Summary
Inactivated oral cholera vaccines (OCVs) are a cornerstone of international efforts to control cholera, and are currently deployed from a global stockpile for the control of epidemics and endemic hotspots, as well as for humanitarian emergencies. One inactivated OCV (with tradenames Shanchol™ and Euvichol-Plus™) is used in the stockpile, but the number of available doses is inadequate to meet the rapidly rising demand for OCVs from countries affected by cholera. Newer, simplified inactivated OCVs under development offer the possibilities of lower expense and higher production yields, and could expand the stockpile. However, their clinical development is made complex because placebo-controlled randomised trials of OCV efficacy are no longer ethically permissible and because the serum vibriocidal antibodies used to measure OCV responses are not correlates of OCV protection against cholera. Here, we propose an observational study design with features to enhance methodological rigor to provide credible evidence of protection against cholera by these newer vaccines.
Keywords: Cholera vaccines, Observational studies, Vaccine evaluation, Legacy vaccines
Search strategy and selection criteria.
References for this Viewpoint were searched in PubMed using the search terms “cholera vaccine”, “vaccine evaluation”, “observational evaluations” and “non-experimental evaluations” from January, 1990 through September, 2023. Relevant publications were also sought from the authors’ personal files. Only papers published in English were reviewed. The final reference list was based on relevance to the topic of this essay.
Introduction
The global control of cholera has been enhanced in recent years by the introduction of an inactivated, oral cholera vaccine (OCV) consisting of killed Vibrio cholerae cells. This vaccine, licensed under the tradenames Shanchol™ and Euvichol-Plus™ by two producers, has been proven safe and effective, both in clinical trials and post-licensure deployments.1 To date, this is the only OCV to have been used in the WHO-coordinated Global OCV Stockpile created in 2013 and supported by Gavi.2 More than 150 million OCV doses have been deployed from the stockpile for use in cholera epidemics, endemic cholera “hotspots”, and humanitarian emergencies in over 20 countries in Asia, Africa, the Gulf, and the Caribbean.
However, since the stockpile’s inception, demand for the inactivated OCV has greatly exceeded the supply, a disparity exacerbated by the unprecedented number and size of major cholera outbreaks reported from mid-2021 until the present day.3 As a consequence, there is consensus on the need for a far greater number of OCV doses from more producers. One strategy to achieve this goal would be to increase the supply of the current OCV, which consists of three inactivated O1 serogroup strains that are either heat-inactivated or formalin-inactivated, as well as one formalin-inactivated O139 serogroup strain (Table 1). Another approach would be to simplify the current formulation by including only one or two component inactivated O1 strains expressing both the Inaba and Ogawa antigens, since only 01 cholera of either serotype currently causes cholera globally. While this change is not anticipated to increase vaccine protection, it could lower vaccine cost and increase the output of vaccine production, because the efficiency of production and quality control is augmented by fermentation of fewer batches of cholera vibrios per dose.
Table 1.
Comparison of Euvichol-Plus, an OCV currently used by the global OCV stockpile, and two new, simplified OCVs, Euvchol-S and Hillchol.
| Euvichol-Plus | Euvichol-S | Hillchol | |
|---|---|---|---|
| Description | Vaccine consisting of killed whole cells of V. cholerae O1 (formalin-inactivated Inaba El Tor strain, heat-inactivated Ogawa classical strain, formalin-inactivated Ogawa classical strain and heat-inactivated Inaba classical strain) and formalin-inactivated O139 strain | Simplified formulation of Euvichol-Plus containing only two components: formalin-inactivated O1 Inaba and O1 Ogawa | Vaccine composed of formaldehyde-inactivated V. cholerae O1 Hikojima |
| Recommended age | 1 year and older | 1 year and older | 1 year and older |
| Delivery | Oral | Oral | Oral |
| Doses | 2 given ≥2 weeks apart | 2 given ≥2 weeks apart | 2 given ≥2 weeks apart |
| Storage temperature | 2–8 °C | 2–8 °C | 2–8 °C |
| Packaging | Single dose 1.5 ml/plastic tube | Single dose 1.5 ml | Single dose 1.5 ml |
| WHO pre-qualification | Yes (2017) | No | No |
OCV = oral cholera vaccine.
Two newer, simplified inactivated whole-cell vaccines have been developed and are undergoing clinical testing. One is a single strain OCV, trade name Hillchol™, which is produced by Bharat in India and consists of a single inactivated El Tor biotype strain that has been genetically engineered to express both the Ogawa and Inaba antigens. Hillchol™ was found to be safe and to elicit serum vibriocidal antibody responses that were non-inferior to Shanchol™ in a phase 1/2 trial in Bangladesh, and has recently been tested in India (findings not yet available).4 The other simplified OCV, Euvichol-S™, produced by Eubiologics in South Korea, contains only two (El Tor Inaba and El Tor Ogawa) of the component strains currently in Euvichol-Plus™. A randomised clinical trial assessing the non-inferiority of Euvichol-S in comparison with Shanchol™ has recently been completed in Nepal.5 Results from these recent trials, for which serum vibriocidal antibody responses—not clinical protection against cholera—is the endpoint, have not yet been reported. The characteristics of Euvichol-Plus™ and the two newer inactivated OCVs under development are compared in Table 1.
Since neither vaccine is identical to the OCVs currently in the stockpile and because the test used to measure immune responses to OCVs, serum vibriocidal antibodies, is not a correlate of vaccine protection, we have argued that demonstration of clinical protection against cholera should be required before putting the newer vaccines into general use through the stockpile.6 Double-blinded, randomised controlled trials (RCT) of clinical protection in which controls receive an agent that does not protect against cholera would offer the strongest approach for this assessment. Unfortunately, such trials are no longer ethically permissible since the currently stockpiled OCVs are internationally licensed, and are prequalified by WHO for purchase by UN agencies and in general use. We have, therefore, proposed that methodologically strengthened observational studies, using a closed cohort design similar to an RCT, could be used to measure clinical protection against cholera.6
Here, we provide a more detailed justification for this recommendation and outline methodological features that can be used to enhance the scientific strength of these observational studies.
Strengthening the design and conduct of an observational demonstration project for measurement of OCV protection
It is axiomatic that the methodological strength of an observational study is best judged by how well the study design captures the methodological strengths of the corresponding RCT of the same topic.7,8 Elsewhere, it has been argued that the most direct way for an observational study of therapy to do this is to conduct a closed cohort study with all of the features of an RCT, minus randomisation and double-blinding.9
How might the design and analysis of a closed cohort evaluation of OCV protection against cholera capture the strengths of the corresponding RCT? In such a study, persons at risk for cholera and eligible for OCV would, as in a typical Phase III trial of OCV efficacy, be offered OCV via mass immunisation at baseline, and OCV vaccinees and otherwise eligible non-vaccinees from the baseline population would then be followed longitudinally and concurrently with uniform procedures to detect and diagnose the comparative incidence of cholera.
If such a study is to produce a scientifically valid estimate of OCV protection, it must overcome several major threats that can bias estimates of OCV protection in analysed OCV recipients versus non-recipients: 1) imbalances in the baseline risk of cholera; 2) misclassification of whether a person received OCV; 3) imbalances in the receipt of other preventive interventions against cholera (cointerventions); 4) imbalances in the post-baseline loss from the baseline cohort via migration, refusal to continue or death; 5) imbalances in the tendency of study participants to attend health care facilities used to diagnose cholera; 6) imbalances in how patients are enrolled and fecal specimens are collected once patients present for care; 7) imbalances in how specimens are processed and tested, or inaccuracy or imbalances in how the diagnosis of cholera is made in microbiological tests of stools; 8) inaccurate or biased linkage of the patient’s identity to baseline census/vaccination data files; 9) lack of an a priori analytic plan for analysis of OCV protection, with primary and secondary analyses; 10) failure to formally lock the dataset before undertaking analyses; and 11) selective reporting of the study results.
Two of the chief methodological strengths of conventional RCTs of the efficacy of OCVs are randomised assignment of the OCV and control agent under evaluation and double-blinding of participants and investigators, features that for ethical reasons cannot now be used in studies of OCV protection. However, because of the many other scientific strengths of the prospective, closed cohort paradigm apart from randomisation and double-blinding, appropriate adaptation and use of the paradigm can help provide protection against the scientific threats both addressed and not addressed by randomisation and double-blinding.9 Moreover, results of such an OCV demonstration project can be challenged with analyses to evaluate whether the study is weakened by residual bias. These methodological safeguards and analyses to measure bias are cornerstones of our proposed observational approach.
Specific elements of the design of the study
As in an RCT, an observational demonstration project of an OCV would target a population that is large enough to provide adequate statistical power to estimate protection at a desired level of statistical precision. Because the ratio of vaccinees to non-vaccinees cannot be directly controlled as in an RCT, the sample size calculation should be conservative. Accordingly, sample sizes should be calculated for the entire range of values of likely OCV uptake, and should take the largest estimate as the required sample size. The study would also follow the ethical and scientific requirements of ICH Good Clinical Practice.10 These help to ensure that the study is ethically appropriate and formally approved; is conducted by adequately trained staff following a formal, scientifically sound protocol; arranges to handle all data in a manner that allows for accurate reporting, interpretation, and verification; employs quality control and quality assurance measures to measure and help ensure compliance of study activities with the study protocol; and is conducted in a setting that offers adequate access to and quality of treatment for cholera.
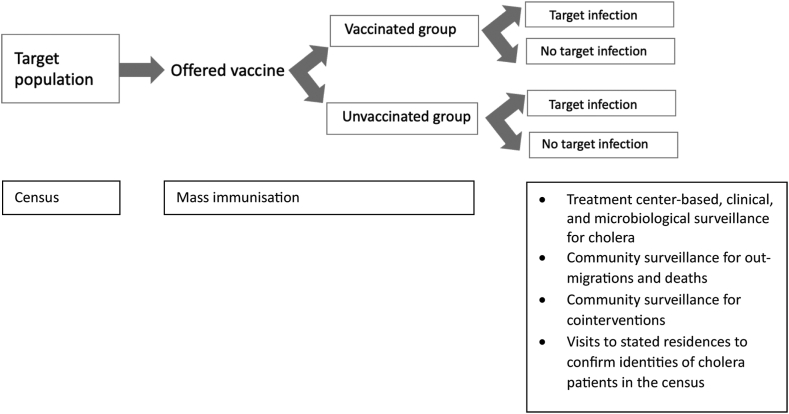
Furthermore, as in an RCT, the study would be designed as a prospective, closed cohort study, preceded by a population census and with subsequent systematic, community-based demographic surveillance for out-migrations and deaths as well as treatment centre-based surveillance for cholera after OCV delivery begins. Fig. 1 shows the basic study design. Prospective conduct of the study and collection of data in real time as events occur enable implementation of quality control and assurance measures to assure compliance with the protocol, as well as systematic and accurate ascertainment and recording of study events without knowledge whether the participant ultimately developed cholera. The closed cohort design, in which membership in compared cohorts of eligible recipients and non-recipients of OCV is established at baseline, allows for monitoring and assessment of post-baseline losses to follow-up in the compared cohorts, thus permitting appropriate analyses to adjust for biases due to differential losses to follow-up after baseline.
Fig. 1.
A closed cohort study comparing oral cholera vaccine (OCV) recipients versus non-recipients for evaluation of OCV protection againstcholera.
The study would begin with a baseline census conducted shortly before mass vaccination, which defines the population that can be included in the compared cohorts analysed for OCV protection, and also provides unique individual identification numbers for later identification of participants at the time of vaccination and later follow-up. The census would also include comprehensive collection of household and individual level demographic, socioeconomic, and water-sanitation-hygiene (WASH) information that aids in establishment of eligibility for vaccination, and that defines potentially confounding variables for analytic adjustment in the assessment of OCV protection against cholera.
Equal eligibility and exclusion criteria would be established for participants analysed as vaccinees and non-vaccinees, including the population at risk, and excluding persons with past histories of receipt of OCV as well as participants with contraindications to vaccination. This tactic helps to ensure that baseline risk of cholera at the outset of follow-up is similar for the compared groups. Concurrent enrollment of vaccinees and non-vaccinees, as might occur when OCV is delivered in discrete rounds of vaccination at baseline and participants are selected from persons present during the rounds, would be implemented to facilitate the concurrent follow-up of closed cohorts of vaccinees and non-vaccinees. Concurrent follow-up helps to mitigate biases due to imbalances in the secular periods of follow-up of the compared groups. As in an RCT, it would be critical to define a zero-time date for each participant, which marks a time of receipt or non-receipt of vaccine. Zero-time provides an equivalent and unique datemark for each participant. It helps ensure that follow-up is concurrent for the compared groups, and defines a comparable baseline time for vaccinees and non-vaccinated controls for ascertainment of potential confounding variables, as well as variables measuring access to surveillance treatment sites, which may require adjustment in analyses of OCV protection to prevent bias. While the selection of such variables may differ from setting to setting, Table 2 provides an illustrative list of baseline variables that might be considered for ascertainment. In an RCT zero-time may be selected in various ways for OCV vaccinees and recipients of the control agent, sometimes the time of the first dose of the administered regimens. One approach for evaluating protection by receipt of at least one dose of OCV that is delivered during a single mass immunisation campaign would be to select the time of the first dose of the OCV regimen for the OCV cohort and the mid-point of the first round of OCV vaccination, when first doses are delivered to vaccinees, for the control cohort.
Table 2.
An illustrative list of zero-time variables to characterise and to consider for analytic adjustments of OCV protection in the event of imbalances between OCV recipients and non-recipients.
| Individual characteristics | Household characteristics | Healthcare utilization |
|---|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
OCV = oral cholera vaccine.
Usually used instead as an exclusionary variable in the assembly of OCV recipients and non-recipients.
Because cholera is a rare disease, even in highly endemic settings, and because prevention of clinically significant cholera is the major preventive objective of OCVs, surveillance for cholera in RCTs typically relies upon passive surveillance for cholera among persons who present for care to treatment settings, a feature that would be replicated in an observational, closed cohort study. As in RCTs the surveillance system and procedures for cholera diagnosis used in the observational study should be well-established before vaccination is undertaken. Several tactics would be employed to help prevent biased detection of cholera in vaccinees versus non-vaccinees in this surveillance. Firstly, efforts would be made to conduct surveillance in all major sources of care for cholera for the participating population, and to make this care freely accessible to the entire population to help mitigate biases due to different tendencies of vaccinees and non-vaccinees to seek care for treatment of acute watery diarrhea, the clinical syndrome of cholera. Secondly, accurate identification of patients in the baseline census at the time of presentation for care would be ensured. This can be done with the aid of a digitised version of the baseline census, without information on OCV receipt, available at each treatment site. Thirdly, efforts would be made to enroll all patients with acute watery diarrhea who come from the study population for care. Records would be kept for patients who were eligible for vaccination and presented for care, but who were not, for whatever reason, entered into clinical surveillance, to enable later analysis of bias that this non-inclusion might have caused. Fourthly, uniform and validated methods would be used for collection of patient data and fecal specimens, and for laboratory evaluation of the specimens for V. cholerae. Fifthly, to make sure of the identities of study participants who are found to have cholera, who will be relatively small in number and for whom even modest rates of misidentification can create serious biases in estimating OCV protection, visits would be made to the stated residences of patients shortly after receipt of the test results to confirm that those with positive results had indeed sought care on the date of presentation. Finally, to further minimise the potential for biased detection of cholera in OCV recipients versus non-recipients, all activities related to enrollment, evaluation and identification of patients at the surveillance sites would be done by staff who do not have access to records of the identities of persons who had received OCV.
Compensating for the absence of randomised allocation and double-blinding
Here we discuss features of the prospective, closed cohort design that could help to compensate for the absence of randomised allocation and double-blinding. Randomisation serves chiefly to ensure that recipients of OCV and the control agent are well balanced with respect to baseline risk factors—both measured and unmeasured- for cholera, while double-blinding further strengthens baseline comparability of risk by making determination of study eligibility and participant agreement to participate blinded to knowledge of the study agent received. Double-blinding also helps protect against imbalances between vaccinees and non-vaccinees in decisions to implement cointerventions (interventions other than OCV that can prevent cholera), imbalances in post-baseline losses to follow-up due to participant or investigator decisions to withdraw participant participation in the study, imbalances in the tendency of participants to seek care at health care sites used for surveillance, imbalances in the procedures used to ascertain patient identities in the census, imbalanced enrollment of patients and/or collection of fecal specimens for testing at the surveillance sites, and imbalances in the accuracy of interpretation of fecal diagnostic tests for cholera.
Several features of RCTS of OCV efficacy other than randomisation and double-blinding can also help to prevent these biases in an observational closed cohort study. In the absence of randomisation, an observational study would use uniform eligibility and exclusion criteria for OCV recipients and non-vaccinated controls, comprehensively ascertain information on baseline risk factors for cholera in the compared groups, and analytically adjust estimates of OCV protection for imbalanced distributions of risk factors. These tactics are useful to help ensure that estimates of protection are not biased by unbalanced baseline risk for cholera in the compared vaccinated and unvaccinated groups. However, they are not the equal of randomisation balancing both measured and unmeasured risk factors in the groups.
Similarly, there are other tactics that can be used in an observational study of OCV to address many of the functions of double-blinding in prevention of biased estimates of OCV protection. Differential cointerventions in OCV recipients versus controls would be monitored both at baseline and post-baseline in community-based surveillance and adjusted for in analyses of protection. As well, post-zero time losses to follow-up would be assessed in systematic surveillance for demographic events, and imbalances would be analytically adjusted for in measurements of OCV protection. Differential seeking of care for diarrhea at the study surveillance sites would be addressed by adjusting for baseline differences in proximity of households to the care sites, as well as baseline variables assessing care-seeking behavior for acute watery diarrhea. It would also be addressed by secondary analyses of OCV protection against clinically severe cholera for which seeking of care could be assumed to be nearly universal. Finally, the single-blinded tactics that would be deployed in the enrollment, evaluation and identification of patients at the surveillance sites would serve to help prevent detection bias in the measurement of OCV protection. All of these tactics would be essential to prevent bias in observational, closed cohort study of OCV protection. However, in aggregate, they cannot be claimed to fully replace the strength of double-blinding in preventing biased measurement of OCV protection. For example, they cannot fully prevent bias due to selective vaccination of participants or selective willingness of participants to be vaccinated, nor can they prevent differential seeking of health care by OCV recipients versus non-recipients.
Analyses to challenge the findings
Despite the importance of the aforementioned tactics to prevent biased measurement of OCV protection in an observational, closed cohort study, such a study still cannot be claimed to be the equal of a double-blinded RCT of OCV in preventing bias. For this reason, we recommend that the findings of an observational study be “challenged” by several types of additional analyses.
Firstly, a good overall screen for assessing whether the measurement of OCV protection is distorted by biased design and/or conduct of the study employs a bias-indicator outcome.11 In this approach, OCV protection would be assessed in a fashion identical to the analysis of OCV protection against cholera, but instead of cholera, non-cholera cases (bias-indicator outcomes) with acute watery diarrhea that are negative for cholera in microbiological testing are analysed.12, 13, 14 The absence of OCV protection against the bias-indicator outcome would support the argument that protection against cholera is unlikely due to bias. Secondly, such an analysis can also be carried one step further, using a test-negative design analysis to provide a more probing analysis of potential bias due to different utilisation of surveillance sites by OCV recipients versus non-recipients.15 In this approach, which depends for its validity on using a diagnostic test with very high sensitivity and specificity for diagnosis of cholera, cholera cases and patients (controls) who are test-negative for cholera, all assembled from the baseline cohorts of OCV recipients and non-recipients, are contrasted for antecedent receipt of OCV. All participants would have a clinical syndrome compatible with cholera (acute watery diarrhea), would receive the same microbiological test for cholera, and would be identified concurrently from the same surveillance sites. Consistency of the test-negative design estimate of OCV protection, computed in the same fashion as for a case-control study of vaccine protection, with that derived from the conventional analysis of the closed cohorts would further support the absence of bias in the latter. Thirdly, sensitivity analyses would be done to assess whether missing data might have biased estimates of OCV protection. For example, if acute watery diarrheal cases from the closed cohort are missed in the surveillance, it is important to evaluate the possible impact of these missed cases on estimates of OCV protection using information on the patients and their vaccination status from treatment log books and records from the study vaccination file.
Safeguarding against other biases
As in RCTs, to help to provide confidence that publication of the study is not biased by publication bias, these studies would be entered before study onset into official clinical trial or clinical study registries, such as ClinicalTrials.gov. Moreover, to help prevent bias in analyses of OCV protection, final statistical analysis plans would be clearly articulated, with a priori primary, secondary, and exploratory objectives and analyses, before the analysis commences. Finally, the dataset from the study would be edited, cleaned, and then frozen before analysis commences.
Conclusions
In this Viewpoint, we propose observational studies to measure vaccine protection using a design that emulates an RCT to the degree possible. Table 3 summarises the important features that we argue should be incorporated into these observational studies, borrowed from conventional prerequisites of contemporary RCTs of vaccine efficacy, to help to prevent, adjust for, or at least measure the biases that can distort the findings of observational assessments of vaccine protection.
Table 3.
Features of an observational, closed cohort evaluation, other than randomisation and double-blinding, that can help prevent and/or measure bias.a
| Feature | Function |
|
|---|---|---|
| Helps prevent bias | Measures bias | |
| Design/conduct | ||
| Prospective conduct | X | |
| GCPb | X | |
| Baseline census | X | |
| Closed cohort | X | |
| Uniform eligibility and exclusion criteria | X | |
| Concurrent enrollment | X | |
| Uniform definition of zero time | X | |
| Comprehensive ascertainment of potential confounders at zero time | X | |
| Concurrent post-zero follow-up | X | |
| Systematic and uniform procedures for ascertainment of post-zero deaths and outmigrationsc | X | |
| Systematic and uniform procedures for ascertainment of post-zero cointerventionsc | X | |
| Surveillance for cholera in all treatment sites providing care for acute water diarrhea occurring in the study populationc | X | |
| Application of uniform eligibility/exclusion criteria for enrollment of acute watery diarrhea patientsc | X | |
| Accurate ascertainment of census IDs of patientsc | X | |
| Uniform and validated procedures for collection of patient clinical datac | X | |
| Uniform and validated procedures for collection, storage, and transmission of fecal specimensc | X | |
| Uniform and validated procedures for microbiological evaluation of fecal specimens for Vibrio choleraec | X | |
| Analysis | ||
| Analysis of OCV protection according to an a priori, formal statistical analysis plan | X | |
| Analysis of OCV protection only after formal locking of data set | X | |
| Analysis of OCV protection against cholera controlling for imbalances in zero time confounders, post-zero imbalances in cointerventions, and post-zero follow-up | X | X |
| Analysis of OCV protection against a bias-indicator condition | X | |
| Analysis of OCV protection against cholera in test-negative design | X | |
| Sensitivity analyses of OCV protection against cholera accounting for missing data | X | |
| Reporting | ||
| Registration of study prior to enrollment of participants | X | |
| Making dataset publicly available | X | X |
See text for further explanation.
Good Clinical Practice.
All post-zero surveillance procedures would be done by observers without access to vaccination records of participants.
Our recommendations offer a potential way forward to provide the public health community with confidence that several recently developed, simplified inactivated OCVs do confer suitable levels of protection against cholera. Because of the availability of a licensed legacy OCVs (Shanchol™, Euvichol-Plus™), an RCT of the clinical efficacy of these newer, simplified vaccines cannot be done since it would be unethical to withhold the legacy vaccine from a control group for a long enough period of follow-up to measure vaccine protection against cholera. Moreover, because of the low incidence of cholera, sample size considerations make a non-inferiority trial of clinical protection infeasible. A non-inferiority trial of serum vibriocidal antibody responses to newer versus legacy vaccines would overcome the ethical problem of withholding the effective legacy vaccine from the experimental vaccine group, since these responses would be measured shortly after dosing. However, the interpretation of the results of such a trial will be uncertain since serum vibriocidal antibodies are not a correlate of OCV vaccine protection.6 Because of this limitation and the fact that assays of serum vibriocidal antibodies are not internationally standardised, it would be prudent to require for licensure that new vaccines non-inferior to the existing legacy OCV with respect to serum vibriocidal antibody responses also demonstrate acceptable clinical protection using our proposed design. If regulatory authorities are willing to take a leap of faith and rely exclusively on serum vibriocidal antibodies as measures of protection in criteria for licensure, we believe it should be mandatory that rigorous, non-experimental studies of clinical protection be conducted before releasing these vaccines to vulnerable populations from the OCV stockpile. In either case, we do not believe that using rigorous non-experimental studies to demonstrate OCV protection would significantly delay bringing these vaccines to market, as multi-year, post-dosing follow-up in such studies need not be required to prove that a vaccine is in fact protective.
It might be argued that human volunteer challenge studies might circumvent the problem created by the lack of a validated immunological correlate of OCV protection. In such a study, volunteers would be randomised to groups receiving or not receiving OCV, and would then be challenged with virulent cholera vibrios to assess comparative rates of illness for calculation of vaccine protective efficacy. One OCV, PaxVax™ (Emergent Biosolutions), has been licensed on the basis of human volunteer challenges studies as well as extended studies of vaccine safety and immunogenicity done in North American volunteers.16 However, this vaccine failed to protect in its only RCT of efficacy in a population with endemic cholera, raising doubts about the applicability of the volunteer challenge model to populations with endemic disease.17
Our proposal comes with several caveats. First, the prospective, longitudinal design we propose, which is formulated to increase scientific credibility, will likely be more expensive and logistically complex than the typical observational study approaches used to measure vaccine effectiveness in the field. We do not, therefore, propose that this design be used for routine post-licensure studies of OCV effectiveness. Second, the type of study we are proposing can only be planned and reliably conducted in a population with predictable, endemic cholera, preferably in a site with established research infrastructure—features of a population and a site that would be appropriate for a conventional RCT of OCV efficacy. Third, as evaluations undertaken in endemic settings, the findings from the studies we propose may not be applicable to use of OCV in outbreaks. Finally, because the level of OCV coverage of the target population will depend on population uptake and cannot be easily controlled, the proposed design may be subject to biases that affect observational comparisons in which vaccine coverage is too high (e.g., >90%) or too low (e.g., <10%).18 However, such a bias should be made evident in bias-indicator outcome and test negative design analyses. Moreover, previous prospectively conducted, demonstration projects of inactivated OCVs that were preemptively delivered against endemic cholera have observed population coverage levels of 55%–61% with the number of vaccinees ranging from 14,164 to 27,678.12,19,20
This paper focuses specifically on a rigorous, non-experimental approach to estimating vaccine protection that may be particularly well-suited to newer generation inactivate OCVs. However, our proposed non-experimental approach to evaluating vaccine protection in target populations at risk would also seem relevant for the clinical development of several other newer vaccines against diseases for which licensed and used legacy vaccines already exist, but for which immunological correlates of vaccine protection are lacking. Examples of vaccines in this circumstance include newer generation vaccines against pertussis and parenteral vaccines against rotavirus.
Contributors
JC and JD formulated the concept for this paper. All authors participated in editing and revision of the paper, and all authors approved the final version of the paper.
Declaration of interests
All authors declare no competing interests.
Acknowledgments
The authors gratefully acknowledge comments on the manuscript provided by Professor Jan Holmgren.
References
- 1.Bi Q., Ferreras E., Pezzoli L., et al. Protection against cholera from killed whole-cell oral cholera vaccines: a systematic review and meta-analysis. Lancet Infect Dis. 2017;17(10):1080–1088. doi: 10.1016/S1473-3099(17)30359-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pezzoli L. Oral Cholera Vaccine Working Group of the Global Task Force on Cholera C. Global oral cholera vaccine use, 2013-2018. Vaccine. 2020;38(Suppl 1):A132–A140. doi: 10.1016/j.vaccine.2019.08.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO . 2023. Cholera – global situation.https://www.who.int/emergencies/disease-outbreak-news/item/2023-DON437 [Google Scholar]
- 4.Chowdhury F., Ali Syed K., Akter A., et al. A phase I/II study to evaluate safety, tolerability and immunogenicity of Hillchol(R), an inactivated single Hikojima strain based oral cholera vaccine, in a sequentially age descending population in Bangladesh. Vaccine. 2021;39(32):4450–4457. doi: 10.1016/j.vaccine.2021.06.069. [DOI] [PubMed] [Google Scholar]
- 5.Eubiologics OCV production and future development. Presentation at the 8th meeting of the GTFCC working group on OCV. https://www.gtfcc.org/wp-content/uploads/2022/01/8th-meeting-of-the-gtfcc-working-group-on-ocv-2021-day-2-rachel-park.pdf
- 6.Deen J., Holmgren J., Clemens J.D. Evaluating improved inactivated oral cholera vaccines for use in ending endemic cholera by 2030: opportunities and challenges. Lancet Infect Dis. 2022;22(10):e292–e298. doi: 10.1016/S1473-3099(22)00215-8. [DOI] [PubMed] [Google Scholar]
- 7.Concato J., Shah N., Horwitz R.I. Randomized, controlled trials, observational studies, and the hierarchy of research designs. N Engl J Med. 2000;342(25):1887–1892. doi: 10.1056/NEJM200006223422507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sterne J.A., Hernan M.A., Reeves B.C., et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355 doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horwitz R.I., Viscoli C.M., Clemens J.D., Sadock R.T. Developing improved observational methods for evaluating therapeutic effectiveness. Am J Med. 1990;89(5):630–638. doi: 10.1016/0002-9343(90)90182-d. [DOI] [PubMed] [Google Scholar]
- 10.E6(R3) EWG. Good clinical practice (GCP) https://www.ich.org/page/ich-guidelines
- 11.Shapiro E.D. Case-control studies to assess the effectiveness of vaccines. J Pediatr Infect Dis Soc. 2014;3(4):278–279. doi: 10.1093/jpids/piu058. [DOI] [PubMed] [Google Scholar]
- 12.Lucas M.E., Deen J.L., von Seidlein L., et al. Effectiveness of mass oral cholera vaccination in Beira, Mozambique. N Engl J Med. 2005;352(8):757–767. doi: 10.1056/NEJMoa043323. [DOI] [PubMed] [Google Scholar]
- 13.Luquero F.J., Grout L., Ciglenecki I., et al. Use of Vibrio cholerae vaccine in an outbreak in Guinea. N Engl J Med. 2014;370(22):2111–2120. doi: 10.1056/NEJMoa1312680. [DOI] [PubMed] [Google Scholar]
- 14.Ivers L.C., Hilaire I.J., Teng J.E., et al. Effectiveness of reactive oral cholera vaccination in rural Haiti: a case-control study and bias-indicator analysis. Lancet Glob Health. 2015;3(3):e162–e168. doi: 10.1016/S2214-109X(14)70368-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ali M., You Y.A., Sur D., et al. Validity of the estimates of oral cholera vaccine effectiveness derived from the test-negative design. Vaccine. 2016;34(4):479–485. doi: 10.1016/j.vaccine.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 16.Levine M.M., Chen W.H., Kaper J.B., Lock M., Danzig L., Gurwith M. PaxVax CVD 103-HgR single-dose live oral cholera vaccine. Expert Rev Vaccines. 2017;16(3):197–213. doi: 10.1080/14760584.2017.1291348. [DOI] [PubMed] [Google Scholar]
- 17.Richie E.E., Punjabi N.H., Sidharta Y.Y., et al. Efficacy trial of single-dose live oral cholera vaccine CVD 103-HgR in North Jakarta, Indonesia, a cholera-endemic area. Vaccine. 2000;18(22):2399–2410. doi: 10.1016/s0264-410x(00)00006-2. [DOI] [PubMed] [Google Scholar]
- 18.Patel M.K., Bergeri I., Bresee J.S., et al. Evaluation of post-introduction COVID-19 vaccine effectiveness: summary of interim guidance of the World Health Organization. Vaccine. 2021;39(30):4013–4024. doi: 10.1016/j.vaccine.2021.05.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wierzba T.F., Kar S.K., Mogasale V.V., et al. Effectiveness of an oral cholera vaccine campaign to prevent clinically-significant cholera in Odisha State, India. Vaccine. 2015;33(21):2463–2469. doi: 10.1016/j.vaccine.2015.03.073. [DOI] [PubMed] [Google Scholar]
- 20.Khatib A.M., Ali M., von Seidlein L., et al. Effectiveness of an oral cholera vaccine in Zanzibar: findings from a mass vaccination campaign and observational cohort study. Lancet Infect Dis. 2012;12(11):837–844. doi: 10.1016/S1473-3099(12)70196-2. [DOI] [PubMed] [Google Scholar]