Abstract
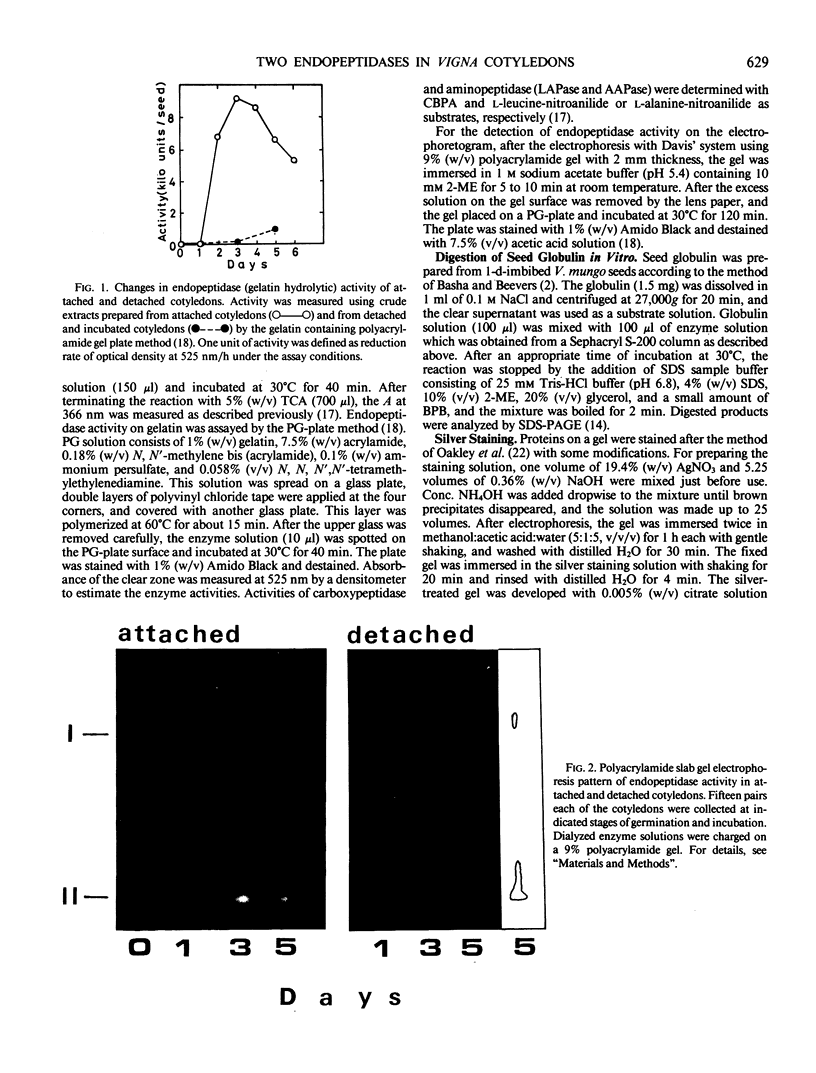
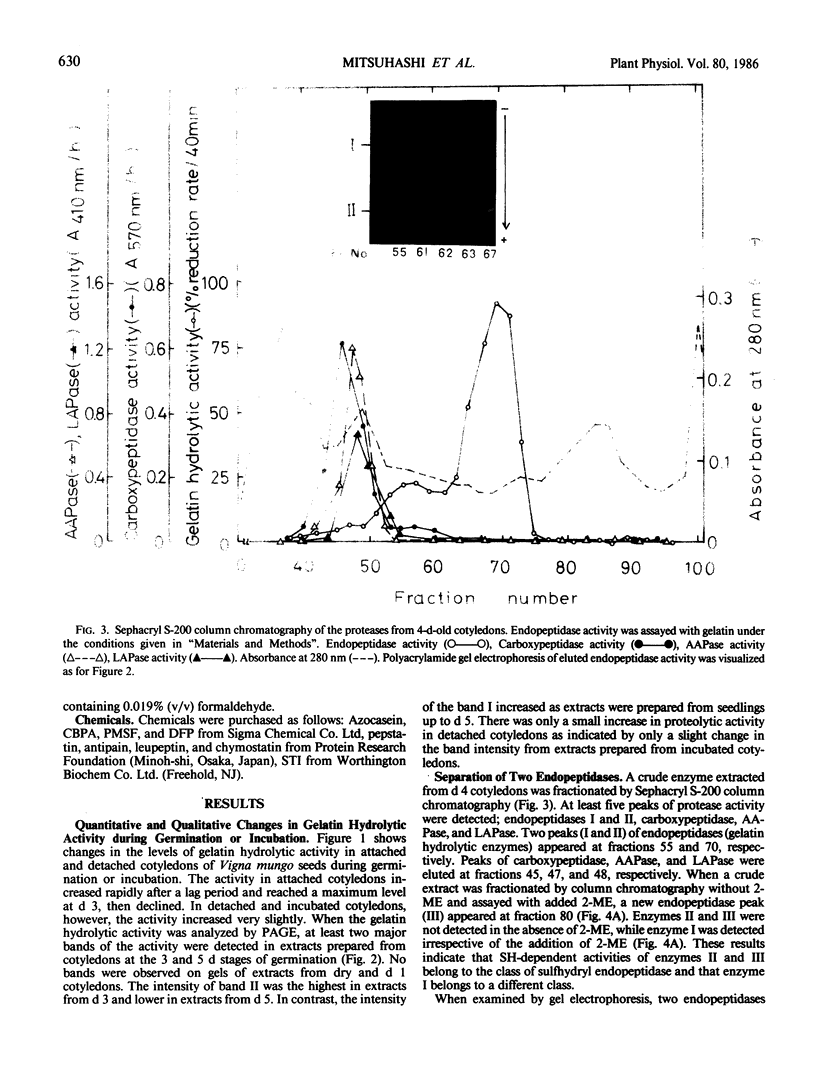
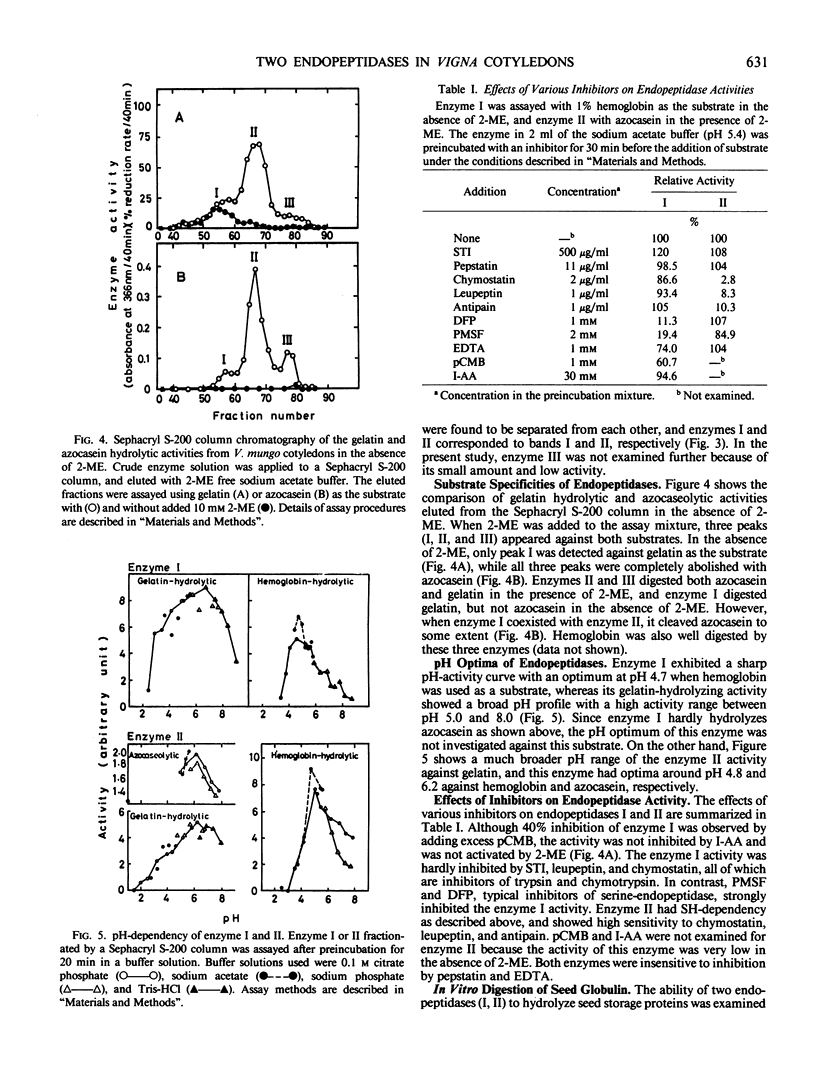
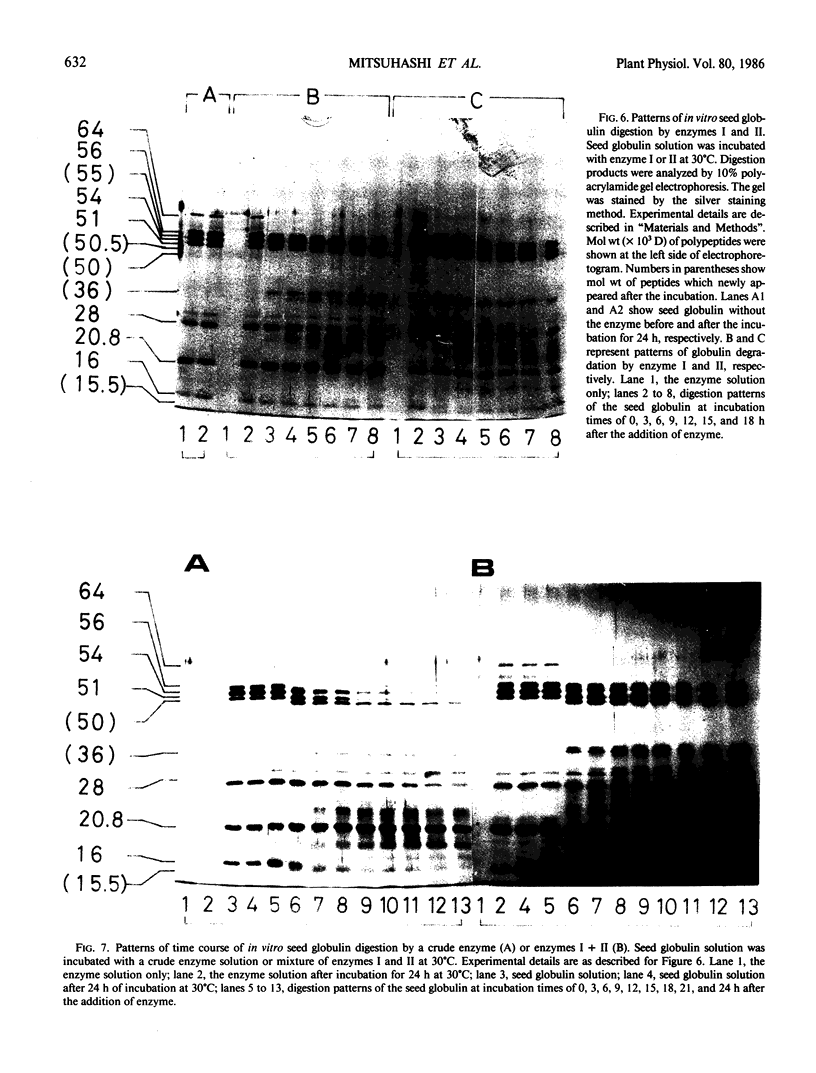
Two major endopeptidases were present in cotyledons of germinating Vigna mungo seeds, as detected by the zymogram after polyacrylamide gel electrophoresis. They were not detectable in cotyledons of dry seeds, but their intensities on the zymogram increased during germination. During incubation of detached cotyledons, however, the activities showed only a slight increase for 5 days. These two endopeptidases could be separated by Sephacryl S-200 column chromatography. One of them was found to be a serine-endopeptidase as judged by phenylmethylsulfonylfluoride and diisopropyl fluorophosphate inhibition. The other was a sulfhydryl-endopeptidase because of its dependency on 2-mercaptoethanol and inhibition by leupeptin, chymostatin, and antipain. Analysis by sodium dodecyl sulfate polyacrylamide gel electrophoresis indicatd that the two endopeptidases digested the Vigna mungo seed globulin subunits at different rates. The serine enzyme digested the 56 kilodalton subunit at first, but the sulfhydryl enzyme digested the 54 kilodalton peptide more efficiently than the 56 kilodalton peptide. The pattern of digestion of globulin by the combination of the serine- and sulfhydryl-endopeptidases was similar to that using crude enzyme extracts.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumgartner B., Chrispeels M. J. Purification and characterization of vicilin peptidohydrolase, the major endopeptidase in the cotyledons of mung-bean seedlings. Eur J Biochem. 1977 Jul 15;77(2):223–233. doi: 10.1111/j.1432-1033.1977.tb11661.x. [DOI] [PubMed] [Google Scholar]
- Bond H. M., Bowles D. J. Characterization of soybean endopeptidase activity using exogenous and endogenous substrates. Plant Physiol. 1983 Jun;72(2):345–350. doi: 10.1104/pp.72.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catsimpoolas N., Campbell T. G., Meyer E. W. Immunochemical study of changes in reserve proteins of germinating soybean seeds. Plant Physiol. 1968 May;43(5):799–805. doi: 10.1104/pp.43.5.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrispeels M. J., Baumgartner B., Harris N. Regulation of reserve protein metabolism in the cotyledons of mung bean seedlings. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3168–3172. doi: 10.1073/pnas.73.9.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csoma C., Polgár L. Proteinase from germinating bean cotyledons. Evidence for involvement of a thiol group in catalysis. Biochem J. 1984 Sep 15;222(3):769–776. doi: 10.1042/bj2220769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg G. K., Virupaksha T. K. Acid protease from germinated sorghum. 1. Purification and characterization of the enzyme. Eur J Biochem. 1970 Nov;17(1):4–12. doi: 10.1111/j.1432-1033.1970.tb01124.x. [DOI] [PubMed] [Google Scholar]
- Harris N., Chrispeels M. J. Histochemical and biochemical observations on storage protein metabolism and protein body autolysis in cotyledons of germinating mung beans. Plant Physiol. 1975 Aug;56(2):292–299. doi: 10.1104/pp.56.2.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mahaboob Basha S. M., Cherry J. P. Proteolytic enzyme activity and storage protein degradation in cotyledons of germinating peanut (Arachis hypogaea L.) seeds. J Agric Food Chem. 1978 Jan-Feb;26(1):229–234. doi: 10.1021/jf60215a045. [DOI] [PubMed] [Google Scholar]
- Murachi T. Bromelain enzymes. Methods Enzymol. 1976;45:475–485. doi: 10.1016/s0076-6879(76)45042-5. [DOI] [PubMed] [Google Scholar]
- Nielsen S. S., Liener I. E. Degradation of the Major Storage Protein of Phaseolus vulgaris during Germination : Role of Endogenous Proteases and Protease Inhibitors. Plant Physiol. 1984 Mar;74(3):494–498. doi: 10.1104/pp.74.3.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Tully R. E., Beevers H. Proteases and Peptidases of Castor Bean Endosperm: Enzyme Characterization and Changes during Germination. Plant Physiol. 1978 Nov;62(5):746–750. doi: 10.1104/pp.62.5.746. [DOI] [PMC free article] [PubMed] [Google Scholar]