Abstract
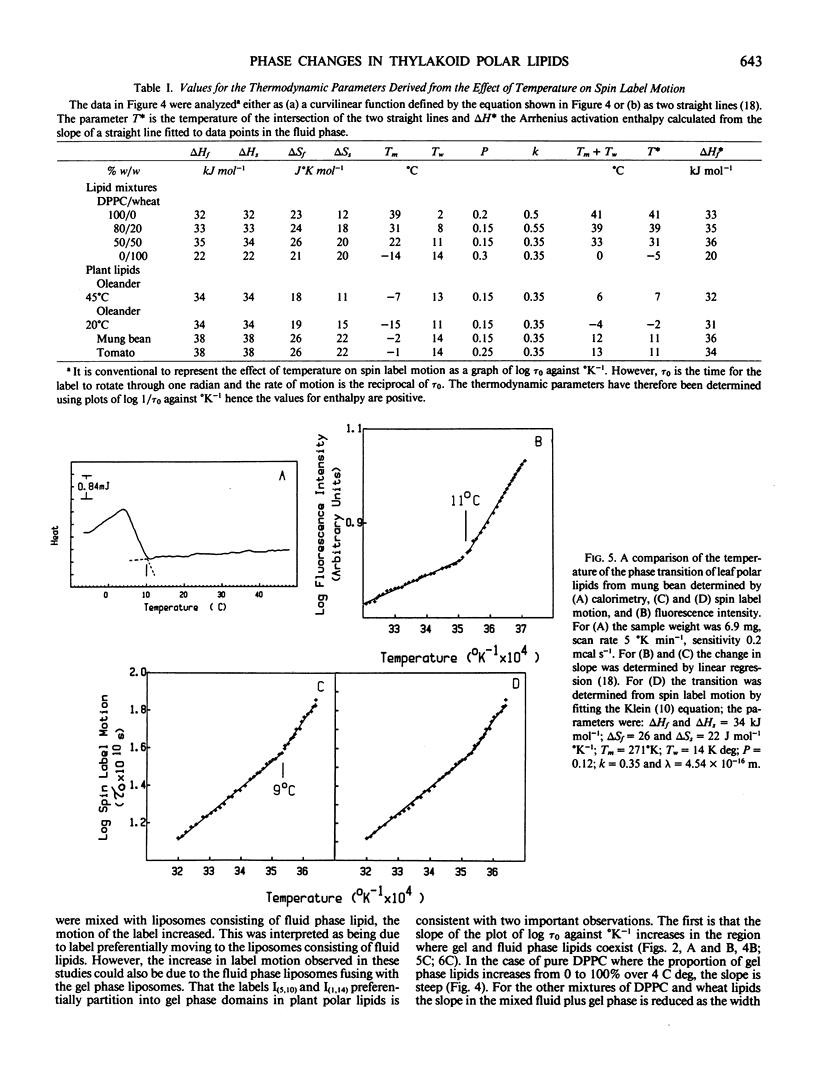
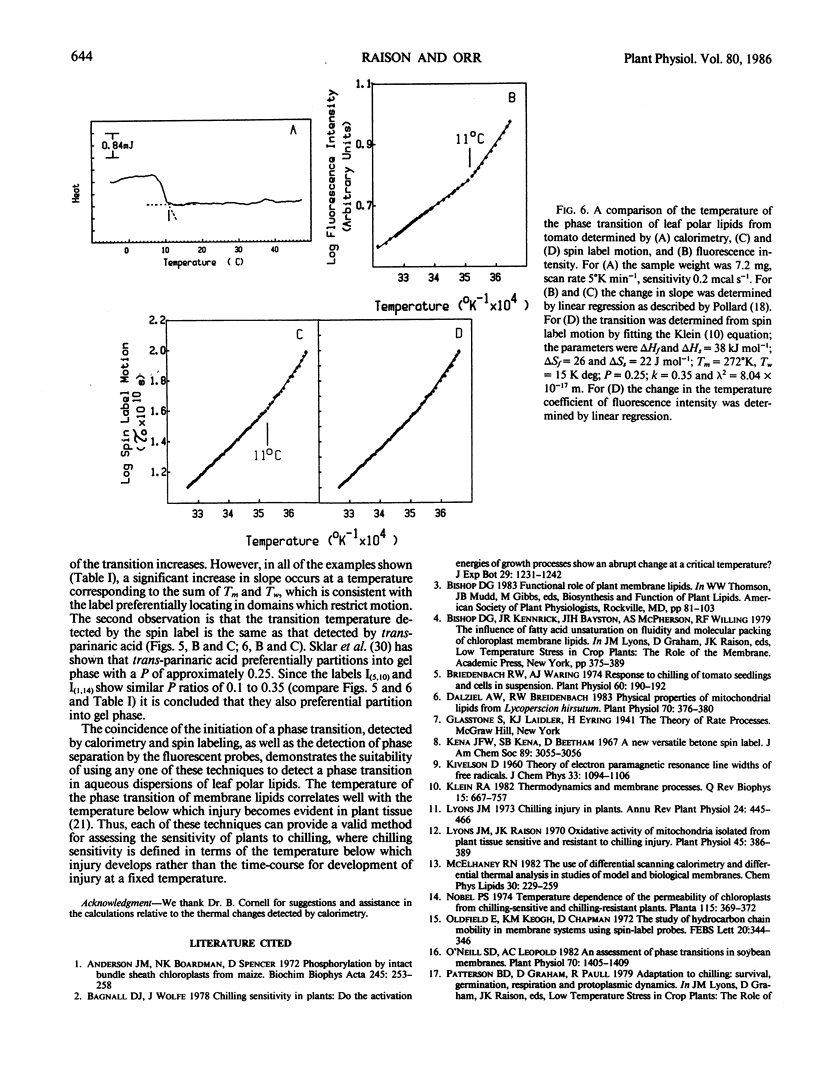
The phase behavior of thylakoid polar lipids from plants sensitive to chilling injury was investigated by calorimetry, electron spin resonance spectroscopy of spin labels, and fluorescence intensity after labeling with trans-parinaric acid. The plants used were oleander (Nerium oleander), mung bean (Vigna radiata L. var Mungo), and tomato (Lycopersicon esculentum cv Grosse Lisse). For all plants the initiation temperature for the calorimetric exotherm was coincident (±1°C) with the transition determined by the increase in the temperature coefficient of spin label motion and fluorescence intensity of trans-parinaric acid. For oleander plants, grown at 45°C, the transition was at 7°C while for plants from the same clone, grown at 20°C, it was at −2°C. For mung bean and tomato the transition was between 9 and 12°C. The similarity in the transition detected by spin labeling and fluorescence intensity suggest that spin labels, like the fluorescent label trans-parinaric acid, preferentially partition into domains of ordered lipid. The coincidence of the temperature for initiation of the transition, determined by the three techniques, shows that each is a valid method of assessing a phase transition in membrane polar lipids.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. M., Boardman N. K., Spencer D. Phosphorylation by intact bundle sheath chloroplasts from maize. Biochim Biophys Acta. 1971 Aug 6;245(1):253–258. doi: 10.1016/0005-2728(71)90032-6. [DOI] [PubMed] [Google Scholar]
- Breidenbach R. W., Waring A. J. Response to chilling of tomato seedlings and cells in suspension cultures. Plant Physiol. 1977 Aug;60(2):190–192. doi: 10.1104/pp.60.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalziel A. W., Breidenbach R. W. Physical Properties of Mitochondrial Lipids from Lycopersicon hirsutum. Plant Physiol. 1982 Aug;70(2):376–380. doi: 10.1104/pp.70.2.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R. A. Thermodynamics and membrane processes. Q Rev Biophys. 1982 Nov;15(4):667–757. doi: 10.1017/s0033583500003772. [DOI] [PubMed] [Google Scholar]
- Lyons J. M., Raison J. K. Oxidative activity of mitochondria isolated from plant tissues sensitive and resistant to chilling injury. Plant Physiol. 1970 Apr;45(4):386–389. doi: 10.1104/pp.45.4.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElhaney R. N. The use of differential scanning calorimetry and differential thermal analysis in studies of model and biological membranes. Chem Phys Lipids. 1982 May;30(2-3):229–259. doi: 10.1016/0009-3084(82)90053-6. [DOI] [PubMed] [Google Scholar]
- O'neill S. D., Leopold A. C. An assessment of phase transitions in soybean membranes. Plant Physiol. 1982 Nov;70(5):1405–1409. doi: 10.1104/pp.70.5.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield E., Keough K. M., Chapman D. The study of hydrocarbon chain mobility in membrane systems using spin-label probes. FEBS Lett. 1972 Feb 15;20(3):344–346. doi: 10.1016/0014-5793(72)80103-0. [DOI] [PubMed] [Google Scholar]
- Pomeroy M. K., Raison J. K. Maintenance of Membrane Fluidity during Development of Freezing Tolerance of Winter Wheat Seedlings. Plant Physiol. 1981 Aug;68(2):382–385. doi: 10.1104/pp.68.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison J. K., Lyons J. M., Mehlhorn R. J., Keith A. D. Temperature-induced phase changes in mitochondrial membranes detected by spin labeling. J Biol Chem. 1971 Jun 25;246(12):4036–4040. [PubMed] [Google Scholar]
- Raison J. K., Lyons J. M., Thomson W. W. The influence of membranes on the temperature-induced changes in the kinetics of some respiratory enzymes of mitochondria. Arch Biochem Biophys. 1971 Jan;142(1):83–90. doi: 10.1016/0003-9861(71)90261-x. [DOI] [PubMed] [Google Scholar]
- Raison J. K., Pike C. S., Berry J. A. Growth Temperature-Induced Alterations in the Thermotropic Properties of Nerium oleander Membrane Lipids. Plant Physiol. 1982 Jul;70(1):215–218. doi: 10.1104/pp.70.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreier S., Polnaszek C. F., Smith I. C. Spin labels in membranes. Problems in practice. Biochim Biophys Acta. 1978 Dec 15;515(4):395–436. doi: 10.1016/0304-4157(78)90011-4. [DOI] [PubMed] [Google Scholar]
- Shneyour A., Raison J. K., Smillie R. M. The effect of temperature of the rate of photosynthetic electron transfer in chloroplasts of chilling-sensitive and chilling-resistant plants. Biochim Biophys Acta. 1973 Jan 18;292(1):152–161. doi: 10.1016/0005-2728(73)90259-4. [DOI] [PubMed] [Google Scholar]
- Sklar L. A., Miljanich G. P., Dratz E. A. Phospholipid lateral phase separation and the partition of cis-parinaric acid and trans-parinaric acid among aqueous, solid lipid, and fluid lipid phases. Biochemistry. 1979 May 1;18(9):1707–1716. doi: 10.1021/bi00576a012. [DOI] [PubMed] [Google Scholar]


