This study |
| pΔYSCB1 | pYSCB1 digested with HpaI and religated with insertion of an 8-bp PstI linker (5′-GCTGCAGC-3′), resulting in the elimination of a 194-bp Hpa fragment within yscB | This study |
| pUK4134.P1 | 2.15-kb PvuII fragment of pΔYSCB1 carrying ΔyscB (aa 61–125) cloned into the unique EcoRV site of pUK4134; Apr | This study |
| pYOPN1 | 986-bp ClaI-Eco47III fragment of pGP2 (42) cloned into ClaI-EcoRV-digested pBCKSII−; Cmr | This study |
| pYOPN2 | 2.1-kb BamHI-BstBI fragment of pGP2 (42) cloned into BamHI-ClaI-digested pBluescript SK(−); Apr | This study |
| pUK4134.6 | 2.2-kb EcoRV fragment of pΔYopN.1 carrying ΔyopN (aa 48–197) cloned into pUK4134; Apr | 44 |
| pYSCB6×HIS | 440-bp PCR fragment generated with primers YscB1 and YscB2, digested with NcoI and BamHI, and cloned into NcoI-BamHI-digested pTRC99a | This study |
All Y. pestis strains are Pgm− (62). Native plasmids of Y. pestis include the LCR plasmid pCD1 (6, 16, 23), the Pla-encoding pPCP1 (59), and pMT1 (46), which encodes the capsular protein.
Numbers in brackets give the amino acids (aa) deleted from the protein.
See Fig. 1 for restriction sites at ends of cloned DNA.
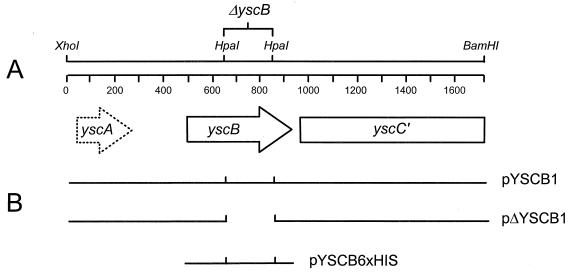
FIG. 1.
Physical and genetic maps of the yscBC′ region of the Y. pestis plasmid pCD1. The approximately 1.7-kb XhoI-BamHI fragment contains the yscB gene and a portion of the upstream yscC gene. In Y. enterocolitica an additional putative gene, designated yscA, was identified upstream of YscB. HpaI restriction endonuclease sites were utilized to create an in-frame deletion in yscB. Plasmids pYSCB1, pΔYSCB1, and pYSCB6×HIS were used in complementation studies.
DNA methods.
Cloning methods, including the use of restriction endonucleases and T4 DNA ligase, were performed as described by Maniatis et al. (31) with modifications as noted. Plasmid DNA was isolated by the method of Kado and Liu (29), by the PERFECT Prep method (5 Prime→3 Prime, Inc., Boulder, Colo.) or with Qiagen (Chatsworth, Calif.) columns. DNA fragments were isolated and purified from agarose gels by using the Qiaex DNA purification kit (Qiagen). Electroporation of E. coli and Y. pestis was carried out as previously described (43). PCR (by the technique described in reference 37) was performed by using 21- to 48-nucleotide primers and 30 cycles of amplification. Unless stated otherwise, denaturing, annealing, and extending conditions were 94°C for 1 min, 50°C for 1 min, and 72°C for 1 min, respectively. Double-stranded DNA was sequenced by the University of Miami School of Medicine DNA sequencing core facility by using a DyeDeoxy Terminator Cycle Sequencing kit and an ABI model 373A DNA sequencer (Applied Biosystems, Foster City, Calif.). Nucleotide sequences were analyzed with Intelligenetics computer software (IntelliGenetics, Mountain View, Calif.).
Construction of a yscB deletion mutant.
A YscB-encoding 1.66-kb XhoI-BamHI restriction endonuclease fragment of plasmid pPH11 (24) was inserted into XhoI-BamHI-digested pBluescript SKII(−) (Stratagene, La Jolla, Calif.) downstream of the vector lac promoter, generating plasmid pYSCB1 (Fig. 1). Plasmid pΔYSCB1, carrying a 195-bp in-frame deletion in yscB, was constructed by HpaI restriction endonuclease digestion and insertion of an 8-bp PstI linker (New England Biolabs, Beverly, Mass.) to maintain the yscB reading frame (Table 1). pBluescript SKII(−) PvuII restriction endonuclease sites flanking the ΔyscB-encoding XhoI-BamHI fragment were utilized to insert the fragment into the EcoRV site of the suicide vector pUK4134, generating plasmid pUK4134.GP1 (Table 1). This plasmid was introduced into Y. pestis KIM5-3001 by electroporation, and recipient bacteria that had integrated the clone into pCD1 by homologous recombination were selected for by their resistance to ampicillin, as previously described (55). Following passage under nonselective conditions to allow a second crossover, clones which had resolved the cointegrate by excision of the vector sequences were selected for by their resistance to streptomycin. Streptomycin-resistant resolvants were screened for the correct deletion by PCR and restriction endonuclease digestion. Y. pestis KIM5-3001.P1 contained the correct in-frame deletion within pCD1 (Table 1) and was utilized for further study.
Construction of yopN deletion mutants.
Deletions of yopN sequences encoding amino acid residues 2 to 10, 6 to 25, 21 to 40, 51 to 85, and 6 to 100 were constructed by the PCR-ligation-PCR technique (1). Initial PCR amplifications (set A) paired the oligonucleotide primer YopNH1 (5′-CTGGGACAAGCTTATATTGG-3′) with 2-10A (5′-AATACCCCGCTGCATAAT-3′), 6-25A (5′-ATCGTAAATCAGACTCTG-3′), 21-40A (5′-ATAGTCAGCGGCACTCTG-3′), 51-85A (5′-CAGGTTAATCAATACCTT-3′), and 6-100A (5′-CAGAATGTGAGTGAGCTG-3′). A second set (set B) of PCRs paired the vector T3 primer (5′-AATTAACCCTCACTAAAGGG-3′) with 2-10B (5′-CATAACTACTCCCTGAGA-3′), 6-25B (5′-ATGAAGCGTCGTCATAAC-3′), 21-40B (5′-CTCTGGACGCTCATTATG-3′), 51-85B (5′-AGCTATAGACTGCAGAGT-3′), and 6-100B (5′-ATGAAGCGTCGTCATAAC-3′). The resultant amplification products were purified from agarose gels and phosphorylated by using T4 polynucleotide kinase, and the respective set A and set B amplification products were ligated together by using T4 DNA ligase (New England Biolabs). Ligated fragments were subsequently used as a template for a second amplification reaction using only the outside primers YopNH1 and T3. The resultant amplification products, which contained defined in-frame deletions within yopN, were purified from agarose gels, digested with ApaI and HindIII, and inserted into ApaI-HindIII-digested pYOPN1, creating plasmids pYOPN1.2 (with amino acids 2 to 10 deleted [Δ2-10]), pYOPN1.3 (Δ6-25), pYOPN1.4 (Δ21-40), pYOPN1.5 (Δ51-85) and pYOPN1.6 (Δ6-100). To increase the amount of homologous flanking DNA surrounding each deletion, a ClaI-BglII fragment of each plasmid was cloned into ClaI-BglII-cut pYOPN2 (Table 1), resulting in plasmids pYOPN2.2 (Δ2-10), pYOPN2.3 (Δ6-25), pYOPN2.4 (Δ21-40), pYOPN2.5 (Δ51-85), and pYOPN2.6 (Δ6-100), which provided a minimum of 650 to 1,100 bp of flanking DNA on either side of the deletions. PvuII fragments containing the deletion and flanking DNA were then inserted into EcoRV-digested pUK4134. The resulting suicide plasmids pUK4134.P2 (Δ2-10), pUK4134.P3 (Δ6-25), pUK4134.P4 (Δ21-40), pUK4134.P5 (Δ51-85), pUK4134.P6 (Δ6-100), and pUK4134.P7 (Δ48-197) were utilized to move the yopN deletions into plasmid pCD1 of Y. pestis KIM8-3002 essentially as described for the yscB deletion mutant. The resultant yopN deletion mutants KIM8-3002.P2 (Δ2-10), KIM8-3002.P3 (Δ6-25), KIM8-3002.P4 (Δ21-40), KIM8-3002.P5 (Δ51-85), KIM8-3002.P6 (Δ6-100), and KIM8-3002.P7 (Δ48-197) were used in protein cross-linking and immunoprecipitation procedures.
Cell fractionation.
Yersinia strains were grown at 26°C in TMH with or without calcium (2.5 mM CaCl2) to an optical density at 620 nm of 0.15 to 0.20. Cultures were then shifted to 37°C for an additional 5 h. Cell pellets and culture supernatants were separated by centrifugation at 12,200 × g for 10 min at 4°C. Cell pellets were washed once with ice-cold buffer A (20 mM Tris-HCl, pH 8.0) and pelleted by centrifugation. Culture supernatant proteins were precipitated with 10% (vol/vol) trichloroacetic acid (TCA) (for 1 h, on ice) and collected by centrifugation at 12,200 × g for 10 min at 4°C. Periplasmic fractions were prepared by the osmotic shock procedure as described by Nossal and Heppel (39). Briefly, cell pellets (0.1 g [wet weight]) were resuspended in 4 ml of 33 mM Tris-HCl, pH 7.1, containing 20% (wt/vol) sucrose, and EDTA was added to a 0.1 mM final concentration. After being stirred for 10 min at room temperature (RT), cells were collected by centrifugation at 12,200 × g for 10 min at 4°C, and the cell pellet was rapidly dispersed in 8 ml of ice-cold 0.5 mM MgCl2. Following osmotic shock the cell pellets were collected by centrifugation at 12,200 × g for 10 min at 4°C, and the supernatants containing the osmotic shock proteins were TCA precipitated. To obtain membrane and cytoplasmic (soluble) fractions, cell pellets were resuspended in buffer A and lysed by a single passage through a chilled French pressure cell at 20,000 lb/in2. Unlysed whole cells and large debris were removed by centrifugation at 8,800 × g for 5 min at 4°C. Total membranes were separated from the soluble fractions of the cleared lysates by ultracentrifugation in a TLA-100.3 rotor at 263,800 × g for 20 min at 4°C (Beckman Instruments, Fullerton, Calif.). Membrane preparations were resuspended and stored in buffer A.
YscB overexpression, purification, and anti-YscB antibody preparation.
A nucleotide sequence encoding six consecutive histidine residues was added to the portion of yscB encoding carboxyl-terminal domain by using PCR methodologies and primers YscB1 (5′-ATATACCATGGAAAATTTACTAAAAAACTTGGCAGCC-3′) and YscB2 (5′-CGGGATCCTTAATGGTGATGGTGATGGTGATTCCACCCCACGCGAGAC-3′). The resultant PCR product was digested with NcoI and BamHI restriction endonucleases and ligated into NcoI/BamHI-digested pTrc99a (Pharmacia, Piscataway, N.J.), generating the expression plasmid pYSCB6×HIS (Table 1). Plasmid pYSCB6×HIS was electroporated into E. coli DH5α for high-level expression of polyhistidine-tagged YscB (YscB6×HIS). The resultant strain was grown at 37°C in Luria-Bertani medium to an optical density at 620 nm of 0.5, and expression of YscB6×HIS was induced by addition of isopropyl-β-d-thiogalactopyranoside (IPTG) to 0.5 mM followed by incubation at 37°C for an additional 4 h. Cultures were harvested by centrifugation at 12,200 × g for 10 min at 4°C. Cell pellets were resuspended in 1× binding buffer (Novagen, Inc., Madison, Wis.) and lysed by two passages through a chilled French pressure cell at 20,000 lb/in2. Unlysed cells, large debris, and inclusion bodies were removed by centrifugation at 8,800 × g for 5 min at 4°C. The YscB6×HIS protein was found almost exclusively in the 8,800 × g pellet, indicating that the protein was primarily present in inclusion bodies. Pellet fractions containing YscB6×HIS were solubilized with 1× binding buffer containing 6 M urea, insoluble material was removed by ultracentrifugation at 263,800 × g for 20 min at 4°C, and the YscB6×HIS protein was purified to homogeneity using the His-Bind Resin and Buffer kit (Novagen). Polyclonal antisera specific for YscB were raised in female New Zealand White rabbits by using the purified YscB6×HIS protein (Animal Pharm Services, Healdsburg, Calif.). Polyclonal antisera specific for YscB were used in immunoprecipitations and to detect YscB in immunoblot analyses at a dilution of 1:10,000.
SDS-PAGE and immunoblotting.
Volumes of cellular fractions corresponding to equal numbers of bacteria were mixed 1:1 (vol/vol) with 2× electrophoresis sample buffer and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblot analysis essentially as previously described (43). Those samples to be analyzed with polyclonal antisera specific for YscB were electrophoresed on 14% (wt/vol) acrylamide gels. Y. pestis LCR proteins were visualized as previously described (43), by using polyclonal antisera or purified antibodies specific for YopM, V antigen, YopN, YopE, or YscB.
Cross-linking and immunoprecipitation of YscB and YopN.
Cultures grown in 25 ml of TMH with or without calcium were harvested by centrifugation at 12,200 × g for 10 min at 4°C, washed once with 20 mM HEPES–100 mM NaCl (pH 7.4), resuspended in 3 ml of immunoprecipitation buffer A (20 mM HEPES, 100 mM NaCl, 0.1% Triton X-100 [pH 7.4]), and lysed by two passages through a chilled French pressure cell at 20,000 lb/in2. Unlysed cells, large debris, and membranes were removed by ultracentrifugation in a TLA-100.3 rotor at 263,800 × g for 20 min at 4°C (Beckman Instruments). Soluble proteins (500 μl) were cross-linked with the thio-cleavable amine reactive cross-linker dithiobis (succinimidyl propionate) (DSP) (Pierce Chemical Co., Rockford, Ill.) at a final concentration of 1 mM (20 mM stock solution in dimethyl sulfoxide) for 20 min at RT. Cross-linking reactions were quenched by addition of Tris-HCl (pH 8.0) to a final concentration of 50 mM. Samples were immunoprecipitated with 10 μl of rabbit polyclonal antisera specific for YopN, YscB, or YopE or with preimmune serum from the corresponding rabbit for 2 h at 4°C. Antigen-antibody complexes were collected by addition of 100 μl of 10% (wt/vol) protein A-Sepharose CL-4B (Pharmacia) in immunoprecipitation buffer B (20 mM HEPES, 250 mM NaCl, 0.5% Triton X-100, 0.1% SDS [pH 7.4]) for 2 h at 4°C. Protein A-Sepharose antigen-antibody complexes were pelleted by centrifugation in a microcentrifuge for 15 s at RT, washed three times with 1 ml of immunoprecipitation buffer B, eluted with 100 μl of electrophoresis sample buffer containing 5% β-mercaptoethanol (cleaves DSP cross-links), boiled for 3 min, and subjected to SDS-PAGE and immunoblot analysis.
RESULTS
Construction of a nonpolar yscB deletion mutant.
To investigate the role of YscB in the expression and delivery of plasmid pCD1-encoded virulence factors, we constructed a 195-bp in-frame internal deletion within yscB that was designed to specifically disrupt the structure of the yscB gene product without having polar effects on the expression of downstream genes (Fig. 1). The mutation was constructed in plasmid pΔYSCB1 (Fig. 1 and Table 1) and introduced into pCD1 by allelic exchange (55). The resultant strain, KIM5-3001.P1 (Table 1), should express a yscB gene product lacking amino acids 61 to 125, but containing three additional alanine residues (amino acid residues 61 to 63 of the deleted gene product) due to the insertion of an 8-bp PstI linker. Plasmids pYSCB1, pΔYSCB1, and pYSCB6×HIS were used in complementation studies.
Growth phenotype of the yscB deletion mutant.
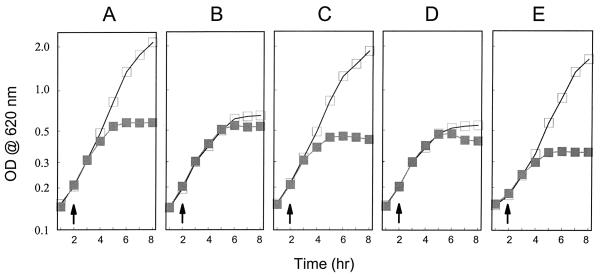
Figure 2 shows growth curves for the parent strain Y. pestis KIM5-3001, the yscB deletion mutant Y. pestis KIM5-3001.P1, and the yscB deletion mutant complemented with plasmid pYSCB1, pΔYSCB1, or pYSCB6×HIS. All strains exhibited full growth yield at 26°C in the presence or absence of calcium (data not shown). The parent strain KIM5-3001 showed a calcium-dependent growth pattern typical for the TMH growth medium (23). In contrast, the yscB deletion strain KIM5-3001.P1 underwent growth restriction at 37°C both in the presence and in the absence of calcium (calcium-blind or temperature-sensitive growth phenotype [23]). These data suggest that the wild-type LCR growth phenotype is dependent upon a functional yscB gene product. Providing plasmid pYSCB1 (yscBC′) or pYSCB6×HIS (yscB) in trans resulted in complete restoration of the wild-type growth phenotype, while providing plasmid pΔYSCB1 (yscΔBC′) in trans had no effect on the growth phenotype. These results indicate that the growth defects associated with the yscB deletion strain were due solely to disruption of yscB and not to polar effects on downstream genes or to spontaneous mutations in other ysc or lcr loci.
FIG. 2.
Growth of the parent strain Y. pestis KIM5-3001 (A), the yscB deletion strain KIM5-3001.P1 (B), and KIM5-3001.P1 complemented with plasmid pYSCB1 (C), pΔYSCB1 (D), or pYSCB6×HIS (E). Y. pestis strains were grown in the presence (open squares) or absence (shaded squares) of calcium in the defined medium TMH. The temperature was shifted from 26 to 37°C when the optical density at 620 nm of the culture reached approximately 0.2 (arrows).
Secretion of YopM, YopN, and V antigen by the yscB deletion mutant.
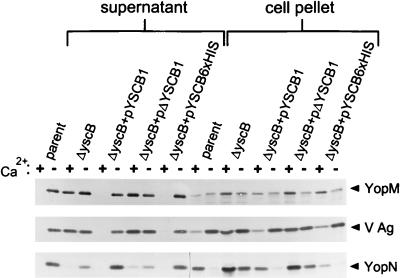
Secretion of YopM, YopN, and V antigen was analyzed by immunoblot analysis of TCA-precipitated culture supernatant proteins from the parent strain, Y. pestis KIM5-3001, the yscB deletion mutant Y. pestis KIM5-3001.P1, and the yscB deletion mutant complemented with pYSCB1, pΔYSCB1, or pYSCB6×HIS in trans. Y. pestis strains were grown in TMH with or without calcium at 26°C and then shifted to 37°C for 5 h. The parent strain, KIM5-3001, secreted YopM, YopN, and V antigen into the culture supernatant when grown at 37°C in the absence of calcium (Fig. 3); however, as expected, no secretion of Yops or V antigen was detected in the presence of calcium. The yscB deletion mutant grown at 37°C in the presence or absence of calcium secreted YopM and V antigen at levels comparable to those of the parent strain grown at 37°C in the absence of calcium. These results demonstrate that secretion of Yops and V antigen by the yscB deletion mutant was no longer blocked in the presence of calcium (calcium-blind secretion phenotype). Interestingly, secretion of YopN by the yscB deletion mutant grown at 37°C in the presence or absence of calcium was specifically reduced compared to that by the parent strain grown in the absence of calcium. In contrast, the amount of YopN found associated with the cell pellet was increased in the yscB deletion mutant, indicating that the reduction in YopN found in the culture supernatant was most likely due to a reduction in YopN export and not to a defect in YopN expression.
FIG. 3.
Immunoblot analysis of YopM, V antigen, and YopN in the supernatant and cell pellet fractions from Y. pestis KIM5-3001 (parent), the yscB deletion strain KIM5-3001.P1, (ΔyscB) and KIM5-3001.P1 complemented with plasmid pYSCB1, pΔYSCB1, or pYSCB6×HIS. Strains were grown in either the presence (+) or in the absence (−) of calcium at 37°C in the defined medium TMH. Polyclonal antisera specific for YopM, V antigen, and YopN were used to detect the presence of these proteins in the supernatant and cell pellet fractions.
Providing the yscB-complementing plasmid pYSCB1 or pYSCB6×HIS in trans to the yscB deletion mutant completely restored calcium-regulated expression and secretion of YopM, YopN, and V antigen; however, plasmid pΔYSCB1, which does not express a functional yscB gene product, failed to complement the expression and secretion defects associated with this strain. These data indicate that the reduction in YopN secretion, as well as the defects in the regulation of Yop and V antigen secretion, was due solely to disruption of yscB and not to polar effects on downstream genes or to spontaneous mutations in other ysc or lcr loci. Furthermore, the ability of pYSCB6×HIS to complement the yscB deletion mutant indicates that the addition of six histidine residues to the carboxyl terminus of YscB does not affect its ability to complement YscB function.
Secretion of YopM, YopN, and V antigen by the yscB deletion mutant and other mutants defective in the regulation of Yop secretion.
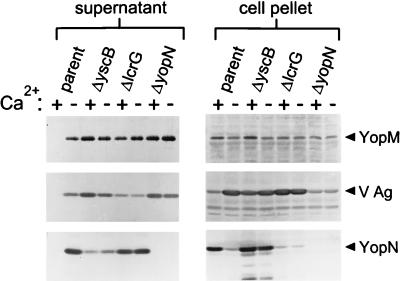
To determine whether the reduction of YopN secretion was a unique property of the yscB deletion mutant or a property common to mutants defective in calcium-regulated Yop secretion, the secretion of YopM, YopN, and V antigen was analyzed in the parent strain (KIM5-3001) and in the yscB (KIM5-3001.P1), lcrG (KIM5-3001.5), and yopN (KIM5-3001.6) deletion mutants (Fig. 4). The parent strain secreted YopM, YopN, and V antigen only in the absence of calcium; however, as expected, the three calcium-blind mutants secreted these proteins both in the presence and in the absence of calcium. The yscB deletion mutant secreted reduced levels of YopN in comparison to the parent strain and the lcrG deletion mutant, while the yopN deletion mutant failed to express a stable yopN gene product. The amount of YopN found in the cell pellet of the yscB deletion mutant was similar to the amount found in that of the parent strain grown under conditions in which Yop secretion is blocked (at 37°C in the presence of calcium). These data demonstrate that YscB was specifically required for the efficient secretion of YopN in Y. pestis. In addition, YscB was required directly or indirectly to prevent Yop secretion in the presence of calcium.
FIG. 4.
Immunoblot analysis of YopM, V antigen, and YopN in the supernatant and cell pellet fractions from Y. pestis KIM5-3001 (parent), the yscB deletion strain KIM5-3001.P1 (ΔyscB), the lcrG deletion strain KIM5-3001.5 (ΔlcrG), and the yopN deletion strain KIM5-3001.6 (ΔyopN). Strains were grown in either the presence (+) or in the absence (−) of calcium at 37°C in the defined medium TMH. Polyclonal antisera specific for YopM, V antigen, and YopN were used to detect the presence of these proteins in the supernatant and cell pellet fractions.
Identification and localization of the yscB gene product.
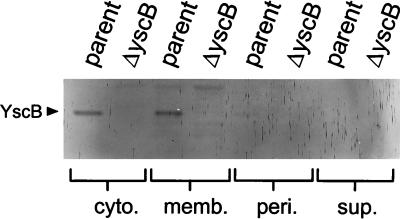
Polyclonal antiserum raised against a polyhistidine-tagged yscB gene product was utilized to identify YscB in immunoblots prepared from fractionated Y. pestis cultures. Cytoplasmic, membrane, periplasmic, and culture supernatant fractions prepared from both the parent strain and the yscB deletion mutant were analyzed for the presence of YscB (Fig. 5). The approximately 15.5-kDa yscB gene product localized equally in the cytoplasmic and membrane fractions of the parent strain; however, no YscB was found in the periplasmic fraction or the culture supernatant fraction of the parent strain or in any fraction of the yscB deletion mutant. These results, in conjunction with the lack of a classical Sec-dependent secretion signal and no predicted transmembrane domains, indicate that YscB is a cytoplasmic or peripheral membrane protein that is not exported across the inner membrane.
FIG. 5.
Cellular localization of YscB. Fractionation was performed on Y. pestis KIM5-3001 (parent) and the yscB deletion strain KIM5-3001.P1 (ΔyscB), which had been grown for 5 h at 37°C in the absence of calcium. Cytoplasmic (cyto.), membrane (memb.), periplasmic (peri.), and culture supernatant (sup.) fractions were analyzed by SDS-PAGE and immunoblot analysis.
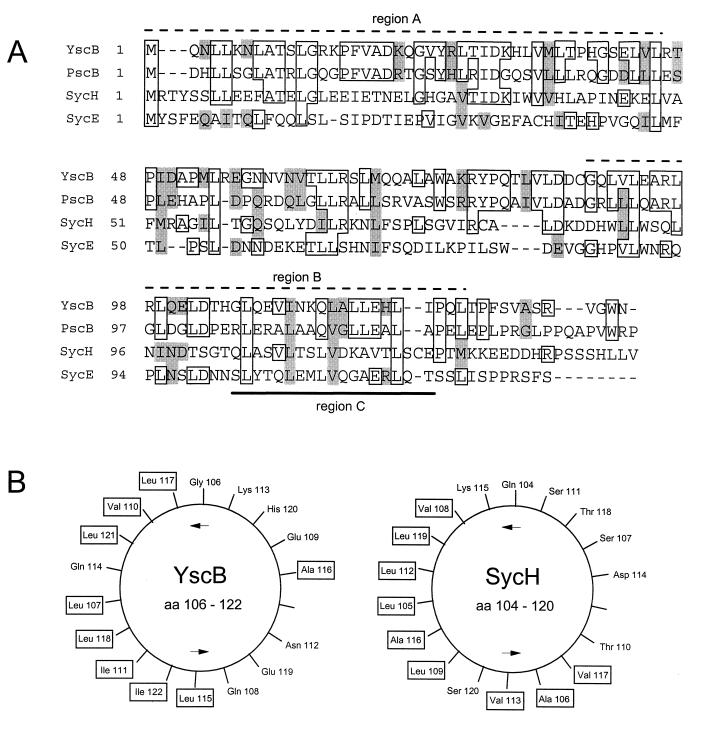
YscB shows extensive similarities with PscB of Pseudomonas aeruginosa and SycE and SycH of Y. enterocolitica, Y. pseudotuberculosis, and Y. pestis.
The specific effect of the yscB deletion on the secretion of YopN was similar to effects reported for sycE (yerA), sycH, and sycD mutant strains on their cognate Yops. Therefore, we attempted to align the amino acid sequence of YscB with those of the other reported Syc and Syc-like proteins (Fig. 6A). YscB showed extensive similarities with SycE and SycH, the specific Yop chaperones for YopE and YopH, respectively, of Y. enterocolitica, Y. pseudotuberculosis, and Y. pestis. The similarity to SycH was highest in the amino-terminal region (33% identity in region A; Fig. 6A), while the greatest similarity to SycE was in the carboxyl-terminal region (30% identity in region B). YscB showed the greatest overall similarity to PscB of P. aeruginosa (41% identity), whose gene is closely linked to gene products which are involved in the secretion of exoenzyme S through a recently discovered type III secretion system (20, 70). Interestingly, a homolog of YopN has also been identified in P. aeruginosa (PopN) and is believed to be associated with the same secretion pathway (20).
FIG. 6.
(A) Alignment of Y. pestis YscB with PscB of P. aeruginosa, SycH of Y. pseudotuberculosis, and SycE of Y. pestis. Boxed residues, sequence identity; shaded residues, related or similar amino acids residues; overlined sequences (dashed lines), regions of YscB with the highest (≥30%) identity to SycH (region A) and SycE (region B); underlined sequence, region predicted to form an amphipathic helix (region C); dashes within sequences, gaps introduced to optimize alignment. (B) Helical wheel representation of region C of YscB and SycH. Hydrophobic amino acid residues are boxed.
YscB also shares many of the common structural and functional features of the Syc and Syc-like family of proteins. Like the other Syc and Syc-like proteins YscB is a small (15.5-kDa) cytoplasmic (or peripheral) protein with a conserved carboxyl-terminal leucine repeat motif and a putative amphipathic alpha-helix (Fig. 6B); however, unlike the previously characterized Syc and Syc-like proteins, YscB is a basic protein (pI 9.3). These data, in conjunction with the unique effect of the yscB deletion on the export of YopN, indicate that YscB may function as a specific chaperone for YopN in Y. pestis.
Interaction of YscB and YopN.
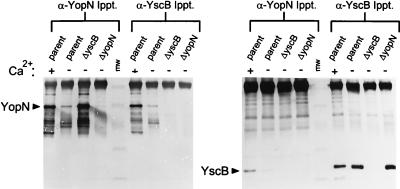
In order to confirm the role of YscB as a chaperone for YopN, we attempted to identify an interaction between YscB and YopN using protein cross-linking reagents and immunoprecipitation. Soluble proteins (cytoplasmic and periplasmic proteins) from the parent strain, Y. pestis KIM5-3001, the yscB deletion mutant (KIM5-3001.P1), and the yopN deletion mutant (KIM5-3001.6) grown at 37°C in the presence or absence of calcium were cross-linked by the addition of the thio-cleavable amine reactive cross-linker DSP to a final concentration of 1 mM for 30 min at RT. Polyclonal antisera specific for YopN, YscB, or YopE were used to immunoprecipitate the corresponding protein and proteins cross-linked with the immunoprecipitated protein (coprecipitating proteins). Cross-linked immunoprecipitates were collected by using protein A-sepharose CL-4B, washed, cleaved by the addition of solubilization buffer containing 5% β-mercaptoethanol, and analyzed by SDS-PAGE and immunoblot analysis (Fig. 7).
FIG. 7.
Coimmunoprecipitation of YopN and YscB following cross-linking with DSP. Soluble extracts from the parent strain, Y. pestis KIM5-3001, grown in the presence (+) or absence (−) of calcium, the yscB deletion strain KIM5-3001.P1 (ΔyscB) grown in the absence of calcium, and the yopN deletion strain KIM5-3001.6 (ΔyopN) grown in the absence of calcium were cross-linked with DSP (1 mM) for 20 min at RT. Samples were processed as described in Materials and Methods, immunoprecipitated with antisera specific for YopN or YscB, and analyzed by SDS-PAGE and immunoblotting with antisera specific for YopN or YscB. DSP cross-links were broken prior to SDS-PAGE analysis by boiling samples in the presence of 5% β-mercaptoethanol. The locations of YopN and YscB are shown by arrows. MW, molecular size standards (45, 32, 25, 16, and 6.5 kDa). Ippt., immunoprecipitation.
Antisera specific for YopN immunoprecipitated YopN from the parent strain and the yscB deletion strain (Fig. 7). The amount of YopN immunoprecipitated from the soluble fraction of the parent strain grown in the absence of calcium was significantly reduced due to the efficient export of YopN under these conditions. Antisera specific for YscB immunoprecipitated approximately equal amounts of YscB from both the parent strain and the yopN deletion strain grown either in the presence or in the absence of calcium. YscB coprecipitated with the immunoprecipitated YopN in the parent strain; however, no YscB was found in the corresponding immunoprecipitate from the yopN deletion strain. Similarly, YopN coprecipitated with the immunoprecipitated YscB in the parent strain but not in the yscB deletion strain. No YopN or YscB was immunoprecipitated with preimmune sera or coprecipitated with control antisera specific for YopE (data not shown). In addition, no coprecipitation of YscB and YopN was observed in the absence of DSP cross-linking. These data indicate that protein cross-linking with DSP stabilized a complex of YopN and YscB that could be efficiently immunoprecipitated with antisera specific for either YscB or YopN, suggesting that these proteins are closely associated or directly interact with one another in Y. pestis.
Localization of the YscB binding region of YopN.
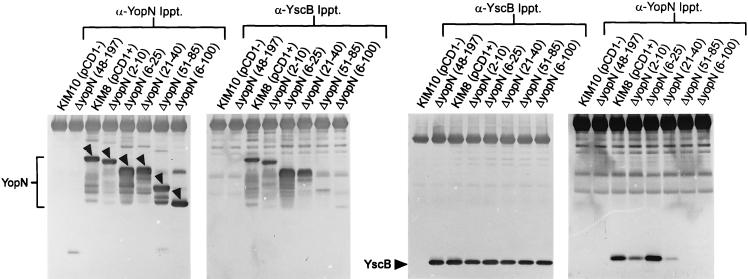
Previous studies with SycE and SycH in Y. enterocolitica and Y. pseudotuberculosis localized the Syc binding region to within amino acids 15 to 100 of YopE and YopH (57, 69), respectively. To begin to map the YscB binding region of YopN, a series of in-frame amino-terminal deletions within yopN were constructed and moved into plasmid pCD1 of Y. pestis KIM8-3002 (pPCP1−) by allelic exchange. Deletions eliminating the coding region for amino acids 2 to 10, 6 to 25, 21 to 40, 51 to 85, and 6 to 100 of yopN were constructed and used in cross-linking and immunoprecipitation studies to localize the YscB binding region of YopN (Fig. 8).
FIG. 8.
Localization of a region of YopN required for YscB binding. Soluble extracts from Y. pestis KIM10 (pPCP1− pCD1−), the yopN deletion strain Y. pestis KIM8-3002.P7 (pPCP1−), Y. pestis KIM8 (pPCP1− pCD1+), and derivatives of Y. pestis KIM8-3002 containing in-frame deletions in yopN eliminating the coding regions for amino acid residues 2 to 10 [ΔyopN (2-10)], 6 to 25 [ΔyopN (6-25)], 21 to 40 [ΔyopN (21-40)], 51 to 85 [ΔyopN (51-85)], and 6 to 100 [ΔyopN (6-100)] were cross-linked with DSP (1 mM) for 20 min at RT. Samples were processed as described in Materials and Methods, immunoprecipitated with antisera specific for YopN or YscB, and analyzed by SDS-PAGE and immunoblotting with antisera specific for YopN or YscB. DSP cross-links were broken prior to SDS-PAGE analysis by boiling samples in the presence of 5% β-mercaptoethanol. The locations of YopN and YscB are shown by arrows. Ippt., immunoprecipitation.
Cross-linking and immunoprecipitation experiments with Y. pestis KIM10 (pCD1− pPCP1−), Y. pestis KIM8 (pCD1+ pPCP1−), the yopN deletion strain KIM8-3002.P7 (pPCP1−), and pPCP1− strains containing in-frame internal deletions within the first 100 amino acids of YopN (Fig. 8 and Table 1) were carried out essentially as described for Fig. 7. Antisera specific for YopN immunoprecipitated YopN or the corresponding deleted derivative of YopN from each of the strains expressing a stable yopN gene product. Y. pestis KIM8 and the yopN deletion strain did not express a stable yopN gene product. YscB coprecipitated with the yopN gene products from Y. pestis KIM8 and the corresponding strains expressing yopN gene products lacking amino acids 2 to 10, 6 to 25, and 21 to 40 following cross-linking with DSP. No YscB coprecipitated with the yopN gene products lacking amino acids 51 to 85 or 6 to 100. Antisera specific for YscB immunoprecipitated approximately equal amounts of YscB from each of the pCD1+ strains of Y. pestis (Fig. 8). YopN or the corresponding deleted derivative of YopN coprecipitated with YscB in Y. pestis KIM8 and the corresponding strains expressing yopN gene products lacking amino acids 2 to 10, 6 to 25, and 21 to 40. A small amount of yopN gene product was also found associated with Y. pestis strains expressing a yopN gene product lacking amino acids 51 to 85 and 6 to 100; however, a similar background level of YopN was also found in control immunoprecipitations using either preimmune serum or antisera specific for YopE (data not shown). These results indicate that the region between amino acids 50 and 86 of YopN is required for the interaction of YopN and YscB.
DISCUSSION
Previous studies have suggested a role for the yscCDEFGIJKL gene products in the secretion of Yops in Y. enterocolitica, Y. pseudotuberculosis, and Y. pestis (4, 24, 32, 33, 44), while the yscH locus was recently shown to encode YopR (4). The present study examined the role of the yscB gene product in Yop secretion and in the regulation of Yop secretion in Y. pestis. A nonpolar yscB deletion mutant exhibited calcium-blind growth, secreted V antigen and YopM both in the presence and in the absence of calcium, and specifically secreted reduced amounts of YopN. The amino acid sequence and predicted secondary structure of YscB showed similarities to those of SycE (21, 65) and SycH (64), the specific Yop chaperones for YopE and YopH, respectively. Furthermore, it was demonstrated that YscB could be efficiently cross-linked to YopN and that this association could be prevented by deletions eliminating the regions encoding amino acids 51 to 85 or 6 to 100 of YopN. Together these results suggest that YscB binds to YopN within the cell and functions as a specific chaperone for YopN in Y. pestis.
Members of the Syc and Syc-like family of protein chaperones are generally small (15 to 20 kDa), acidic, cytoplasmic proteins that specifically bind to an amino-terminal region of their specific target Yop and share a conserved carboxyl-terminal motif consisting of a leucine-rich amphipathic helix (64, 66). YscB shares numerous characteristics with this family of proteins, including size (15.5 kDa), location (cytoplasmic or peripheral membrane protein), a carboxyl-terminal amphipathic helix (Fig. 6B), and a specific interaction with an amino-terminal region of its specific Yop (YopN); however, YscB is a basic protein (pI 9.3) that is required directly or indirectly for the calcium-dependent regulation of Yop secretion in addition to its role in the secretion of YopN. An additional characteristic of the Syc chaperones is that the genes encoding individual Syc chaperones are generally located in close proximity to the gene encoding their cognate Yop (66). In contrast, the yscB locus is the first gene in a multicistronic operon that predominately encodes essential components of the Yop secretion apparatus and is located approximately 8 kb from the yopN locus.
The exact function is not completely understood for any of the individual Syc proteins. Insertional or deletional inactivation of an individual Syc protein results in reduced secretion of the respective Yop in vitro (64, 65). In addition, deletion of the Syc binding region from YopE or YopH hybrid reporter proteins prevented translocation of the reporter proteins into eucaryotic target cells (21, 54, 57, 69). Recent evidence reported by Cheng et al. (10) indicates that the Syc binding region of YopE can function to target YopE for secretion in a SycE-dependent manner, independently of a previously identified SycE-independent secretion signal located at the extreme amino terminus of YopE (57, 69). Deletional mutagenesis of yerA (sycE) reduced YopE export approximately 88%, with the remaining YopE export presumably resulting from the Syc-independent amino-terminal secretion signal (5).
Deletional inactivation of the Y. pestis yscB locus did not prevent export of YopN; however, the amount of YopN exported was specifically reduced. These results mirror those found for YopE export in a sycE (yerA) mutant of Y. enterocolitica (10). In addition, the yscB deletion mutant lost its ability to block Yop secretion in the presence of calcium, a phenotype similar to that of a yopN deletion mutant (19, 63). This phenotype may be a direct effect of YscB loss or, more likely, may be due to the effect of YscB loss on the secretion and/or presentation of YopN. Interestingly, deletional inactivation of yscB only reduced the secretion of YopN; however, the ability of the yscB deletion strain to block Yop secretion in the presence of calcium was essentially lost, with the exception of a small degree of calcium-dependent secretion regulation for YopN itself (Fig. 3). This indicates either that YscB is necessary for presenting YopN in a conformation capable of blocking Yop secretion in the presence of calcium, that YscB is a direct participant in blocking Yop secretion, or that, in order to block Yop secretion, YopN must be targeted efficiently to the secretion apparatus.
It is noteworthy that Boland et al. (9) recently presented data indicating that YopN, unlike YopE, YopH, and YopM, is not translocated into the eucaryotic target cell. This conclusion was based upon the activity of truncated YopN-CyaA fusion proteins. If these data are confirmed by studies utilizing full-length YopN, it would suggest either that YscB differs in function from SycE and SycH or that YscB, SycE, and SycH share a common function and that the role of SycE and SycH in YopE and YopH translocation is indirect. Woestyn et al. (69) suggest that the Syc chaperones may function to prevent nonspecific interactions within the bacterial cell between Yops destined for translocation across the eucaryotic cell membrane and Yops that facilitate this translocation process, namely YopB and/or YopD. Thus, in the absence of the Syc chaperone, premature interaction of the Yop to be secreted with these or other proteins within the bacterial cell renders the interacting Yops incompetent for secretion. In a similar manner, YscB may prevent premature interactions of YopN with LcrG, TyeA, or other components involved in the regulation of Yop secretion. Interestingly, TyeA has recently been shown to bind to a carboxyl-terminal region of YopN (28). In addition, we have recently identified another protein (Orf2 [19, 63]) that also interacts with YopN within the cell (13). Analysis of these interactions will further our understanding of the mechanism and protein-protein interactions involved in the regulation of Yop secretion.
ACKNOWLEDGMENTS
This study was supported by Public Health Service Grant AI39575.
We thank Susan C. Straley (University of Kentucky) for the kind gift of rabbit anti-V antigen antibody, rabbit anti-YopM antibody, and Y. pestis KIM8-3002.
REFERENCES
- 1.Ali S A, Steinkasserer A. PCR-ligation-PCR mutagenesis: a protocol for creating gene fusions and mutations. BioTechniques. 1995;18:746–750. [PubMed] [Google Scholar]
- 2.Allaoui A, Woestyn S, Sluiters C S, Cornelis G R. YscU, a Yersinia enterocolitica inner membrane protein involved in Yop secretion. J Bacteriol. 1994;176:4534–4542. doi: 10.1128/jb.176.15.4534-4542.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allaoui A, Scheen R, Lambert de Rouvroit C, Cornelis G R. VirG, a Yersinia enterocolitica lipoprotein involved in Ca2+ dependency, is related to exsB of Pseudomonas aeruginosa. J Bacteriol. 1995;177:4230–4237. doi: 10.1128/jb.177.15.4230-4237.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allaoui A, Schulte R, Cornelis G R. Mutational analysis of the Yersinia enterocolitica virC operon: characterization of yscE, F, G, I, J, K required for Yop secretion and yscH encoding YopR. Mol Microbiol. 1995;18:343–355. doi: 10.1111/j.1365-2958.1995.mmi_18020343.x. [DOI] [PubMed] [Google Scholar]
- 5.Anderson D M, Schneewind O. A mRNA signal for the type III secretion of Yop proteins by Yersinia enterocolitica. Science. 1997;278:1140–1143. doi: 10.1126/science.278.5340.1140. [DOI] [PubMed] [Google Scholar]
- 6.Ben-Gurion R, Shafferman A. Essential virulence determinants of different Yersinia species are carried on a common plasmid. Plasmid. 1981;5:183–187. doi: 10.1016/0147-619x(81)90019-6. [DOI] [PubMed] [Google Scholar]
- 7.Bergman T, Erickson K, Galyov E, Persson C, Wolf-Watz H. The lcrB (yscN/U) gene cluster of Yersinia pseudotuberculosis is involved in Yop secretion and shows high homology to the spa gene clusters of Shigella flexneri and Salmonella typhimurium. J Bacteriol. 1994;176:2619–2626. doi: 10.1128/jb.176.9.2619-2626.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bliska J B, Guan K, Dixon J E, Falkow S. Tyrosine phosphate hydrolysis of host proteins by an essential Yersinia virulence determinant. Proc Natl Acad Sci USA. 1991;88:1187–1191. doi: 10.1073/pnas.88.4.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boland A, Sory M P, Iriarte M, Kerbourch C, Wattiau P, Cornelis G R. Status of YopM and YopN in the Yersinia Yop virulon: YopM of Y. enterocolitica is internalized inside the cytosol of PU5-1.8 macrophages by the YopB, D, N delivery apparatus. EMBO J. 1996;15:5191–5201. [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng L W, Anderson D M, Schneewind O. Two independent type III secretion mechanisms for YopE in Yersinia enterocolitica. Mol Microbiol. 1997;24:757–765. doi: 10.1046/j.1365-2958.1997.3831750.x. [DOI] [PubMed] [Google Scholar]
- 11.Cornelis G R, Biot T, Lambert de Rouvroit C, Michiels T, Mulder B, Sluiters C, Sory M-P, Van Bouchaute M, Vanooteghem J-C. The Yersinia yop regulon. Mol Microbiol. 1989;3:1455–1459. doi: 10.1111/j.1365-2958.1989.tb00129.x. [DOI] [PubMed] [Google Scholar]
- 12.Cornelis G R, Wolf-Watz H. The Yersinia Yop virulon: a bacterial system for subverting eukaryotic cells. Mol Microbiol. 1997;23:861–867. doi: 10.1046/j.1365-2958.1997.2731623.x. [DOI] [PubMed] [Google Scholar]
- 13.Day, J. B., and G. V. Plano. Unpublished data.
- 14.Fallman M, Andersson K, Hakansson S, Magnusson K E, Stendahl O, Wolf-Watz H. Yersinia pseudotuberculosis inhibits Fc receptor-mediated phagocytosis in J774 cells. Infect Immun. 1995;63:3117–3124. doi: 10.1128/iai.63.8.3117-3124.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fallman M, Persson C, Wolf-Watz H. Yersinia proteins that target host cell signaling pathways. J Clin Investig. 1997;99:1153–1157. doi: 10.1172/JCI119270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferber D M, Brubaker R R. Plasmids in Yersinia pestis. Infect Immun. 1981;31:839–841. doi: 10.1128/iai.31.2.839-841.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fields K, Plano G V, Straley S C. A low-Ca2+ response (LCR) secretion (ysc) locus lies within the lcrB region of the LCR plasmid in Yersinia pestis. J Bacteriol. 1994;176:569–579. doi: 10.1128/jb.176.3.569-579.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forsberg Å, Rosqvist R, Wolf-Watz H. Regulation and polarized transfer of the Yersinia outer proteins (Yops) involved in antiphagocytosis. Trends Microbiol. 1994;2:14–19. doi: 10.1016/0966-842x(94)90339-5. [DOI] [PubMed] [Google Scholar]
- 19.Forsberg Å, Vitanen A-M, Skurnik M, Wolf-Watz H. The surface-located YopN protein is involved in calcium signal transduction in Yersinia pseudotuberculosis. Mol Microbiol. 1991;5:977–986. doi: 10.1111/j.1365-2958.1991.tb00773.x. [DOI] [PubMed] [Google Scholar]
- 20.Frank D W. The exoenzyme S regulon of Pseudomonas aeruginosa. Mol Microbiol. 1997;26:621–629. doi: 10.1046/j.1365-2958.1997.6251991.x. [DOI] [PubMed] [Google Scholar]
- 21.Frithz-Lindsten E, Rosqvist R, Johansson L, Forsberg Å. The chaperone-like protein YerA of Yersinia pseudotuberculosis stabilizes YopE in the cytoplasm but is dispensable for targeting to the secretion loci. Mol Microbiol. 1995;16:635–647. doi: 10.1111/j.1365-2958.1995.tb02426.x. [DOI] [PubMed] [Google Scholar]
- 22.Gaudriault S, Malandrin L, Paulin J P, Barny M A. DspA, an essential pathogenicity factor of Erwinia amylovora showing homology with AvrE of Pseudomonas syringae, is secreted via the Hrp secretion pathway in a DspB-dependent way. Mol Microbiol. 1997;26:1057–1069. doi: 10.1046/j.1365-2958.1997.6442015.x. [DOI] [PubMed] [Google Scholar]
- 23.Goguen J D, Yother J, Straley S C. Genetic analysis of the low calcium response in Yersinia pestis Mud1 (Ap lac) insertion mutants. J Bacteriol. 1984;160:842–848. doi: 10.1128/jb.160.3.842-848.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haddix P L, Straley S C. Structure and regulation of the Yersinia pestis yscBCDEF operon. J Bacteriol. 1992;174:4820–4828. doi: 10.1128/jb.174.14.4820-4828.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hakansson S, Galyov E E, Rosqvist R, Wolf-Watz H. The Yersinia YpkA Ser/Thr kinase is translocated and subsequently targeted to the inner surface of the HeLa cell plasma membrane. Mol Microbiol. 1996;20:593–603. doi: 10.1046/j.1365-2958.1996.5251051.x. [DOI] [PubMed] [Google Scholar]
- 26.Hakansson S, Schesser K, Persson C, Galyov E E, Rosqvist R, Homble F, Wolf-Watz H. The YopB protein of Yersinia pseudotuberculosis is essential for the translocation of Yop effector proteins across the target cell plasma membrane and displays a contact-dependent membrane disrupting activity. EMBO J. 1996;15:5812–5823. [PMC free article] [PubMed] [Google Scholar]
- 27.Holmstrom A, Petterson J, Rosqvist R, Hakansson S, Tafazoli F, Fallman M, Magnusson K E, Wolf-Watz H, Forsberg A. YopK of Yersinia pseudotuberculosis controls translocation of Yop effectors across the eukaryotic cell membrane. Mol Microbiol. 1997;24:73–91. doi: 10.1046/j.1365-2958.1997.3211681.x. [DOI] [PubMed] [Google Scholar]
- 28.Iriarte M, Sory M P, Boland A, Boyd A P, Mills S D, Lambermont I, Cornelis G R. TyeA, a protein involved in control of Yop release and in translocation of Yersinia Yop effectors. EMBO J. 1998;17:1907–1918. doi: 10.1093/emboj/17.7.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kado C I, Liu S T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981;145:1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lindler L E, Klempner M S, Straley S C. Yersinia pestis pH 6 antigen: genetic, biochemical, and virulence characterization of a protein involved in the pathogenesis of bubonic plague. Infect Immun. 1990;58:2569–2577. doi: 10.1128/iai.58.8.2569-2577.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 32.Michiels T, Vanooteghem J-C, de Rouvroit C L, China B, Gustin A, Boudry P, Cornelis G R. Analysis of virC, an operon involved in the secretion of Yop proteins by Yersinia enterocolitica. J Bacteriol. 1991;173:4994–5009. doi: 10.1128/jb.173.16.4994-5009.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Michiels T, Wattiau P, Brasseur R, Ruysschaert J-M, Cornelis G. Secretion of Yop proteins by Yersiniae. Infect Immun. 1990;58:2840–2849. doi: 10.1128/iai.58.9.2840-2849.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller V L, Mekalanos J J. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J Bacteriol. 1988;170:2575–2583. doi: 10.1128/jb.170.6.2575-2583.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mills S D, Boland A, Sory M P, van der Smissen P, Kerbourch C, Finlay B B, Cornelis G R. Yersinia enterocolitica induces apoptosis in macrophages by a process requiring functional type III secretion and translocation mechanisms and involving YopP, presumably acting as an effector protein. Proc Natl Acad Sci USA. 1997;94:12638–12643. doi: 10.1073/pnas.94.23.12638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Monack D M, Mecsas J, Ghori N, Falkow S. Yersinia signals macrophages to undergo apoptosis and YopJ is necessary for this cell death. Proc Natl Acad Sci USA. 1997;94:10385–10390. doi: 10.1073/pnas.94.19.10385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mullis K B, Faloona F A. Specific synthesis of DNA in vitro via a polymerase catalyzed chain reaction. Methods Enzymol. 1987;155:335–350. doi: 10.1016/0076-6879(87)55023-6. [DOI] [PubMed] [Google Scholar]
- 38.Nilles M L, Williams A W, Skrzypek E, Straley S C. Yersinia pestis LcrV forms a stable complex with LcrG and may have a secretion-related regulatory role in the low-Ca2+ response. J Bacteriol. 1997;179:1307–1316. doi: 10.1128/jb.179.4.1307-1316.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nossal N G, Heppel L A. The release of enzymes by osmotic shock from Escherichia coli in exponential phase. J Biol Chem. 1966;241:3055–3062. [PubMed] [Google Scholar]
- 40.Persson C, Carballeira N, Wolf-Watz H, Fallman M. The PTPase YopH inhibits uptake of Yersinia, tyrosine phosphorylation of p130Cas and FAK, and the associated accumulation of these proteins in peripheral focal adhesions. EMBO J. 1997;16:2307–2318. doi: 10.1093/emboj/16.9.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Persson C, Nordfelth R, Holmstrom A, Hakansson S, Rosqvist R, Wolf-Watz H. Cell-surface-bound Yersinia translocate the protein tyrosine phosphatase YopH by a polarized mechanism into the target cell. Mol Microbiol. 1995;18:135–150. doi: 10.1111/j.1365-2958.1995.mmi_18010135.x. [DOI] [PubMed] [Google Scholar]
- 42.Plano G V, Barve S S, Straley S C. LcrD, a membrane-bound regulator of the Yersinia pestis low-calcium response. J Bacteriol. 1991;173:7293–7303. doi: 10.1128/jb.173.22.7293-7303.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Plano G V, Straley S C. Multiple effects of lcrD mutations in Yersinia pestis. J Bacteriol. 1993;175:3536–3545. doi: 10.1128/jb.175.11.3536-3545.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Plano G V, Straley S C. Mutations in yscC, yscD, and yscG prevent high-level expression and secretion of V antigen and Yops in Yersinia pestis. J Bacteriol. 1995;177:3843–3854. doi: 10.1128/jb.177.13.3843-3854.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Price S B, Straley S C. lcrH, a gene necessary for virulence of Yersinia pestis and for the normal response of Y. pestis to ATP and calcium. Infect Immun. 1989;57:1491–1498. doi: 10.1128/iai.57.5.1491-1498.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Protsenko O A, Anisimov P L, Mozharov O T, Konnov N P, Popov Y A, Kokooshkin A M. Detection and characterization of Yersinia pestis plasmids determining pesticin I, fraction I antigen, and “mouse” toxin synthesis. Genetica. 1983;19:1081–1090. [PubMed] [Google Scholar]
- 47.Rimpiläinen M, Forsberg Å, Wolf-Watz H. A novel protein, LcrQ, involved in the low-calcium response of Yersinia pseudotuberculosis shows extensive homology to YopH. J Bacteriol. 1992;174:3355–3363. doi: 10.1128/jb.174.10.3355-3363.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rosqvist R, Bölin I, Wolf-Watz H. Inhibition of phagocytosis in Yersinia pseudotuberculosis: a virulence plasmid-encoded ability involving the Yop2b protein. Infect Immun. 1988;56:2139–2143. doi: 10.1128/iai.56.8.2139-2143.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rosqvist R, Forsberg Å, Rimpiläinen M, Bergman T, Wolf-Watz H. The cytotoxic protein YopE of Yersinia obstructs the primary host defence. Mol Microbiol. 1990;4:657–667. doi: 10.1111/j.1365-2958.1990.tb00635.x. [DOI] [PubMed] [Google Scholar]
- 50.Rosqvist R, Forsberg Å, Wolf-Watz H. Intracellular targeting of the Yersinia YopE cytotoxin in mammalian cells induces actin microfilament disruption. Infect Immun. 1991;59:4562–4569. doi: 10.1128/iai.59.12.4562-4569.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rosqvist R, Magnusson K, Wolf-Watz H. Target cell contact triggers expression and polarized transfer of Yersinia YopE cytotoxin into mammalian cells. EMBO J. 1994;13:964–972. doi: 10.1002/j.1460-2075.1994.tb06341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ruckdeschel K, Roggenkamp A, Lafont V, Maugeat P, Heesemann J, Rouot B. Interaction of Yersinia enterocolitica with macrophages leads to macrophage cell death through apoptosis. Infect Immun. 1997;65:4813–4821. doi: 10.1128/iai.65.11.4813-4821.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sarker M R, Neyt C, Stainier I, Cornelis G R. The Yersinia Yop virulon: LcrV is required for extrusion of the translocators YopB and YopD. J Bacteriol. 1998;180:1207–1214. doi: 10.1128/jb.180.5.1207-1214.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schesser K, Frithz-Lindsten E, Wolf-Watz H. Delineation and mutational analysis of the Yersinia pseudotuberculosis YopE domains which mediate translocation across bacterial and eukaryotic cellular membranes. J Bacteriol. 1996;178:7227–7233. doi: 10.1128/jb.178.24.7227-7233.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Skrzypek E, Haddix P L, Plano G V, Straley S C. New suicide vector for gene replacement in yersiniae and other gram-negative bacteria. Plasmid. 1993;29:160–163. doi: 10.1006/plas.1993.1019. [DOI] [PubMed] [Google Scholar]
- 56.Skrzypek E, Straley S C. LcrG, a secreted protein involved in negative regulation of the low-calcium response in Yersinia pestis. J Bacteriol. 1993;175:3520–3528. doi: 10.1128/jb.175.11.3520-3528.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sory M P, Boland A, Lambermont I, Cornelis G R. Identification of the YopE and YopH domains required for secretion and internalization into the cytosol of macrophages, using the cyaA gene fusion approach. Proc Natl Acad Sci USA. 1995;92:11998–12002. doi: 10.1073/pnas.92.26.11998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stainier I, Iriarte M, Cornelis G R. YscM1 and YscM2, two Yersinia enterocolitica proteins causing downregulation of yop transcription. Mol Microbiol. 1997;26:833–843. doi: 10.1046/j.1365-2958.1997.6281995.x. [DOI] [PubMed] [Google Scholar]
- 59.Straley S C, Bowmer W S. Virulence genes regulated at the transcriptional level by Ca2+ in Yersinia pestis include structural genes for outer membrane proteins. Infect Immun. 1986;51:445–454. doi: 10.1128/iai.51.2.445-454.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Straley S C, Plano G V, Skrzypek E, Haddix P L, Fields K A. Regulation by Ca2+ in the Yersinia low-Ca2+ response. Mol Microbiol. 1993;8:1005–1010. doi: 10.1111/j.1365-2958.1993.tb01644.x. [DOI] [PubMed] [Google Scholar]
- 61.Straley S C, Skrzypek E, Plano G V, Bliska J B. Yops of Yersinia spp. pathogenic for humans. Infect Immun. 1993;61:3105–3110. doi: 10.1128/iai.61.8.3105-3110.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Une T, Brubaker R R. In vivo comparison of avirulent Vwa− and Pgm− or Pstr phenotypes of yersiniae. Infect Immun. 1984;43:895–900. doi: 10.1128/iai.43.3.895-900.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Viitanen A-M, Toivanen P, Skurnik M. The lcrE gene is part of an operon in the lcr region of Yersinia enterocolitica O:3. J Bacteriol. 1990;172:3152–3162. doi: 10.1128/jb.172.6.3152-3162.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wattiau P, Bernier B, Deslee P, Michiels T, Cornelis G R. Individual chaperones required for Yop secretion by Yersinia. Proc Natl Acad Sci USA. 1994;91:10493–10497. doi: 10.1073/pnas.91.22.10493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wattiau P, Cornelis G R. SycE, a chaperone-like protein of Yersinia enterocolitica involved in the secretion of YopE. Mol Microbiol. 1993;8:123–131. doi: 10.1111/j.1365-2958.1993.tb01209.x. [DOI] [PubMed] [Google Scholar]
- 66.Wattiau P, Woestyn S, Cornelis G R. Customized secretion chaperones in pathogenic bacteria. Mol Microbiol. 1996;20:255–262. doi: 10.1111/j.1365-2958.1996.tb02614.x. [DOI] [PubMed] [Google Scholar]
- 67.Williams A W, Straley S C. YopD of Yersinia pestis plays a role in negative regulation of the low-calcium response in addition to its role in translocation of Yops. J Bacteriol. 1998;180:350–358. doi: 10.1128/jb.180.2.350-358.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Woestyn S, Allaoui A, Wattiau P, Cornelis G R. YscN, the putative energizer of the Yersinia Yop secretion machinery. J Bacteriol. 1994;176:1561–1569. doi: 10.1128/jb.176.6.1561-1569.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Woestyn S, Sory M P, Boland A, Lequenne O, Cornelis G R. The cytosolic SycE and SycH chaperones of Yersinia protect the region of YopE and YopH involved in translocation across eukaryotic cell membranes. Mol Microbiol. 1996;6:1261–1271. doi: 10.1111/j.1365-2958.1996.tb02645.x. [DOI] [PubMed] [Google Scholar]
- 70.Yahr T L, Goranson J, Frank D W. Exoenzyme S of Pseudomonas aeruginosa is secreted by a type III pathway. Mol Microbiol. 1996;22:991–1003. doi: 10.1046/j.1365-2958.1996.01554.x. [DOI] [PubMed] [Google Scholar]