Highlights
-
•
Viral screening was conducted against isolated ascomycetous macrofungi.
-
•
Two dsRNA viruses, one quadrivirus and one partitivirus were newly detected.
-
•
Three mitoviruses, one endornavirus and one virgavirus were newly detected.
Keywords: Mycovirus, Ascomycetous macrofungi, FLDS analyses, Mitoviridae, Virgaviridae
Abstract
Mycoviruses have been described in all major fungal taxonomic groups. There has been much focus on commercially cultivated basidiomycetous macrofungi, while attention to viruses from ascomycetous macrofungi is lacking. Therefore, in this study, we conducted viral screening against fungal mycelia that were regenerated from ascomycetous macrofungi using agarose gel electrophoresis (AGE) and fragmented and primer-ligated dsRNA sequencing (FLDS). Among the 57 isolates, four isolates were detected with virus-like bands through screening with AGE, and subsequent FLDS analyses determined the viral sequences. Other isolates without virus-like bands in AGE were pooled to check for viral sequences. Using FLDS analysis, a total of seven new mycoviruses were identified, including two double-stranded RNA (dsRNA) viruses belonging to Quadriviridae and Partitiviridae, five positive-sense single-stranded RNA (ssRNA) viruses (three belonging to Mitoviridae, one belonging to Endornaviridae and one belonging to Virgaviridae). All viruses characterized in this study are novel species, and all the hosts are firstly reported to be infected by mycoviruses. These findings expand our knowledge of the diversity of mycoviruses from macrofungi in natural environments.
1. Introduction
Mycoviruses have been described in all major fungal taxonomic groups. The majority of identified mycoviruses have an RNA genome and a persistent-type life cycle, in which the virus is transmitted by cell division and fusion (Ghabrial and Suzuki, 2009; Ghabrial et al., 2015; Urayama et al., 2022). Since the first mycovirus was discovered in the commercially cultivated mushroom Agaricus bisporus (Hollings, 1962), an increasing number of novel mycoviruses have been identified in recent years (Gilbert et al., 2019; Sato and Suzuki, 2023; Ayllón and Vainio, 2023). Mycoviruses from plant pathogenic fungi have received considerable attention due to their potential applications as biocontrol tools, because some mycoviruses are associated with hypovirulence and can be used to reduce host virulence. Several human pathogenic fungi have also been investigated (Kotta-Loizou and Coutts 2017; Chiba et al., 2021). In addition, mycoviruses from fungal isolates in natural environments have been the focus of attention in recent years. For example, mycorrhizal fungi were screened for viral infection and a novel negative-sense ssRNA virus [(-)ssRNA] of the order Bunyavirales with a unique ssRNA genome was reported (Sutela and Vainio, 2020). Subsequently, a novel mycovirus with a similar genome was discovered from an isolated wild basidiomycetous macrofungi (Zhao et al., 2023). This illustrates that viral screening of poorly studied fungal groups could uncover novel viral groups. Therefore, viral screening of fungal isolates from natural environments could expand our knowledge of RNA viruses, both in terms of evolutionary path and diversity.
Basidiomycetous or ascomycetous macrofungi, which all possess visible fruiting bodies, are a source for mycovirus screening and thus have been investigated in several reports. In particular, commercially cultivated basidiomycetous macrofungi were extensively screened because of their ecological and industrial importance (Ro et al., 2006; Magae and Sunagawa, 2010; Magae, 2012; Lin et al., 2019; Qiu et al., 2010; Revill et al., 1994; Komatsu et al., 2019; Li et al., 2020). Wild edible macrofungi, such as the commercially valuable ascomycetous genus Tuber (true truffles) (Stielow et al., 2011a; 2011b; 2012; Stielow and Menzel, 2010) and the basidiomycetous genus Lactarius (Sutela and Vainio, 2020), have also been reported to harbor mycoviruses. In addition, several wild basidiomycetous macrofungi, which are not widely considered to be edible, have also been reported to harbor mycoviruses (Heinze, 2012; Vainio et al., 2017; Vainio and Sutela, 2020; Vainio and Hantula, 2016; Zhao et al., 2023).
Compared with the numerous studies of basidiomycetous macrofungi, mycoviruses from ascomycetous macrofungi are rarely reported. Gilbert et al. (2019) conducted a comprehensive survey of the subphylum Pezizomycotina using publicly available fungal RNA-seq datasets and discovered several novel viral sequences from ascomycetous macrofungi (such as Gyromitra esculenta, Loramyces juncicola, Morchella importuna and Rutstroemia firma). This indicated that the ascomycetous macrofungi may extensively coexist with undiscovered mycoviruses, and further viral screening could uncover novel viral groups.
This study aimed to obtain a broader understanding of mycoviruses in ascomycetous macrofungi, and 57 fungal isolates obtained from wild ascomycetous macrofungi in Japan were investigated. Seven novel RNA viruses with complete genome sequences from Cordyceps militaris, Dicephalospora rufocornea, Stromatinia cryptomeriae, Ciborinia camelliae, Dialonectria episphaeria and Crassitunica tubakii were described, and their phylogenetic positions were discussed.
2. Materials & methods
2.1. Sample collection
Fifty-seven isolates of ascomycetous fungi isolated from natural environments in Japan were obtained from the ascomycete collection of the National Museum of Nature and Science (Tsukuba, Japan). The isolates included a wide range of identified species of pyrenomycetes and discomycetes (Table S1).
2.2. Detection of RNA viruses by dsRNA agarose gel electrophoresis and sequencing
The mycelia of each isolate were harvested after growth in potato dextrose broth (PDB) with reciprocal shaking (120 rpm) for two weeks at 25 °C. Total nucleic acids of individual isolates were extracted from 0.2 g of cultured mycelia. The obtained mycelia were ground into powder in the presence of liquid nitrogen, then suspended in extraction buffer (20 mM Tris–HCl, pH 6.8, 200 mM NaCl, 2 mM EDTA, 1 % SDS and 0.1 % (v/v) β-mercaptoethanol). Following extraction, samples were subjected to phenol/chloroform/isoamyl alcohol (25:24:1) extraction twice for nucleic acid purification. The dsRNA fraction was then purified using a microspin column (empty Bio-spin column; Bio-Rad) containing cellulose powder (Cellulose D; ADVAN-TEC) as described previously (Urayama et al., 2018; Chiba et al., 2021; Zhao et al., 2023). In total, 300 μL of purified dsRNA elution was obtained. A 50-μL portion was stored in a -80 °C freezer for fragmented and primer-ligated dsRNA sequencing (FLDS) (Urayama et al., 2018; Chiba et al., 2021). The remaining 250 μL was precipitated using 100 % ethanol and then used for agarose gel electrophoresis (AGE). AGE was performed at 100 V for 85 min, and the gel was stained with GelRed (Biotium, Inc.).
When virus-like band(s) were detected through AGE, the dsRNA samples from each isolate were separately processed for viral genome sequencing by FLDS. While if no distinct virus-like bands were identified, the dsRNA samples from up to twenty isolates were pooled into a single sample following FLDS analysis (referred to as pooled-FLDS analysis). dsRNA was converted into dscDNA by the FLDS method (Urayama et al., 2018). In brief, the U2 primer was ligated to the 3′ end of the fragmented dsRNA, and cDNA was synthesized using the SMARTer RACE 5′/3′ Kit (Takara Bio) with the U2-comp primer. After PCR amplification, the cDNA was fragmented using an ultrasonicator (Covaris S220). Illumina sequencing libraries were then constructed using KAPA Hyper Prep Kit Illumina platforms (Kapa Bio-systems). Individual 300-bp paired-end reads were determined with the Illumina platform (Illumina, CA, USA).
2.3. Data processing
The obtained raw sequence reads were stripped of low-quality, adaptor, rRNA, and low-complexity sequences, as described previously (Hirai et al., 2021). The remaining cleaned reads were then assembled using de novo assembly with CLC Genomics Workbench version 11.0 (CLC Bio, Aarhus, Denmark). The assembled sequences were manually examined and extended using the Tablet viewer (Milne et al., 2010). Where the terminal end of a contig ended with same bases for more than ten reads or a polyA sequence was present, the position was recognized as the terminal end of the RNA genome. When a contig had termini at both ends, it was considered that the segment of the RNA genome had been fully sequenced. The obtained contigs were blasted against non-redundant (nr) databases available from GenBank at the National Center for Biotechnology Information (NCBI). Open reading frame (ORF) prediction was performed by ORFfinder of NCBI.
2.4. Phylogenetic analysis
Phylogenetic trees were constructed using the maximum-likelihood method in RAxML (Stamatakis, 2014), based on the deduced amino acid sequences of RNA-dependent RNA polymerase (RdRp) domains. Multiple sequence alignments of amino acid sequences of viral RdRp proteins were obtained using MUSCLE (Edgar, 2004) with the default parameters. Ambiguous positions in the alignment were removed using trimAl with the option gt=1 (Capella-Gutiérrez et al., 2009). The best-fitting model of amino acid substitutions was tested in Aminosan (Tanabe, 2011) and judged according to the corrected Akaike information criterion (Sugiura, 1978). Bootstrap tests were conducted with 1000 samplings. FigTree (Rambaut, 2014) was used to illustrate the resulting phylogenies.
2.5. Reverse transcription PCR (RT-PCR)
To confirm the sequences obtained from FLDS, RT-PCR analysis was conducted using a specific primer set of each segment (Table S3). RT-PCR was performed using the Super-Script III One-Step RT-PCR System with Platinum Taq (Invitrogen) according to the manufacturer's protocol. Total nucleic acids were used as the template. PCR products were run on 1.2 % agarose gels and the visualized fragments were excised, purified using the FastGene Gel/PCR Extraction Kit (Nippon Genetics, Tokyo, Japan), and then used for direct Sanger sequencing.
3. Results
3.1. Mycoviruses detected by dsRNA agarose gel electrophoresis and sequencing
A total of 57 isolates that were conserved in the Department of Botany, National Museum of Nature and Science (Japan; Table S1) were screened for dsRNA mycoviruses using AGE. The results showed that virus-like bands were detected in four strains (Fig. 1). Except the remaining genomic DNA and rRNA bands, isolate FC-1571 (Cordyceps militaris) showed three clearly visible bands around 4.4 kbp; isolate FC-2948 (Dicephalospora rufocornea) showed two clear bands smaller than 2.0 kbp; isolates FC-1840 and FC-2977 (all identified as Stromatinia cryptomeriae) showed a similar result, with one clear band around 2.0 kbp. FLDS analysis was performed to determine dsRNA sequences in the four isolates that showed virus-like bands in AGE. Details of the obtained viral sequences are summarized in Table 1, and all the viral segments were confirmed by RT-PCR (Fig. S1, Table S3).
Fig. 1.
Detection of dsRNA by agarose gel electrophoresis (AGE). Lane 1: FC-2948 (Dicephalospora fufocornea); Lane 2: FC-1840 (Stromatinia cryptomeriae); Lane 3: FC-1571 (Cordyceps militaris); Lane 4: FC-2977 (S. cryptomeriae). Lane M: RNA size marker. The arrows indicate potential viral RNAs bands.
Table 1.
Summary of RNA viral sequences detected in the present study.
| Virus name | Segment | Length (bp) | Function of the ORF | Accession no. | Host identification | Host isolate ID |
|---|---|---|---|---|---|---|
| Cordyceps militaris quadrivirus 1 (CorQV1) | RNA1 | 4899 | hypothetical protein | LC776592 | Cordyceps militaris | FC-1571 |
| RNA2 | 4402 | RdRP | LC776593 | |||
| RNA3 | 3557 | putative capsid protein | LC776594 | |||
| RNA4 | 4470 | coat protein | LC776595 | |||
| Dicephalospora rufocornea partitivirus 1 (DicePV1) | RNA1 | 1949 | RdRp | LC776598 | Dicephalospora rufocornea | FC-2948 |
| RNA2 | 1801 | CP | LC776599 | |||
| Stromatinia cryptomeriae mitovirus1-I (StroMV1-I) | RNA1 | 2692 | RdRP | LC776596 | Stromatinia cryptomeriae | FC-1840 |
| Stromatinia cryptomeriae mitovirus1-II (StroMV1-II) | RNA1 | 2767 | RdRP | LC776597 | Stromatinia cryptomeriae | FC-2977 |
| Ciborinia camelliae endornavirus 1 (CibEV1) | RNA1 | 9661 | polyprotein | LC776600 | Ciborinia camelliae | FC-1860 |
| Dialonectria episphaeria virga-like virus 1 (DiaVV1) | RNA1 | 9786 | polyprotein | LC776601 | Dialonectria episphaeria | FC-1637 |
| Crassitunica tubakii mitovirus 1 (CraMV1) | RNA1 | 2537 | RdRP | LC776602 | Crassitunica tubakii | FC-2843 |
| Crassitunica tubakii mitovirus 2 (CraMV2) | RNA1 | 2525 | RdRP | LC776603 |
For the 53 other isolates that did not yield virus-like bands in AGE, the dsRNAs were pooled and sequenced by FLDS. Several viral sequences were obtained from the pooled samples, and RT-PCR analyses were conducted using specific primer sets for each viral sequence to confirm the host. As a result, three isolates, FC-1860 (Ciborinia camelliae), FC-1637 (Dialonectria episphaeria) and FC-2843 (Crassitunica tubakii) were determined to possess virus sequences (Table 1).
3.2. Characterization of two novel dsRNA viruses of the families Quadriviridae and Partitiviridae
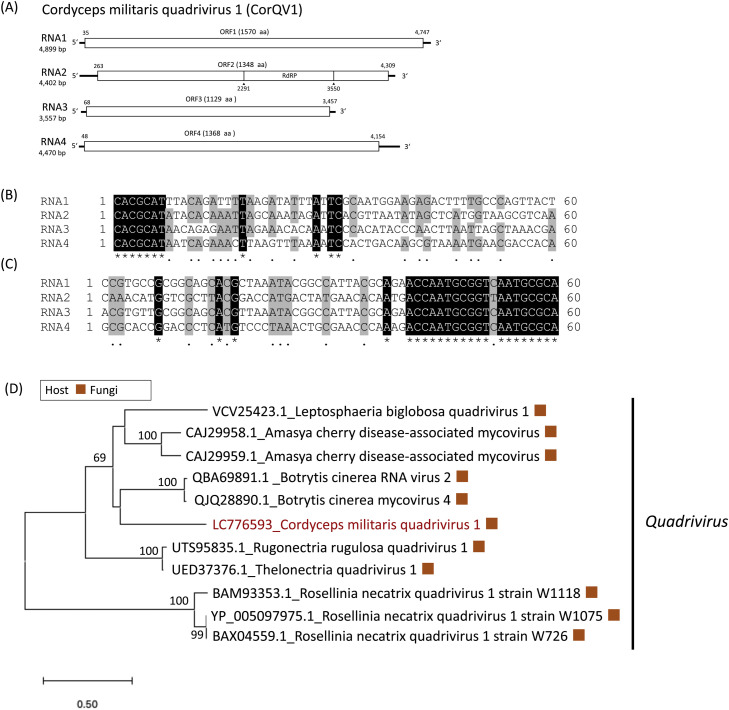
Cordyceps militaris quadrivirus 1 (CorQV1) was obtained from isolate FC-1571 (Table 1). It had four segments with sizes ranging from 3557 to 4899 bp, which corresponded with previously reported Quadrivirus spp. of 3.5 to 5.0 kbp in length (Chiba et al., 2018). Each of the four dsRNA segments contains a single ORF on the positive-sense RNA strand (RNA1∼4, Fig. 2A). The first 7 nucleotides at the 5ʹ-end (Fig. 2B) and the last 20 nucleotides at the 3ʹ-end of CorQV1 segments are highly conserved (Fig. 2C). Based on NCBI BlastX homology search, RNA2 showed the highest similarity (45.71 % identity) with the RdRp of Botrytis cinerea RNA virus 2 (Unpublished). RNA2 also showed similarity with known quadriviruses, such as Leptosphaeria biglobosa quadrivirus 1 (LbQV-1, Shah et al., 2018), Thelonectria quadrivirus 1 (Lutz et al., 2022), Rugonectria rugulosa quadrivirus 1 (Pielhop et al., 2022) and Rosellinia necatrix quadrivirus 1 (Lin et al., 2012), with 43.02 %, 44.18 %, 44.10 % and 34.03 % protein-sequence identities, respectively. The three other segments also showed the highest similarity with known proteins of quadrivirus (Table S2). In the phylogenetic tree, CorQV1 was included in the highly supported clade with (LbQV-1) (Fig. 2D). Although there is no official demarcation value for the determination of quadrivirus (Chiba et al., 2018), CorQV1 was identified as a novel quadrivirus species.
Fig. 2.
Genome organization and phylogeny analysis of viruses from Quadriviridae.
(A): The RNA genome structure model of CorQV1. The predicted ORFs are indicated by white boxes. (B): Alignments of the 5′-terminal regions of the RNA sequences. Nucleotide positions with 100 % matches among the sequences are depicted by black shading. (C): Alignments of the 3′-terminal regions of the RNA sequences. Nucleotide positions with 100 % matches among the sequences are depicted by black shading. (D): An unrooted phylogenetic tree from maximum likelihood-based methodology. The numbers indicate the percentage of bootstrap support from 1000 RAxML bootstrap replicates. The best-fitting amino acid substitution model was RtREV+G + F. The viral sequences obtained from the present research are shown in red font.
Dicephalospora rufocornea partitivirus 1 (DicePV1) was obtained from isolate FC-2948 (Table 1). The larger segment (RNA1; 1949 bp) was predicted to encode an RdRp, the smaller segment (RNA2; 1801 bp) was predicted to encode a CP (Fig. 3A). The terminal sequences of each segment were highly conserved in the 5′-untranslated regions (UTRs), and the interrupted poly(A) which all end with “GT” in the 3′-UTRs (Fig. 3B), indicating that these segments originated from a single virus. Based on an NCBI BlastX homology search, RNA1 showed the highest similarity with the RdRp of Rhizoctonia solani partitivirus 10 (71.05 % identity, Li et al., 2022b), whereas RNA2 showed high similarity with the CP of Gyromitra esculenta partitivirus 1 (54.95 % identity; Table S2; Sahin et al., 2021). According to the species differentiation criteria for partitiviruses (≤ 90% aa sequence identity in the RdRp and ≤ 80% aa sequence identity in the CP) (Vainio et al., 2018), DicePV1 was identified as a novel partitivirus. In the phylogenetic tree, DicePV1 was included in the highly supported Alphapartitivirus clade, showing it belongs to the genus Alphapartitivirus (Fig. 3C).
Fig. 3.
Genome organization and phylogeny analysis of viruses from Partitiviridae.
(A): The RNA genome structure model of DicePV1. The predicted ORFs are indicated by white boxes. (B): Alignments of the 5′- or 3′-terminal regions of the RNA sequences. Nucleotide positions with 100 % matches among the sequences are depicted by black shading. (C): An unrooted phylogenetic tree produced using a maximum likelihood-based methodology. The numbers indicate the percentage of bootstrap support from 1000 RAxML bootstrap replicates. The best-fitting amino acid substitution model was RtREV+G + F. The viral sequences obtained from the present research are shown in red font.
3.3. Characterization of five (+)ssRNA viruses of the families Mitoviridae, Endornaviridae and Virgaviridae
Three novel mycoviruses belonging to Mitoviridae were detected from three isolates (FC-1840, FC-2977 and FC-2843) (Table 1). All three mycoviruses have a small (2525–2767 nt), non-segmented (+ss)RNA genome that codes for a single RdRp protein, a known feature of Mitoviridae species (Hillman and Cohen, 2020; Wolf et al., 2018).
Two viral sequences obtained from different isolates (FC-1840 and FC-2977) of S. cryptomeriae shared 94.01 % nucleotide sequence identity and 92.81 % amino acid (aa) identity. Therefore, they were identified as belonging to the identical viral species. We named it Stromatinia cryptomeriae mitovirus 1 (StroMV1). The sequences corresponded in size to the 2.0 kbp bands detected by AGE (Fig. 1). The viral sequences of StroMV1-I (2692 nt) and StroMV1-II (2767 nt) were obtained from isolates FC-1840 and FC-2977, respectively. Both viruses encoded a single ORF with a Mitovir_RNA_pol (Pfam05919) domain (Fig. 4A). Based on a homology search with the predicted amino acid (polyprotein) sequence, the two sequences share 56.60 % and 54.95 % identity with an unidentified mitovirus (Table S2). Based on the phylogenetic tree, StroMV1-I and StroMV1-II were included in the genus Unuamitovirus cluster (Fig. 4B), suggesting that StroMV1 represents an Unuamitovirus species.
Fig. 4.
Genome organization and phylogeny analysis of viruses from Mitoviridae.
(A): The RNA genome structure model of StroMV1, CraMV1, and CraMV2. The predicted ORFs are indicated by white boxes. (B): An unrooted phylogenetic tree produced using a maximum likelihood-based methodology. The numbers indicate the percentage of bootstrap support from 1000 RAxML bootstrap replicates. The best-fitting amino acid substitution model was RtREV+G + F. The viral sequences obtained from the present research are shown in red font.
Two different mitoviruses, Crassitunica tubakii mitovirus 1 (CraMV1) and Crassitunica tubakii mitovirus 2 (CraMV2), were identified as infecting isolate FC-2843. Both CraMV1 (2537 nt) and CraMV2 (2525 nt) exhibited a single ORF encoding a Mitovir_RNA_pol domain (Fig. 4A). No similarity was discovered between the two nucleotide sequences, while with 34.42 % aa identity. Similar to StroMV1, the phylogenetic tree indicated that CraMV1 and CraMV2 were included in the genus Unuamitovirus cluster, with the three viruses belonging to different subclusters (Fig. 4B).
A complete viral sequence (9661 nt), identified as Ciborinia camelliae endornavirus 1 (CibEV1), was obtained from isolate FC-1860. A single ORF was found starting at nucleotide 214 and ending at nucleotide 9612, which potentially encodes a polyprotein (Fig. 5A). The conserved domain search in Pfam (Lu et al., 2020) showed that this polyprotein sequence has two conserved domain motifs, including a viral helicase (Pfam01443) and an Endornaviridae_RdRp (Pfam00978) located at the C-terminus. Based on a homology search with the predicted amino acid (polyprotein) sequence, CibEV1 shares 44.88 % identity with Inner Mongolia sediment alphaendornavirus 3 (Chen et al., 2022; Table S2). According to the ICTV species demarcation criteria for endornaviruses, members of different species have an overall nucleotide sequence identity below 75 %, and CibEV1 did not show > 75 % nucleotide identity to known viral sequences. Therefore, we proposed CibEV1 as a new species of Endornaviridae and, according to the phylogenetic tree, CibEV1 was included in the genus Alphaendornavirus (Fig. 5B).
Fig. 5.
Genome organization and phylogeny analysis of viruses from Endornaviridae.
(A): The RNA genome structure model of CibEV1. The predicted ORFs are indicated by white boxes. (B): An unrooted phylogenetic tree produced using a maximum likelihood-based methodology. The numbers indicate the percentage of bootstrap support from 1000 RAxML bootstrap replicates. The best-fitting amino acid substitution model was LG+I + G + F. The viral sequences obtained from the present research are shown in red font.
A complete viral sequence (9786 nt) that identified as Dialonectria episphaeria virga-like virus 1 (DiaVV1) was obtained from isolate FC-1637 (Table 1). A single ORF was found starting at nucleotide 93 and ending at nucleotide 9686, which potentially encodes a polyprotein (Fig. 6A). Based on the conserved domain search in Pfam (Lu et al., 2020), this polyprotein sequence including a viral helicase (Pfam01443) and an RdRp (Pfam00978) located at the C-terminus. Based on a homology search with the predicted amino acid (polyprotein) sequence, the present sequence shares 33.70 % identity with Plasmopara viticola lesion-associated virga-like virus 1 (PvLaVLV1) (Chiapello et al., 2020; Table S2), which was obtained from the total RNA-seq of Plasmopara viticola mycelia-associated leaf lesions (Chiapello et al., 2020). The phylogenetic tree showed that DiaVV1 was included in the unclassified Virgaviridae cluster (Fig. 6B), showing a close relationship with other virga-like viruses.
Fig. 6.
Genome organization and phylogeny analysis of viruses from the order Virgaviridae.
(A): The RNA genome structure model of DiaVV1. The predicted ORFs are indicated by white boxes. (B): A rooted phylogenetic tree produced using a maximum likelihood-based methodology. The numbers indicate the percentage of bootstrap support from 1000 RAxML bootstrap replicates. The best-fitting amino acid substitution model was RtREV+I + G + F. The viral sequences obtained from the present research are shown in red font.
4. Discussion
In the present study, we detected seven novel mycoviruses from isolates of ascomycetous macrofungi using AGE and FLDS. FLDS enabled us to determine full-length genomic sequence of the viruses based on its high specificity and depth of the sequence reads (Hirai et al., 2021; Chiba et al., 2021; Urayama et al., 2018). This is the first report of mycovirus infections for these hosts. These findings have expanded our knowledge of the diversity of mycoviruses from ascomycetous macrofungi in natural environments.
In our mycovirus screening of 57 fungal isolates spanning 13 species, seven isolates belonging to six species (Table 1, S1) were found to harbor viruses. Gilbert et al. (2019) conducted a comprehensive survey of the subphylum Pezizomycotina using publicly available fungal RNA-seq datasets and discovered several novel viral sequences from ascomycetous macrofungi. Of the 569 RNA-Seq samples, 59 complete mycoviral genomes were identified in 57 datasets (∼10 % detection efficiency) (Gilbert et al., 2019). Considering a significant portion of the isolates used in the screening had been conserved for a long-term period, some fungi originally carrying virusese might have lost them during storage. Although the detection efficiency depends on the screening target and the conducted method, we hypothesize that the quantity of mycoviruses infecting ascomycetous macrofungi in their natural habitat may be underestimated, warranting further exploration. Detecting mycoviruses in non-cultivated macrofungi could significantly contribute to our knowledge of mycovirus diversity in the environment, ultimately restoring their acknowledged role in the ecosystem.
Previously, most fungal viruses were considered as only having dsRNA genomes (Pearson et al., 2009); recently, however, numerous new ssRNA viruses in plant-associated fungal communities have been identified (Marzano and Domier, 2016; Marzano et al., 2016; Nerva et al., 2019).
In the present study, five (+)ssRNA mycoviruses (three belonging to Mitoviridae, one belonging to Endornaviridae and one belonging to Virgaviridae) were newly detected. Endornaviridae includes unique, persistent viruses with symbiotic properties that are capable of infecting diverse eukaryotes, such as plants, fungi, and protists (Fukuhara, 2019). The genus Alphaendornavirus is capable of infecting basidiomycetes, oomycetes, and plants, with a diverse host range and genome characteristics (Ong et al., 2016; Luo et al., 2022). Alphaendornavirus includes three major subclusters (1a, 1b, 1c in Fig. 5B), whereas CibEV1 in the present study was not included in the major subclusters, showing that more taxonomical research may be needed in the future.
Mitoviridae includes small (2151–4955 nt)-sized viruses with non-segmented (+ss) RNA genomes that encode a single RdRp protein (Hillman and Cohen, 2020; Wolf et al., 2018). Mitoviruses were first found infecting fungi (Rogers et al., 1987), are unique in replicating persistently in host cell mitochondria (Hillman and Cohen, 2020; Nibert, 2017; Nibert et al., 2018; Nerva et al., 2019; Fonseca et al., 2020), and can migrate to the cytoplasm of host cells (Muñoz-Adalia et al., 2018; Shahi et al., 2019). Most of the known fungal mitoviruses are reported from the genera Kvaramitovirus, Triamitovirus and Unuamitovirus. The genus Unuamitovirus includes several mycoviruses with the macrofungi host, such as Sclerotinia sclerotiorum, Monilinia fructicola, Agaricus bisporus and Hymenoscyphus fraxineus, combined with StroMV1, CraMV1 and CraMV2 reported in the present research (all with macrofungi hosts), Unuamitovirus was suggested to have a wide fungi host range, especially in the macrofungi group.
Most members of Virgaviridae infect plants and consist of rod-shaped virions with + ssRNA genomes (Adams et al., 2009; 2017). According to the 2020 ICTV taxonomy report, Virgaviridae contains seven genera including Furovirus, Hordeivirus, Goravirus, Pecluvirus, Pomovirus, Tobamovirus, and Tobravirus. In recent years, several virga-like viruses have been discovered from transcriptomic or metatranscriptomic sequencing without confirmed hosts, which were recognized as not belonging to any of the above known genera; therefore, they were assigned as unclassified Virgaviridae species (Thekke-Veetil et al., 2020; Quintanilha-Peixoto et al., 2021; Chiapello et al., 2020; Li et al., 2022a). In the present phylogenetic tree, these unclassified Virgaviridae species were included in one 54 % supported cluster (Fig. 6B), and two highly supported subclusters (a and b) were included. DiaVV1 and several unclassified Virgaviridae species from metatranscriptomic sequencing of mosquito (UYL94365.1), algae (BBZ90076.1), and Plasmopara viticola mycelia-associated leaf lesions (QHD64722.1) were included in subcluster a, also with an unclassified Virgaviridae species (QOW97229.1) detected from cultured microbial algae, which broadened our understanding of virus diversity in protists (Charon et al., 2020). Isolation of DiaVV1 from the host fungus could support further functional analysis of virga-like viruses and reveal their ecological significance.
Note
Isolates detected with mycoviruses were deposited in the NBRC culture collection. Sequence data supporting the results of this study are available in the GenBank database repository (Accession No. LC776592–LC776603) and the Short Read Archive database (Accession No. DRR494161–DRR494166).
Funding
This research was funded by a grant from the Institute for Fermentation and by a Grant-in-Aid for Scientific Research (22J40078, 22KJ0440, 22H04879, 20H05579, 21K18217) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan.
CRediT authorship contribution statement
Yan-jie Zhao: Formal analysis, Funding acquisition, Visualization, Data curation, Writing – original draft. Tsuyoshi Hosoya: Investigation, Resources. Syunichi Urayama: Methodology, Project administration, Investigation, Writing – review & editing. Daisuke Hagiwara: Project administration, Supervision, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
The authors thank FORTE Science Communications (https://www.forte-science.co.jp/) for English language editing. The authors also thank Ms. Yoshimi Nakazawa for the technical support in library preparation and AGE detection.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.virusres.2023.199290.
Contributor Information
Yan-jie Zhao, Email: zhao.yanjie.gf@u.tsukuba.ac.jp.
Daisuke Hagiwara, Email: hagiwara.daisuke.gb@u.tsukuba.ac.jp.
Appendix. Supplementary materials
Data availability
No data was used for the research described in the article.
References
- Adams M.J., Adkins S., Bragard C., Gilmer D., Li D., MacFarlane S.A., Wong S.M., Melcher U., Ratti C., Ryu K.H. ICTV virus taxonomy profile: Virgaviridae. J. Gen. Virol. 2017;98:1999–2000. doi: 10.1099/jgv.0.000884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams M.J., Antoniw J.F., Kreuze J. Virgaviridae: a new family of rod-shaped plant viruses. Arch. Virol. 2009;154:1967–1972. doi: 10.1007/s00705-009-0506-6. [DOI] [PubMed] [Google Scholar]
- Ayllón M.A., Vainio E.J. Mycoviruses as a part of the global virome: diversity, evolutionary links and lifestyle. Adv. Virus Res. 2023;115:1–86. doi: 10.1016/bs.aivir.2023.02.002. [DOI] [PubMed] [Google Scholar]
- Capella-Gutiérrez S., Silla-Martínez J.M., Gabaldón T. trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics. 2009;25:1972–1973. doi: 10.1093/bioinformatics/btp348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charon J., Marcelino V.R., Wetherbee R., Verbruggen H., Holmes E.C. Metatranscriptomic identification of diverse and divergent RNA viruses in green and chlorarachniophyte algae cultures. Viruses. 2020;12(10):1180. doi: 10.3390/v12101180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.M., Sadiq S., Tian J.H., Chen X., Lin X.D., Shen J.J., et al. RNA viromes from terrestrial sites across China expand environmental viral diversity. Nat. Microbiol. 2022;7(8):1312–1323. doi: 10.1038/s41564-022-01180-2. [DOI] [PubMed] [Google Scholar]
- Chiapello M., Rodríguez-Romero J., Ayllón M.A., Turina M. Analysis of the virome associated to grapevine downy mildew lesions reveals new mycovirus lineages. Virus Evol. 2020;6 doi: 10.1093/ve/veaa058. veaa 058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba S., Castón J.R., Ghabrial S.A., Suzuki N. ICTV virus taxonomy profile: quadriviridae. J. Gen. Virol. 2018;99:1480–1481. doi: 10.1099/jgv.0.001152. [DOI] [PubMed] [Google Scholar]
- Chiba Y., Oiki S., Yaguchi T., Urayama S.I., Hagiwara D. Discovery of divided RdRp sequences and a hitherto unknown genomic complexity in fungal viruses. Virus Evol. 2021;7 doi: 10.1093/ve/veab027. veaa101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar R.C. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca P., Ferreira F., Da Silva F., Oliveira L.S., Marques J.T., Goes-Neto A., Aguiar E., Gruber A. Characterization of a novel mitovirus of the sand fly Lutzomyia longipalpis using genomic and virus–host interaction signatures. Viruses. 2020;13(9) doi: 10.3390/v13010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuhara T. Endornaviruses: persistent dsRNA viruses with symbiotic properties in diverse eukaryotes. Virus Genes. 2019;55:165–173. doi: 10.1007/s11262-019-01635-5. [DOI] [PubMed] [Google Scholar]
- Ghabrial S.A., Castón J.R., Jiang D., Nibert M.L., Suzuki N. 50-plus years of fungal viruses. Virology. 2015;479:356–368. doi: 10.1016/j.virol.2015.02.034. [DOI] [PubMed] [Google Scholar]
- Ghabrial S.A., Suzuki N. Viruses of plant pathogenic fungi. Annu. Rev. Phytopathol. 2009;47:353–384. doi: 10.1146/annurev-phyto-080508-081932. [DOI] [PubMed] [Google Scholar]
- Gilbert K.B., Holcomb E.E., Allscheid R.L., Carrington J.C. Hiding in plain sight: new virus genomes discovered via a systematic analysis of fungal public transcriptomes. PLoS One. 2019;14(7) doi: 10.1371/journal.pone.0219207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinze C. A novel mycovirus from Clitocybe odora. Arch. Virol. 2012;157:1831–1834. doi: 10.1007/s00705-012-1373-0. [DOI] [PubMed] [Google Scholar]
- Hillman B.I., Cohen A.B. 4th ed. 1-5. Elsevier; 2020. Mitoviruses (Mitoviridae) pp. 601–606. (Encyclopedia of Virology). Vol. 1-5, pp. [DOI] [Google Scholar]
- Hirai M., Takaki Y., Kondo F., Horie M., Urayama S., Nunoura T. RNA viral metagenome analysis of subnanogram dsRNA using fragmented and primer ligated dsRNA sequencing (FLDS) Microbes Environ. 2021;36:ME20152. doi: 10.1264/jsme2.ME20152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollings M. Viruses associated with a die-back disease of cultivated mushroom. Nature. 1962;196:962–965. doi: 10.1038/196962a0. [DOI] [Google Scholar]
- Komatsu A., Kondo H., Sato M., Kurahashi A., Nishibori K., Suzuki N., Fujimori F. Isolation and characterization of a novel mycovirus infecting an edible mushroom, Grifola frondosa. Mycoscience. 2019;60:211–220. doi: 10.1016/j.myc.2019.01.005. [DOI] [Google Scholar]
- Kotta-Loizou I., Coutts R.H. Mycoviruses in Aspergilli: a comprehensive review. Front. Microbiol. 2017;8:1699. doi: 10.3389/fmicb.2017.01699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Guo J., Ye Z., Zhao Z., Chen J., Yang J. Molecular characterization of a novel virga-like virus associated with wheat. Arch. Virol. 2022;167(9):1909–1913. doi: 10.1007/s00705-022-05473-z. [DOI] [PubMed] [Google Scholar]
- Li X., Sui K., Xie J., Hai D., Yin W., Sossah F.L., Jiang D., Song B., Li Y. Molecular characterization of a novel fusarivirus infecting the edible fungus Auricularia heimuer. Arch. Virol. 2020;165:2689–2693. doi: 10.1007/s00705-020-04781-6. [DOI] [PubMed] [Google Scholar]
- Li Y., Li S., Zhao Y., Zhou T., Wu X., Zhao C. Six novel mycoviruses containing positive single-stranded RNA and double-stranded RNA genomes co-infect a single strain of the Rhizoctonia solani AG-3 PT. Viruses. 2022;14(4):813. doi: 10.3390/v14040813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y.H., Chiba S., Tani A., Kondo H., Sasaki A., Kanematsu S., Suzuki N. A novel quadripartite dsRNA virus isolated from a phytopathogenic filamentous fungus, Rosellinia necatrix. Virology. 2012;426(1):42–50. doi: 10.1016/j.virol.2012.01.013. [DOI] [PubMed] [Google Scholar]
- Lin Y.H., Fujita M., Chiba S., Hyodo K., Andika I.B., Suzuki N., Kondo H. Two novel fungal negative-strand RNA viruses related to mymonaviruses and phenuiviruses in the shiitake mushroom (Lentinula edodes) Virology. 2019;533:125–136. doi: 10.1016/j.virol.2019.05.008. [DOI] [PubMed] [Google Scholar]
- Lu S., Wang J., Chitsaz F., Derbyshire M.K., Geer R.C., Gonzales N.R., Gwadz M., Hurwitz D.I., Marchler G.H., Song J.S., Thanki N., Yamashita R.A., Yang M., Zhang D., Zheng C., Lanczycki C.J., Marchler-Bauer A. CDD/SPARCLE: the con-served domain database in 2020. Nucleic Acids Res. 2020;48:D265–D268. doi: 10.1093/nar/gkz991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X., Jiang D., Xie J., Jia J., Duan J., Cheng J., Fu Y., Chen T., Yu X., Li B., Lin Y. Genome Characterization and Phylogenetic Analysis of a Novel Endornavirus That Infects Fungal Pathogen Sclerotinia sclerotiorum. Viruses. 2022;14:456. doi: 10.3390/v14030456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz T., Langer G., Heinze C. Complete genome sequence of a new quadrivirus infecting a member of the genus Thelonectria. Arch. Virol. 2022;167:691–694. doi: 10.1007/s00705-021-05353-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magae Y. Molecular characterization of a novel mycovirus in the cultivated mushroom, Lentinula edodes. Virol. J. 2012;9:1–6. doi: 10.1186/1743-422X-9-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magae Y., Sunagawa M. Characterization of a mycovirus associated with the brown discoloration of edible mushroom, Flammulina velutipes. Virol. J. 2010;7:1–8. doi: 10.1186/1743-422X-7-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzano S.Y.L., Domier L.L. Novel mycoviruses discovered from metatranscriptomics survey of soybean phyllosphere phytobiomes. Virus Res. 2016;213:332–342. doi: 10.1016/j.virusres.2015.11.002. [DOI] [PubMed] [Google Scholar]
- Marzano S.Y.L., Nelson B.D., Ajayi-Oyetunde O., Bradley C.A., Hughes T.J., Hartman G.L., Eastburn D.M., Domier L.L. Identification of diverse mycoviruses through metatranscriptomics characterization of the viromes of five major fungal plant pathogens. J. Virol. 2016;90:6846–6863. doi: 10.1128/JVI.00357-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne I., Bayer M., Cardle L., Shaw P., Stephen G., Wright F., Marshall D. Tablet—next generation sequence assembly visualization. Bioinformatics. 2010;26:401–402. doi: 10.1093/bioinformatics/btp666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Adalia E.J., Diez J.J., Fernández M.M., Hantula J., Vainio E.J. Characterization of small RNAs originating from mitoviruses infecting the conifer pathogen Fusarium circinatum. Arch. Virol. 2018;163:1009–1018. doi: 10.1007/s00705-018-3712-2. [DOI] [PubMed] [Google Scholar]
- Nerva L., Vigani G., Di Silvestre D., Ciuffo M., Forgia M., Chitarra W., Turina M. Biological and molecular characterization of chenopodium quinoa mitovirus 1 reveals a distinct small rna response compared to those of cytoplasmic RNA viruses. J. Virol. 2019;93 doi: 10.1128/jvi.01998-18. e01998-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nibert M.L. Mitovirus UGA(Trp) codon usage parallels that of host mitochondria. Virology. 2017;507:96–100. doi: 10.1016/j.virol.2017.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nibert M.L., Vong M., Fugate K.K., Debat H.J. Evidence for contemporary plant mitoviruses. Virology. 2018;518:14–24. doi: 10.1016/j.virol.2018.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong J.W., Li H., Sivasithamparam K., Dixon K.W., Jones M.G., Wylie S.J. Novel Endorna-like viruses, including three with two open reading frames, challenge the membership criteria and taxonomy of the Endornaviridae. Virology. 2016;499:203–211. doi: 10.1016/j.virol.2016.08.019. [DOI] [PubMed] [Google Scholar]
- Pearson M.N., Beever R.E., Boine B., Arthur K. Mycoviruses of filamentous fungi and their relevance to plant pathology. Mol. Plant Pathol. 2009;10:115–128. doi: 10.1111/j.1364-3703.2008.00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pielhop T.P., Popp C., Knierim D., et al. Three new mycoviruses identified in the apple replant disease (ARD)-associated fungus Rugonectria rugulosa. Virus Genes. 2022;58:423–435. doi: 10.1007/s11262-022-01924-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu L., Li Y., Liu Y., Gao Y., Qi Y., Shen J. Particle and naked RNA mycoviruses in industrially cultivated mushroom Pleurotus ostreatus in China. Fungal Biol. 2010;114:507–513. doi: 10.1016/j.funbio.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Quintanilha-Peixoto G., Fonseca P.L.C., Raya F.T., Marone M.P., Bortolini D.E., Mieczkowski P., et al. The Sisal virome: uncovering the viral diversity of agave varieties reveals new and organ-specific viruses. Microorganisms. 2021;9(8):1704. doi: 10.3390/microorganisms9081704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambaut, A., 2014. FigTree v1. 4.2, a graphical viewer of phylogenetic trees. Available from left angle bracket. http://tree.bio.ed.ac.uk/software/figtree/right.angle.bracket.
- Revill P.A., Davidson A.D., Wright P.J. The nucleotide sequence and genome organization of mushroom bacilliform virus: a single-stranded RNA virus of Agaricus bisporus (Lange) Imbach. Virology. 1994;202:904–911. doi: 10.1006/viro.1994.1412. [DOI] [PubMed] [Google Scholar]
- Ro H.S., Lee N.J., Lee C.W., Lee H.S. Isolation of a novel mycovirus OMIV in Pleurotus ostreatus and its detection using a triple antibody sandwich-ELISA. J. Virol. Methods. 2006;138:24–29. doi: 10.1016/j.jviromet.2006.07.016. [DOI] [PubMed] [Google Scholar]
- Rogers H.J., Buck K.W., Brasier C.M. A mitochondrial target for double-stranded RNA in diseased isolates of the fungus that causes Dutch elm disease. Nature. 1987;329:558–560. doi: 10.1038/329558a0. [DOI] [Google Scholar]
- Sahin E., Keskin E., Akata I. Molecular characterization of a novel partitivirus hosted by the false morel mushroom Gyromitra esculenta. Arch. Virol. 2021;166:1247–1251. doi: 10.1007/s00705-021-04978-3. [DOI] [PubMed] [Google Scholar]
- Sato Y., Suzuki N. Continued mycovirus discovery expanding our understanding of virus lifestyles, symptom expression, and host defense. Curr. Opin. Microbiol. 2023;75 doi: 10.1016/j.mib.2023.102337. [DOI] [PubMed] [Google Scholar]
- Shah U.A., Kotta-Loizou I., Fitt B.D., Coutts R.H. Identification, molecular characterization, and biology of a novel quadrivirus infecting the phytopathogenic fungus Leptosphaeria biglobosa. Viruses. 2018;11(1):9. doi: 10.3390/v11010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahi S., Eusebio-Cope A., Kondo H., Hillman B.I., Suzuki N. Investigation of host range of and host defense against a mitochondrially replicating Mitovirus. J. Virol. 2019;93:e01503–e01518. doi: 10.1128/JVI.01503-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stielow B., Klenk H.P., Menzel W. Complete genome sequence of the first endornavirus from the ascocarp of the ectomycorrhizal fungus Tuber aestivum Vittad. Arch. Virol. 2011;156:343–345. doi: 10.1007/s00705-010-0875-x. [DOI] [PubMed] [Google Scholar]
- Stielow B., Klenk H.P., Winter S., Menzel W. A novel Tuber aestivum (Vittad.) mitovirus. Arch. Virol. 2011;156:1107–1110. doi: 10.1007/s00705-011-0998-8. [DOI] [PubMed] [Google Scholar]
- Stielow B., Menzel W. Complete nucleotide sequence of TaV1, a novel totivirus isolated from a black truffle ascocarp (Tuber aestivum Vittad.) Arch. Virol. 2010;155:2075–2078. doi: 10.1007/s00705-010-0824-8. [DOI] [PubMed] [Google Scholar]
- Stielow J.B., Bratek Z., Klenk H.P., Winter S., Menzel W. A novel mitovirus from the hypogeous ectomycorrhizal fungus Tuber excavatum. Arch. Virol. 2012;157:787–790. doi: 10.1007/s00705-012-1228-8. [DOI] [PubMed] [Google Scholar]
- Sugiura N. Further analysts of the data by akaike's information criterion and the finite corrections. Commun. Stat. Theory Methods. 1978;7:13–26. doi: 10.1080/03610927808827599. [DOI] [Google Scholar]
- Sutela S., Vainio E.J. Virus population structure in the ectomycorrhizal fungi Lactarius rufus and L. tabidus at two forest sites in Southern Finland. Virus Res. 2020;285 doi: 10.1016/j.virusres.2020.197993. [DOI] [PubMed] [Google Scholar]
- Tanabe A.S. Kakusan4 and Aminosan: two programs for comparing nonpartitioned, proportional and separate models for combined molecular phylogenetic analyses of multilocus sequence data. Mol. Ecol. Resour. 2011;11:914–921. doi: 10.1111/j.1755-0998.2011.03021.x. [DOI] [PubMed] [Google Scholar]
- Thekke-Veetil T., Lagos-Kutz D., McCoppin N.K., Hartman G.L., Ju H.K., Lim H.S., Domier L.L. Soybean thrips (Thysanoptera: thripidae) harbor highly diverse populations of arthropod, fungal and plant viruses. Viruses. 2020;12(12):1376. doi: 10.3390/v12121376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urayama S.I., Takaki Y., Chiba Y., Zhao Y., Kuroki M., Hagiwara D., Nunoura T. Eukaryotic microbial RNA viruses—acute or persistent? Insights into their function in the aquatic ecosystem. Microbes Environ. 2022;37 doi: 10.1264/jsme2.ME22034. ME22034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urayama S.I., Takaki Y., Nishi S., Yoshida-Takashima Y., Deguchi S., Takai K., Nunoura T. Unveiling the RNA virosphere associated with marine microorganisms. Mol. Ecol. Resour. 2018;18:1444–1455. doi: 10.1111/1755-0998.12936. [DOI] [PubMed] [Google Scholar]
- Vainio E.J., Pennanen T., Rajala T., Hantula J. Occurrence of similar mycoviruses in pathogenic, saprotrophic and mycorrhizal fungi inhabiting the same forest stand. FEMS Microbiol. Ecol. 2017;93 doi: 10.1093/femsec/fix003. fix003. [DOI] [PubMed] [Google Scholar]
- Vainio E.J., Chiba S., Ghabrial S.A., Maiss E., Roossinck M., Sabanadzovic S., Suzuki N., Xie J., Nibert M. ICTV virus taxonomy profile: partitiviridae. J. Gen. Virol. 2018;99:17. doi: 10.1099/jgv.0.000985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vainio E.J., Hantula J. Taxonomy, biogeography and importance of Heterobasidion viruses. Virus Res. 2016;219:2–10. doi: 10.1016/j.virusres.2015.10.014. [DOI] [PubMed] [Google Scholar]
- Vainio E.J., Sutela S. Mixed infection by a partitivirus and a negative-sense RNA virus related to mymonaviruses in the polypore fungus Bondarzewia berkeleyi. Virus Res. 2020;286 doi: 10.1016/j.virusres.2020.198079. [DOI] [PubMed] [Google Scholar]
- Wolf Y.I., Kazlauskas D., Iranzo J., Lucía-Sanz A., Kuhn J.H., Krupovic M., Dolja V.V., Koonin E.V. Origins and evolution of the Global RNA virome. mBio. 2018;9 doi: 10.1128/mbio.02329-18. e02329-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y.J., Shirouzu T., Chiba Y., Hosaka K., Moriyama H., Urayama S.I., Hagiwara D. Identification of novel RNA mycoviruses from wild mushroom isolates in Japan. Virus Res. 2023;325 doi: 10.1016/j.virusres.2023.199045. 110.1016/j.virusres.2023.199045. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data was used for the research described in the article.