Abstract
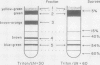
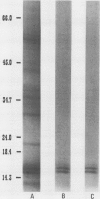
Three pigment-protein complexes were isolated from the marine diatom Phaeodactylum tricornutum (Bohlin) by treatment of thylakoid membrane fragments with 1% Triton X-100 at 4°C followed by centrifugation on sucrose density gradients. The major complex contains chlorophyll a, c1, c2, and the carotenoid fucoxanthin (chlorophyll a: c1: c2: fucoxanthin = 1.0: 0.09: 0.28: 2.22) bound to an apoprotein doublet of 16.4 and 16.9 kilodaltons. This complex accounts for >70% of the total pigment and 20 to 40% of the protein in the thylakoid membranes. Efficient coupling of chlorophyll c and fucoxanthin absorption to chlorophyll a fluorescence supports a light-harvesting function for the complex. A minor light-harvesting complex containing chlorophyll a, c1, and c2 but no fucoxanthin (chlorophyll a: c1: c2 = 1.0: 0.23: 0.26) was also isolated at Triton: chlorophyll a ratios between 20 and 40. These pigments are bound to a similar molecular weight apoprotein doublet. The third complex isolated was the P700-chlorophyll a protein, the reaction center of photosystem I, which showed characteristics similar to those isolated from other plant sources. The yield of the chlorophyll a/c-fucoxanthin complex was shown to respond strongly to changes in light intensity during growth, accounting for most of the changes in cellular pigmentation.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett J., Anderson J. M. The P-700-chlorophyl alpha-protein complex and two major light-harvesting complexes of Acrocarpia paniculata and other brown seaweeds. Biochim Biophys Acta. 1980 May 9;590(3):309–323. doi: 10.1016/0005-2728(80)90202-9. [DOI] [PubMed] [Google Scholar]
- Fish L. E., Jagendorf A. T. High rates of protein synthesis by isolated chloroplasts. Plant Physiol. 1982 Oct;70(4):1107–1114. doi: 10.1104/pp.70.4.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman A. L., Alberte R. S. A diatom light-harvesting pigment-protein complex : purification and characterization. Plant Physiol. 1984 Oct;76(2):483–489. doi: 10.1104/pp.76.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman A. L., Alberte R. S. Biogenesis and light regulation of the major light harvesting chlorophyll-protein of diatoms. Plant Physiol. 1986 Jan;80(1):43–51. doi: 10.1104/pp.80.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUILLARD R. R., RYTHER J. H. Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt, and Detonula confervacea (cleve) Gran. Can J Microbiol. 1962 Apr;8:229–239. doi: 10.1139/m62-029. [DOI] [PubMed] [Google Scholar]
- Holdsworth E. S., Arshad J. H. A manganese-copper-pigment-protein complex isolated from the photosystem II of Phaeodactylum tricornutum. Arch Biochem Biophys. 1977 Oct;183(2):361–373. doi: 10.1016/0003-9861(77)90370-8. [DOI] [PubMed] [Google Scholar]
- Jeffrey S. W. Properties of two spectrally different components in chlorophyll c preparations. Biochim Biophys Acta. 1969 May 6;177(3):456–467. doi: 10.1016/0304-4165(69)90308-0. [DOI] [PubMed] [Google Scholar]
- Jeffrey S. W. Quantitative thin-layer chromatography of chlorophylls and carotenoids from marine algae. Biochim Biophys Acta. 1968 Aug 20;162(2):271–285. doi: 10.1016/0005-2728(68)90109-6. [DOI] [PubMed] [Google Scholar]
- Owens T. G. Light-Harvesting Function in the Diatom Phaeodactylum tricornutum: II. Distribution of Excitation Energy between the Photosystems. Plant Physiol. 1986 Mar;80(3):739–746. doi: 10.1104/pp.80.3.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prézelin B. B., Alberte R. S. Photosynthetic characteristics and organization of chlorophyll in marine dinoflagellates. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1801–1804. doi: 10.1073/pnas.75.4.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read S. M., Northcote D. H. Minimization of variation in the response to different proteins of the Coomassie blue G dye-binding assay for protein. Anal Biochem. 1981 Sep 1;116(1):53–64. doi: 10.1016/0003-2697(81)90321-3. [DOI] [PubMed] [Google Scholar]
- Vierling E., Alberte R. S. P(700) Chlorophyll a-Protein : Purification, Characterization, and Antibody Preparation. Plant Physiol. 1983 Jul;72(3):625–633. doi: 10.1104/pp.72.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi A., Katoh S. Two chlorophyll-binding subunits of the photosystem 2 reaction center complex isolated from the thermophilic cyanobacterium Synechococcus sp. Arch Biochem Biophys. 1983 Sep;225(2):836–846. doi: 10.1016/0003-9861(83)90096-6. [DOI] [PubMed] [Google Scholar]