Abstract
Sections excised from maize coleoptiles incorporated radioactivity from proline, tyrosine, and phenylalanine into structural components of the cell wall. Only about 2% of radioactivity from proline taken up by sections was incorporated into cell wall; about 24% of that incorporated was in hydroxyproline and the rest remained in proline. In contrast, as much as 40% of the radioactivity from phenylalanine and 30% from tyrosine was incorporated into cell wall material. Most of this radioactivity was in saponifiable ferulic acid. Small amounts of p-coumaric and diferulic acid were found, but only a small fraction of the hemicellulose can possibly be immobilized directly through cross-linking of diferulic esters. Substantial amounts of radioactivity from aromatic amino acids remained insoluble after strong alkali extractions of wall material, and a large fraction of polysaccharide was solubilized by dilute alkali following oxidation of phenolics by acidic NaClO2. Hence, hemicellulosic material in the cell walls of maize coleoptiles may be organized and cross-linked primarily through alkali-resistant etherified aromatics.
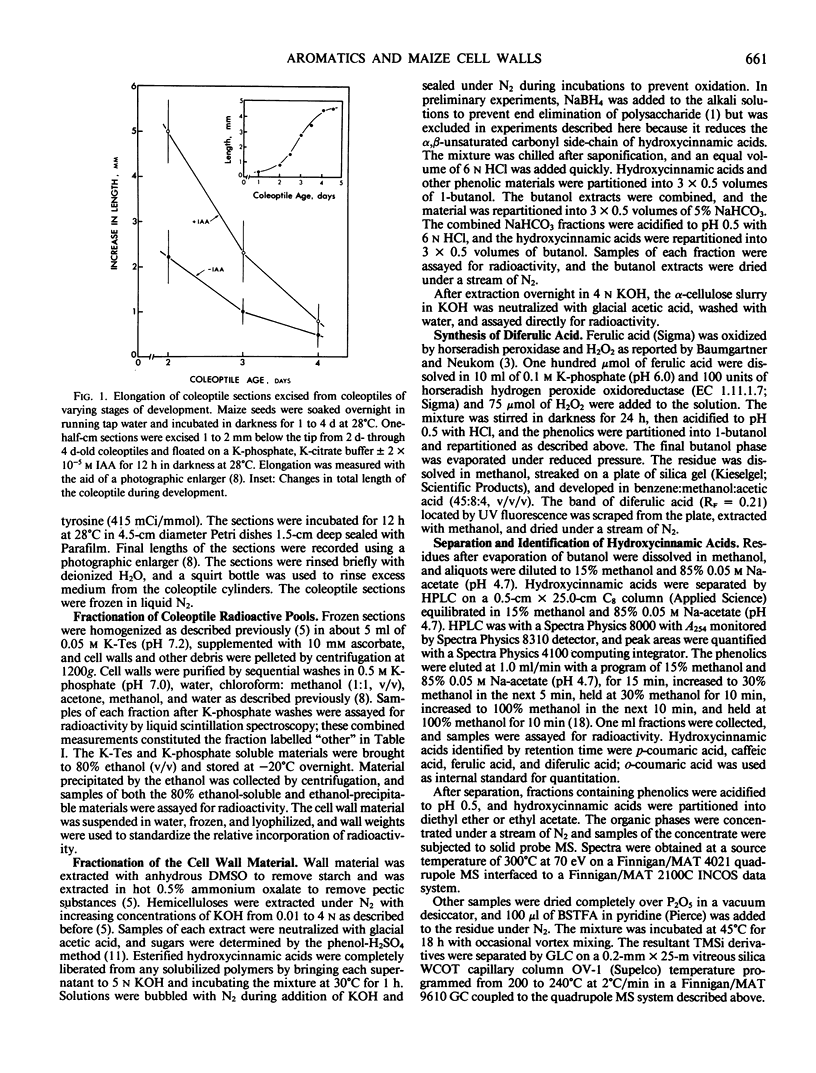
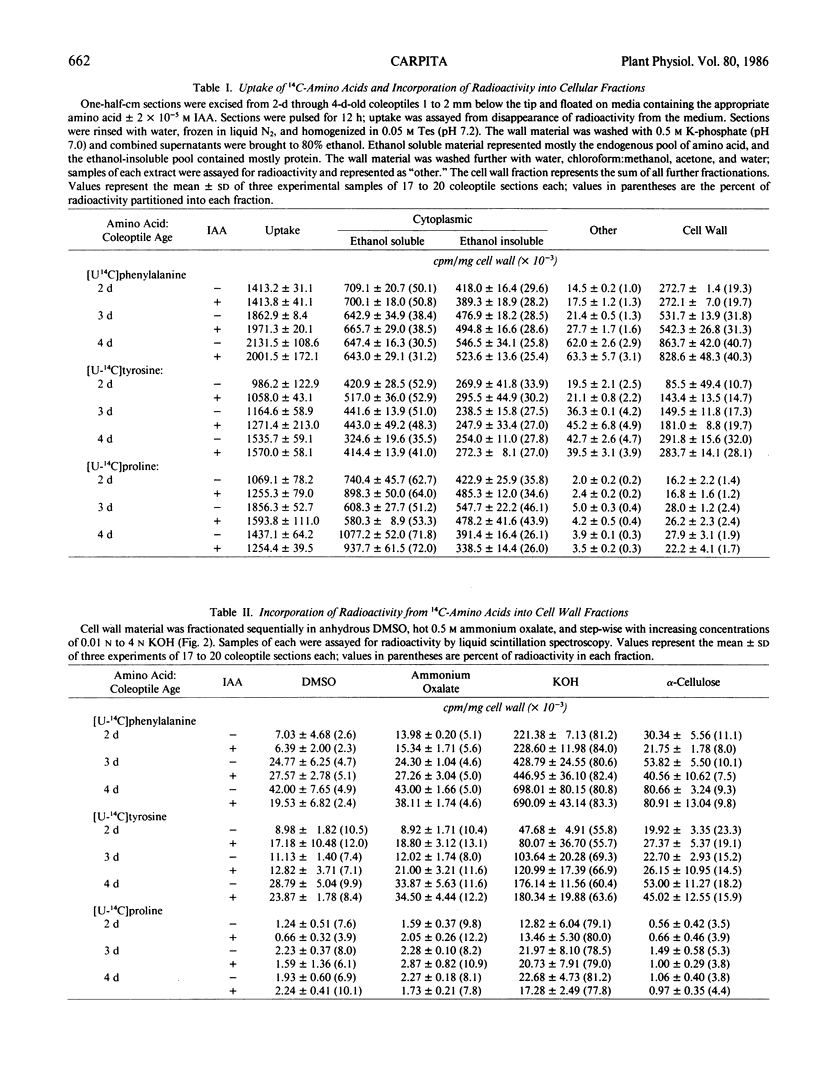
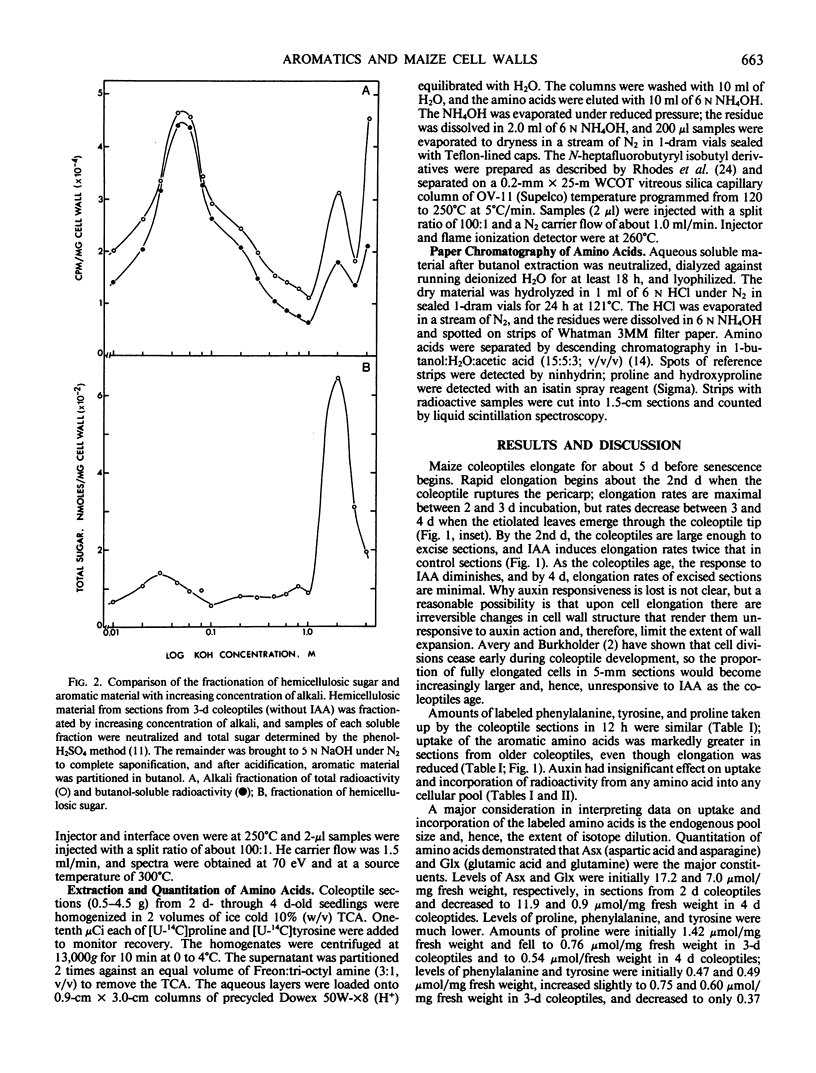
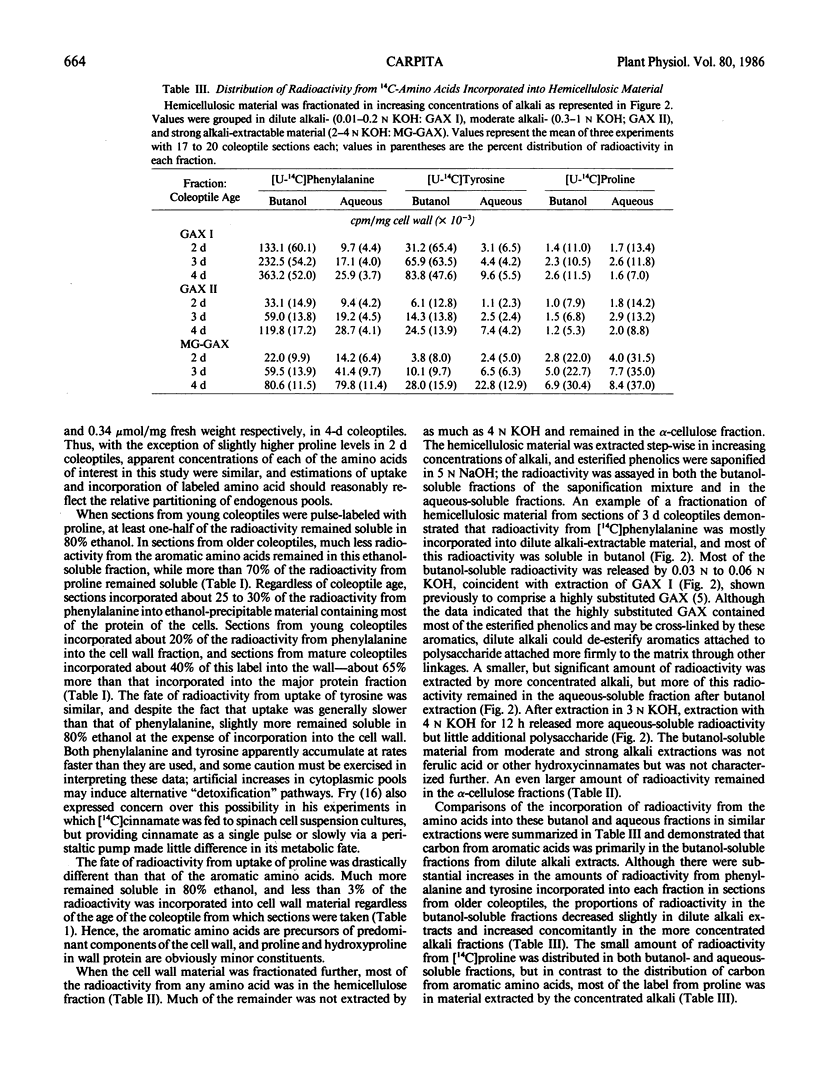
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carpita N. C., Brown R. A., Weller K. M. Uptake and Metabolic Fate of Glucose, Arabinose, and Xylose by Zea mays Coleoptiles in Relation to Cell Wall Synthesis. Plant Physiol. 1982 May;69(5):1173–1180. doi: 10.1104/pp.69.5.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpita N. C. Cell wall development in maize coleoptiles. Plant Physiol. 1984 Sep;76(1):205–212. doi: 10.1104/pp.76.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpita N. C. Hemicellulosic polymers of cell walls of zea coleoptiles. Plant Physiol. 1983 Jun;72(2):515–521. doi: 10.1104/pp.72.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland R. E. The control of cell enlargement. Symp Soc Exp Biol. 1977;31:101–115. [PubMed] [Google Scholar]
- Cooper J. B., Varner J. E. Insolubilization of hydroxyproline-rich cell wall glycoprotein in aerated carrot root slices. Biochem Biophys Res Commun. 1983 Apr 15;112(1):161–167. doi: 10.1016/0006-291x(83)91811-9. [DOI] [PubMed] [Google Scholar]
- Fry S. C. Isodityrosine, a new cross-linking amino acid from plant cell-wall glycoprotein. Biochem J. 1982 May 15;204(2):449–455. doi: 10.1042/bj2040449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes D., Myers A. C., Jamieson G. Gas Chromatography-Mass Spectrometry of N- Heptafluorobutyryl Isobutyl Esters of Amino Acids in the Analysis of the Kinetics of [N]H(4) Assimilation in Lemna minor L. Plant Physiol. 1981 Nov;68(5):1197–1205. doi: 10.1104/pp.68.5.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmore F. W. Phenolic acids in wheat coleoptile cell walls. Plant Physiol. 1974 May;53(5):728–731. doi: 10.1104/pp.53.5.728. [DOI] [PMC free article] [PubMed] [Google Scholar]


