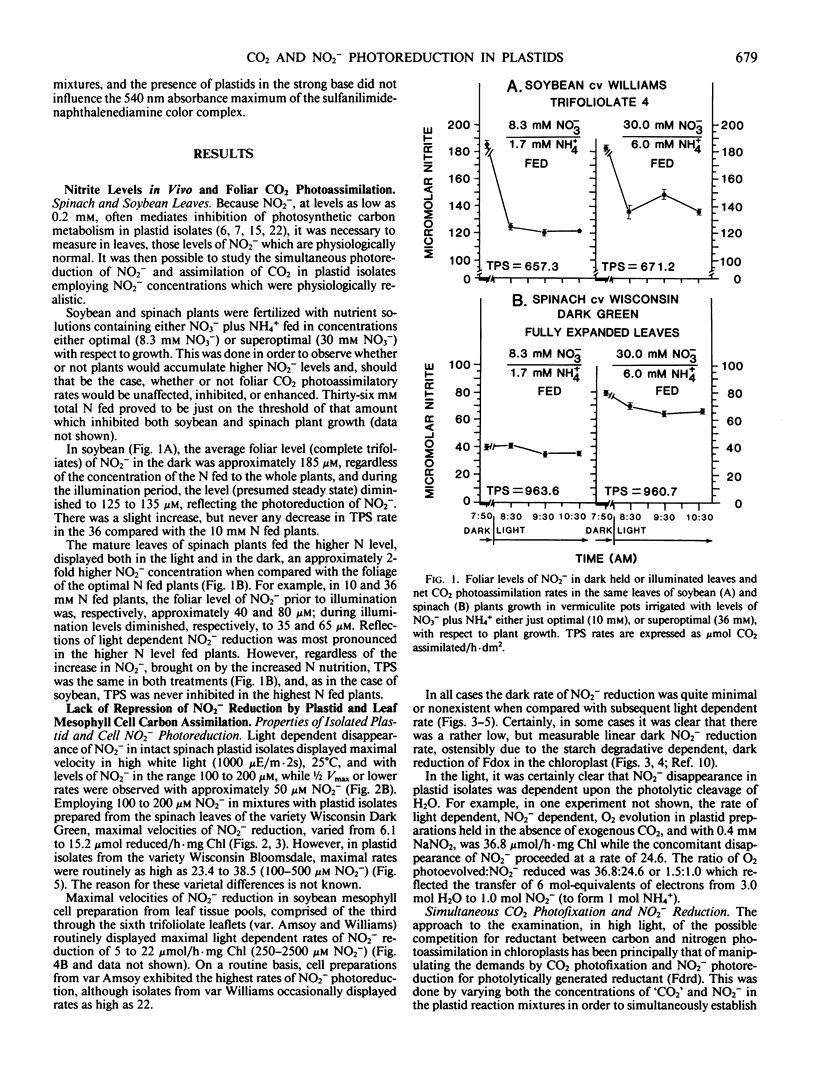
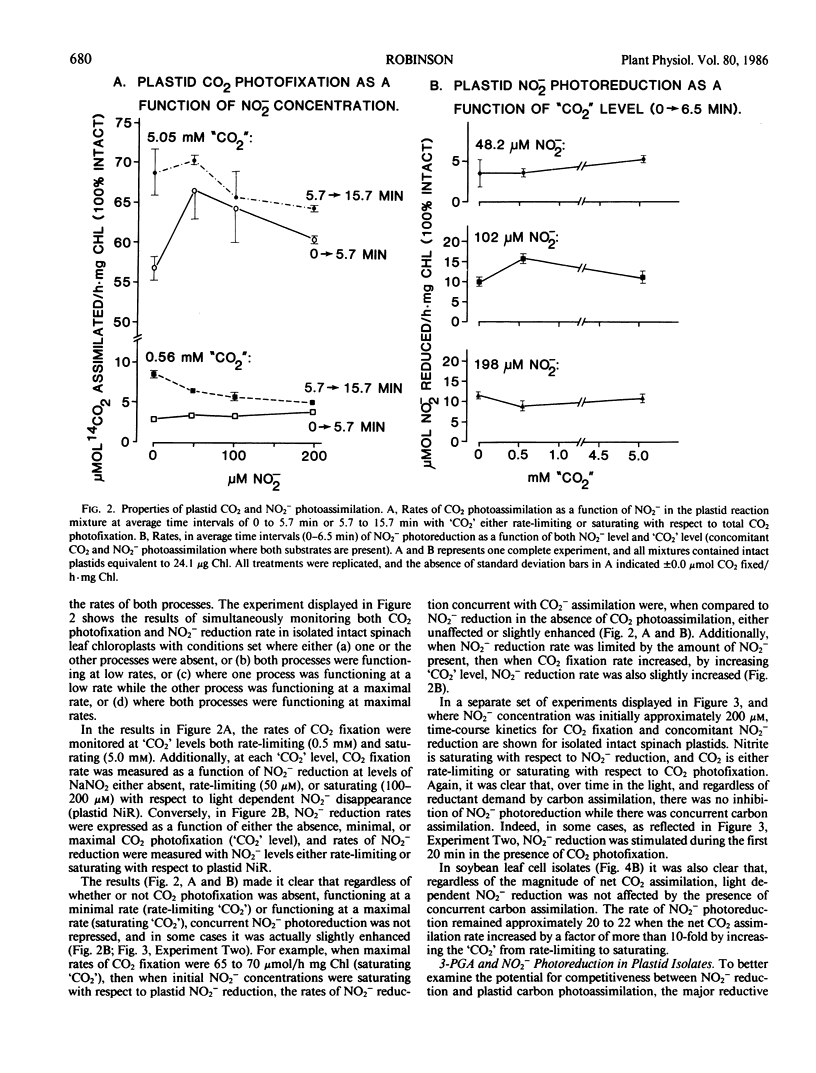
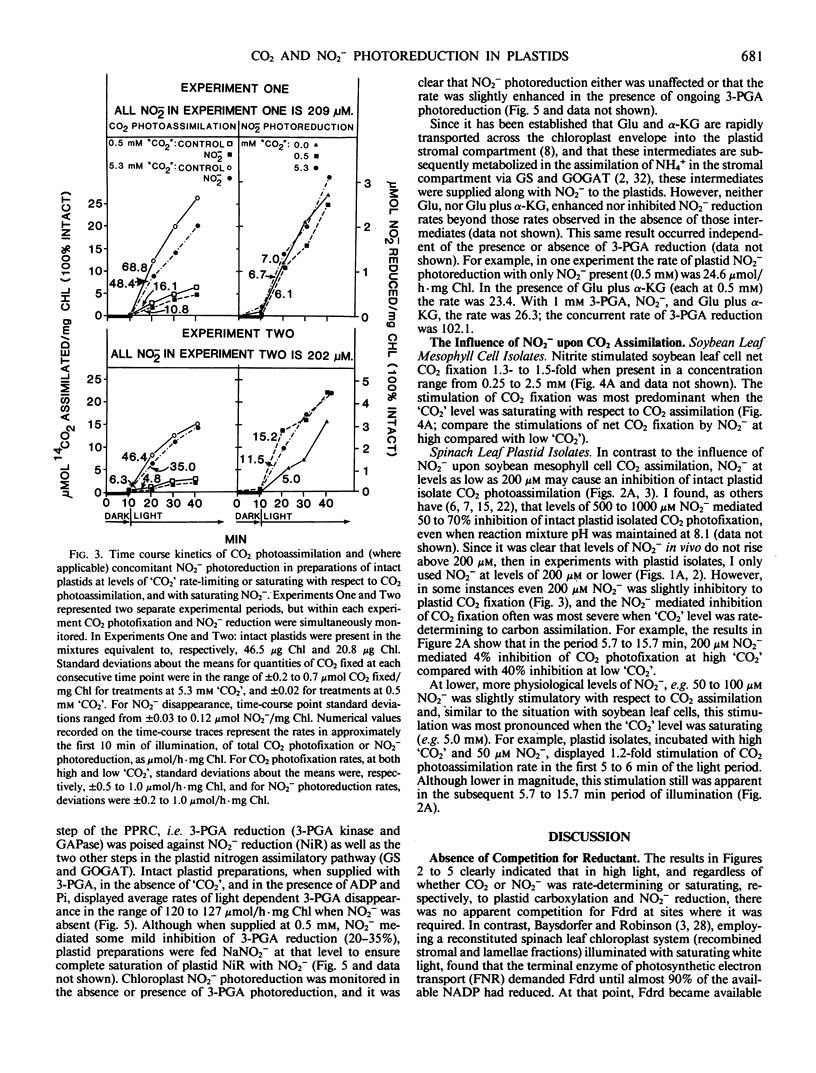
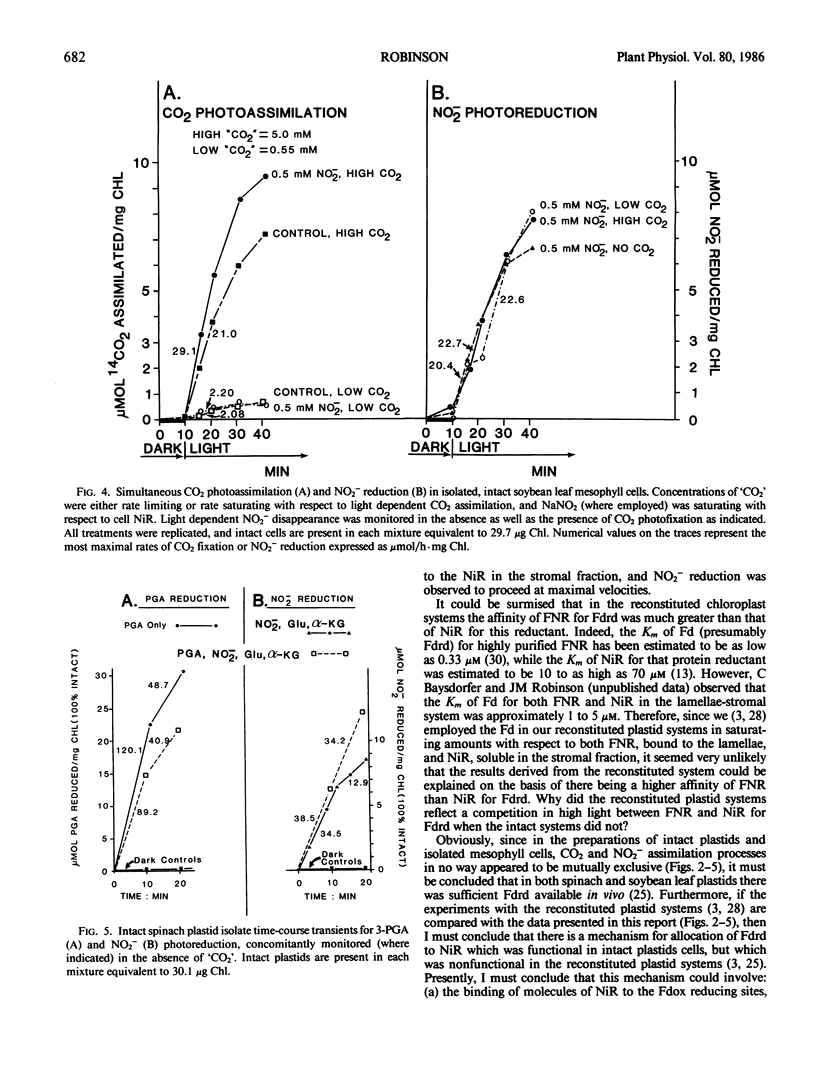
Abstract
Previously, C Baysdorfer and JM Robinson (1985 Plant Physiol 77: 318-320) demonstrated that, in a reconstituted spinach chloroplast system, NADP photoreduction functioning at most maximal rate and reductant demand, was the successful competitor with NO2− photoreduction for reduced ferredoxin. This resulted in a repression of NO2− reduction until all NADP available had been almost totally reduced. Further experiments, employing isolated, intact spinach leaf plastids and soybean leaf mesophyll cells, were conducted to examine competition for reductant between CO2 and NO2− photoassimilation, in situ. In isolated, intact plastid preparations, regardless of whether the demand for reductant by CO2 photoassimilation was high (5 millimolar `CO2') with rates of CO2 fixation in the range 40 to 90 micromoles CO2 fixed per hour per milligram chlorophyll, low (0.5 millimolar `CO2') with rates in the range 5 to 8 micromoles CO2 per hour per milligram chlorophyll, or zero (no `CO2'), NO2− photoreduction displayed equal rates in the range of 8 to 22 micromoles per hour per milligram chlorophyll. In the absence of `CO2', but in the presence of saturating white light, 3-phosphoglycerate photoreduction at rates of 82 to 127 micromoles per hour per milligram chlorophyll did not repress, and occasionally stimulated concomitant rates of NO2− reduction which ranged from 23.4 to 38.5. Conversely, in plastid preparations, NO2− at levels of 50 to 100 micromolar, stimulated plastid CO2 fixation when `CO2' was saturating with respect to carboxylation. Further, levels of NO2− in the range 250 to 2500 micromolar, stimulated soybean leaf mesophyll cell net CO2 fixation as much as 1.5-fold if `CO2' was saturating with respect to CO2 fixation. It appeared likely that, in high light in vivo, CO2 and NO2− photoassimilatory processes are not forced to intercompete for reduced ferredoxin in the intact chloroplast.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. W., Done J. Light-dependent Assimilation of Nitrite by Isolated Pea Chloroplasts. Plant Physiol. 1978 Apr;61(4):692–697. doi: 10.1104/pp.61.4.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baysdorfer C., Robinson J. M. Metabolic Interactions between Spinach Leaf Nitrite Reductase and Ferredoxin-NADP Reductase: Competition for Reduced Ferredoxin. Plant Physiol. 1985 Feb;77(2):318–320. doi: 10.1104/pp.77.2.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klepper L., Flesher D., Hageman R. H. Generation of reduced nicotinamide adenine dinucleotide for nitrate reduction in green leaves. Plant Physiol. 1971 Nov;48(5):580–590. doi: 10.1104/pp.48.5.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kow Y. W., Erbes D. L., Gibbs M. Chloroplast Respiration : A MEANS OF SUPPLYING OXIDIZED PYRIDINE NUCLEOTIDE FOR DARK CHLOROPLASTIC METABOLISM. Plant Physiol. 1982 Feb;69(2):442–447. doi: 10.1104/pp.69.2.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latzko E., Gibbs M. Measurement of the intermediates of the photosynthetic carbon reduction cycle, using enzymatic methods. Methods Enzymol. 1972;24:261–268. doi: 10.1016/0076-6879(72)24073-3. [DOI] [PubMed] [Google Scholar]
- Magalhaes A. C., Neyra C. A., Hageman R. H. Nitrite assimilation and amino nitrogen synthesis in isolated spinach chloroplasts. Plant Physiol. 1974 Mar;53(3):411–415. doi: 10.1104/pp.53.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Leary V., Solberg M. Effect of sodium nitrite inhibition on intracellular thiol groups and on the activity of certain glycolytic enzymes in Clostridium perfringens. Appl Environ Microbiol. 1976 Feb;31(2):208–212. doi: 10.1128/aem.31.2.208-212.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaut Z., Lendzian K., Bassham J. A. Nitrite Reduction in Reconstituted and Whole Spinach Chloroplasts during Carbon Dioxide Reduction. Plant Physiol. 1977 Feb;59(2):184–188. doi: 10.1104/pp.59.2.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purczeld P., Chon C. J., Portis A. R., Jr, Heldt H. W., Heber U. The mechanism of the control of carbon fixation by the pH in the chloroplast stroma. Studies with nitrite-mediated proton transfer across the envelope. Biochim Biophys Acta. 1978 Mar 13;501(3):488–498. doi: 10.1016/0005-2728(78)90116-0. [DOI] [PubMed] [Google Scholar]
- Reed A. J., Canvin D. T. Light and Dark Controls of Nitrate Reduction in Wheat (Triticum aestivum L.) Protoplasts. Plant Physiol. 1982 Feb;69(2):508–513. doi: 10.1104/pp.69.2.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed A. J., Canvin D. T., Sherrard J. H., Hageman R. H. Assimilation of [N]Nitrate and [N]Nitrite in Leaves of Five Plant Species under Light and Dark Conditions. Plant Physiol. 1983 Feb;71(2):291–294. doi: 10.1104/pp.71.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J. M. Photosynthetic Carbon Metabolism in Leaves and Isolated Chloroplasts from Spinach Plants Grown under Short and Intermediate Photosynthetic Periods. Plant Physiol. 1984 Jun;75(2):397–409. doi: 10.1104/pp.75.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J. M., Smith M. G., Gibbs M. Influence of Hydrogen Peroxide upon Carbon Dioxide Photoassimilation in the Spinach Chloroplast: I. HYDROGEN PEROXIDE GENERATED BY BROKEN CHLOROPLASTS IN AN "INTACT" CHLOROPLAST PREPARATION IS A CAUSAL AGENT OF THE WARBURG EFFECT. Plant Physiol. 1980 Apr;65(4):755–759. doi: 10.1104/pp.65.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl R. L., Harper J. E., Hageman R. H. Improvements of the nitrite color development in assays of nitrate reductase by phenazine methosulfate and zinc acetate. Plant Physiol. 1974 Jun;53(6):825–828. doi: 10.1104/pp.53.6.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn K. C., Duke S. O. Histochemical localization of nitrate reductase. Histochemistry. 1981;72(2):191–198. doi: 10.1007/BF00517132. [DOI] [PubMed] [Google Scholar]


