Abstract
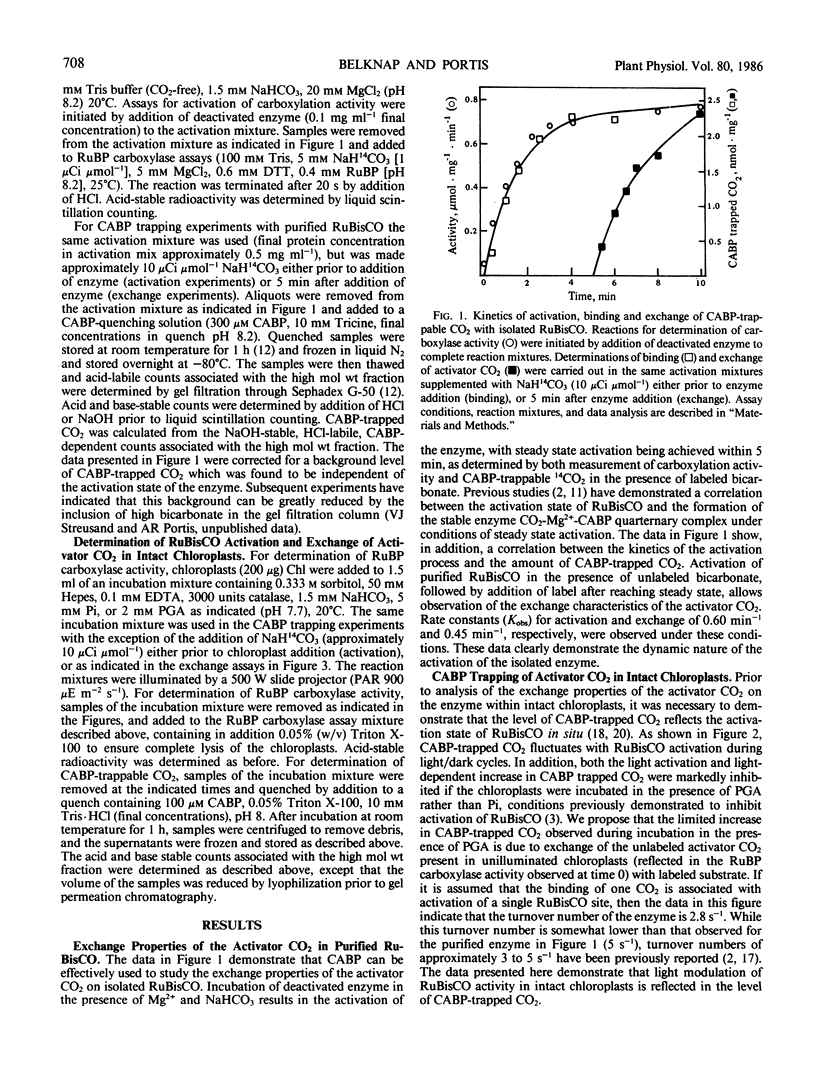
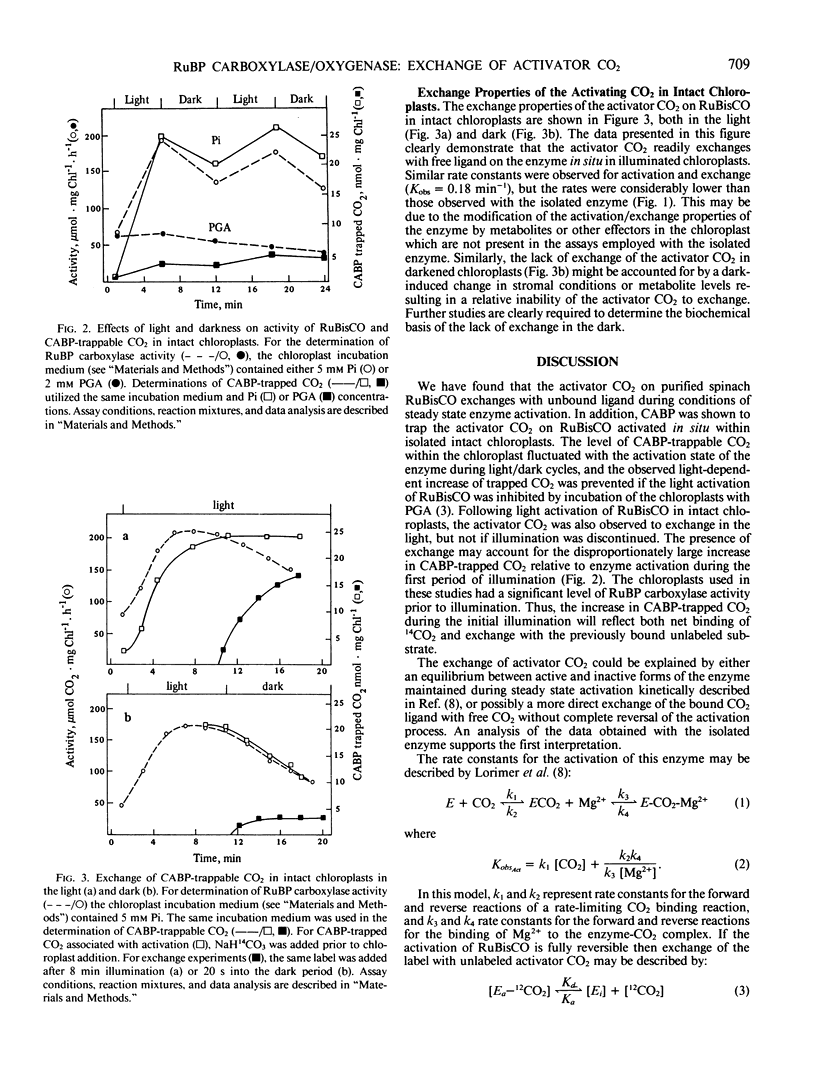
The exchange properties of the activator CO2 of spinach ribulose-1,5-bisphosphate carboxylase/oxygenase were characterized both in vitro with the purified enzyme, and in situ within isolated chloroplasts. Carboxyarabinitol-1,5-bisphosphate, a proposed reaction intermediate analog for the carboxylase activity of the enzyme, was used to trap the activator CO2 on the enzyme both in vitro and in situ. Modulation of ribulose-1,5-bisphosphate carboxylase/oxygenase activity in intact chloroplasts during a light/dark cycle was associated with a similar modulation in carboxyarabinitol-1,5-bisphosphate-trapped CO2. The exchange kinetics of the activator CO2 were monitored by activation of the enzyme to steady state in the presence of 12CO2, followed by addition of 14CO2 and determination of the amount of labeled CO2 trapped on the enzyme by carboxyarabinitol-1,5-bisphosphate. Rate constants (Kobs) for exchange with both the purified enzyme (0.45 min−1) and in illuminated chloroplasts (0.18 min−1) were comparable to the observed rate constants for enzyme activation under the two conditions. A similar exchange of the activator CO2 was not observed in chloroplasts in the dark. Kinetic analysis of the exchange properties of the purified enzyme were consistent with an equilibrium between active and inactive forms of the enzyme during steady state activation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bahr J. T., Jensen R. G. Activation of ribulose bisphosphate carboxylase in intact chloroplasts by CO2 and light. Arch Biochem Biophys. 1978 Jan 15;185(1):39–48. doi: 10.1016/0003-9861(78)90141-8. [DOI] [PubMed] [Google Scholar]
- Hall N. P., Pierce J., Tolbert N. E. Formation of a carboxyarabinitol bisphosphate complex with ribulose bisphosphate carboxylase/oxygenase and theoretical specific activity of the enzyme. Arch Biochem Biophys. 1981 Nov;212(1):115–119. doi: 10.1016/0003-9861(81)90349-0. [DOI] [PubMed] [Google Scholar]
- Heldt H. W., Sauer F. The inner membrane of the chloroplast envelope as the site of specific metabolite transport. Biochim Biophys Acta. 1971 Apr 6;234(1):83–91. doi: 10.1016/0005-2728(71)90133-2. [DOI] [PubMed] [Google Scholar]
- Laing W. A., Christeller J. T. A model for the kinetics of activation and catalysis of ribulose 1,5-bisphosphate carboxylase. Biochem J. 1976 Dec 1;159(3):563–570. doi: 10.1042/bj1590563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorimer G. H., Badger M. R., Andrews T. J. D-Ribulose-1,5-bisphosphate carboxylase-oxygenase. Improved methods for the activation and assay of catalytic activities. Anal Biochem. 1977 Mar;78(1):66–75. doi: 10.1016/0003-2697(77)90009-4. [DOI] [PubMed] [Google Scholar]
- Lorimer G. H., Badger M. R., Andrews T. J. The activation of ribulose-1,5-bisphosphate carboxylase by carbon dioxide and magnesium ions. Equilibria, kinetics, a suggested mechanism, and physiological implications. Biochemistry. 1976 Feb 10;15(3):529–536. doi: 10.1021/bi00648a012. [DOI] [PubMed] [Google Scholar]
- Lorimer G. H. Evidence for the existence of discrete activator and substrate sites for CO2 on ribulose-1,5-bisphosphate carboxylase. J Biol Chem. 1979 Jul 10;254(13):5599–5601. [PubMed] [Google Scholar]
- Lorimer G. H. Ribulosebisphosphate carboxylase: amino acid sequence of a peptide bearing the activator carbon dioxide. Biochemistry. 1981 Mar 3;20(5):1236–1240. doi: 10.1021/bi00508a028. [DOI] [PubMed] [Google Scholar]
- McCurry S. D., Gee R., Tolbert N. E. Ribulose-1,5-bisphosphate carboxylase/oxygenase from spinach, tomato, or tobacco leaves. Methods Enzymol. 1982;90(Pt E):515–521. doi: 10.1016/s0076-6879(82)90178-1. [DOI] [PubMed] [Google Scholar]
- McCurry S. D., Pierce J., Tolbert N. E., Orme-Johnson W. H. On the mechanism of effector-mediated activation of ribulose bisphosphate carboxylase/oxygenase. J Biol Chem. 1981 Jul 10;256(13):6623–6628. [PubMed] [Google Scholar]
- Miziorko H. M., Lorimer G. H. Ribulose-1,5-bisphosphate carboxylase-oxygenase. Annu Rev Biochem. 1983;52:507–535. doi: 10.1146/annurev.bi.52.070183.002451. [DOI] [PubMed] [Google Scholar]
- Miziorko H. M. Ribulose-1,5-biphosphate carboxylase. Evidence in support of the existence of distinct CO2 activator and CO2 substrate sites. J Biol Chem. 1979 Jan 25;254(2):270–272. [PubMed] [Google Scholar]
- Perchorowicz J. T., Raynes D. A., Jensen R. G. Light limitation of photosynthesis and activation of ribulose bisphosphate carboxylase in wheat seedlings. Proc Natl Acad Sci U S A. 1981 May;78(5):2985–2989. doi: 10.1073/pnas.78.5.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce J., Tolbert N. E., Barker R. Interaction of ribulosebisphosphate carboxylase/oxygenase with transition-state analogues. Biochemistry. 1980 Mar 4;19(5):934–942. doi: 10.1021/bi00546a018. [DOI] [PubMed] [Google Scholar]
- Rose I. A., O'Connell E. L., Litwin S. Determination of the rate of hexokinase-glucose dissociation by the isotope-trapping method. J Biol Chem. 1974 Aug 25;249(16):5163–5168. [PubMed] [Google Scholar]
- Seemann J. R., Badger M. R., Berry J. A. Variations in the Specific Activity of Ribulose-1,5-bisphosphate Carboxylase between Species Utilizing Differing Photosynthetic Pathways. Plant Physiol. 1984 Apr;74(4):791–794. doi: 10.1104/pp.74.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicher R. C. Characteristics of light-dependent inorganic carbon uptake by isolated spinach chloroplasts. Plant Physiol. 1984 Apr;74(4):962–966. doi: 10.1104/pp.74.4.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota A., Komura H., Kitaoka S. Intracellular inorganic carbon exists as protein carbamate in photosynthesizing Euglena gracilis z. Biochem Biophys Res Commun. 1983 Mar 16;111(2):544–550. doi: 10.1016/0006-291x(83)90341-8. [DOI] [PubMed] [Google Scholar]


