Abstract
Objective:
Eating disorders commonly co-occur with gastrointestinal problems. This case-control study aimed to 1) document the prevalence of disorders of gut-brain interaction (DGBI) in eating disorders, 2) examine the specific impact of disordered eating behaviors on the risk of DGBI, and 3) explore the impact of current eating disorder psychopathology on DGBI.
Method:
We included 765 cases with eating disorders and 1240 controls. DGBI were assessed via the ROME III questionnaire. Prevalences of DGBI were calculated across eating disorder diagnoses (anorexia nervosa, bulimia nervosa, multiple eating disorders) and in controls. The association between disordered eating behaviors and DGBI was examined using logistic regression models. Lastly, we compared the total number of DGBI in individuals with high versus low current eating disorder symptoms.
Results:
A large majority (88.2–95.5%) of individuals with eating disorders reported at least one DGBI and 34.8–48.7% reported three or more DGBI. Of the DGBI categories, functional bowel disorders were the most commonly endorsed category, and of the individual DGBI, irritable bowel syndrome was the most frequently reported (43.9–58.8%). All investigated disordered eating behaviors showed a positive association with most DGBI categories. Finally, individuals reporting high current eating disorder symptoms reported higher mean number of DGBI (3.03–3.34) than those with low current symptoms (1.60–1.84).
Discussion:
The directionality and mechanisms underlying the nature of the relationship between gastrointestinal and eating disorder symptoms is worthy of further study and clinicians should adopt an integrated approach by attending to both gastrointestinal and eating disorder symptoms in their patients.
Keywords: eating disorders, disorders of gut-brain interaction, functional gastrointestinal disorders, ROME III, body mass index
BACKGROUND
Disorders of gut-brain interaction (DGBI), previously known as functional gastrointestinal disorders (FGID), are a group of gastrointestinal (GI) disorders characterized by chronic or recurrent GI symptoms. They arise as a result of abnormal functioning of the GI tract, and cannot be explained by structural or biochemical abnormalities (Drossman, 2016). The symptoms of DGBI are related to physiological determinants including dysmotility, visceral hypersensitivity, altered mucosal immune and inflammatory function (including alterations in intestinal bacterial flora), and altered central nervous system regulation (Drossman, 2016). Knowledge about the pathophysiology and diagnostic accuracy of DGBI improved with the introduction of the ROME criteria in 1994, and DGBI now have a clear taxonomy that enhances both science and clinical diagnosis and treatment. Several determinants of DGBI have been identified. Psychosocial factors such as stress, psychological state, and social support serve as moderators of patients’ experience and behaviors and, in the end, also the clinical outcome (Drossman, 2006).
Gastrointestinal complaints are common in individuals with eating disorders (Bern, Woods, & Rodriguez, 2016; Hetterich, Mack, Giel, Zipfel, & Stengel, 2019; Riedlinger et al., 2020; Sato & Fukudo, 2015). Problems such as constipation, bloating, nausea, and epigastric discomfort are commonly reported in anorexia nervosa (AN), bulimia nervosa (BN), and binge-eating disorder (BED) (Bern & O’Brien, 2013; Cremonini et al., 2009; Peat et al., 2013; Sato & Fukudo, 2015; Zipfel et al., 2006). In addition, patients with eating disorders are about 3 times more likely than controls to seek treatment for GI problems (Winstead & Willard, 2006). Furthermore, GI problems have been found to be associated with somatization in patients with AN (Kessler et al., 2020). However, the relationship between DGBI and eating disorders is complex and potentially bidirectional.
Although it is clear that some behaviors associated with eating disorders such as restraint, binge eating, and compensatory behaviors can lead to or exacerbate GI symptoms (Cremonini et al., 2009; Wang, Luscombe, Boyd, Kellow, & Abraham, 2014), alterations in feeding behaviors, such as food restriction, can also be initiated in response to GI symptoms in individuals suffering from GI disorders (Wang et al., 2014). The directionality of effect can be particularly challenging to dissect in patients with eating disorders and comorbid GI disorders (Abraham, Boyd, Luscombe, Hart, & Russell, 2007; Lee, Lee, Ngai, Lee, & Wing, 2001). In individuals with both eating disorders and GI symptoms, the GI symptoms may persist in up to 77% of cases, even after weight restoration and improvement in eating behaviors, suggesting that eating behaviors alone cannot explain the variance in GI symptoms (Boyd, Abraham, & Kellow, 2010). Psychological features that have been implicated in DGBI include higher levels of somatization, neuroticism (Boyd, Abraham, & Kellow, 2005; Wang et al., 2014), anxiety (Boyd et al., 2005), and depression (Wang et al., 2014). It has also been suggested that once GI problems develop subsequent to an eating disorder, physiological and psychological factors interact and strengthen thereby contributing to the persistence of GI problems independent of psychopathology (Janssen, 2010).
A comprehensive evaluation of DGBI across various DSM-5 eating disorder presentations in a large sample is needed to clarify the relationship. Existing studies focus on GI symptoms or individual DGBI, have small sample sizes, or lack a control group (Boyd et al., 2005; Wang et al., 2014).
The present study had three aims: 1) to document the prevalence of DGBI in a sample of individuals with eating disorders [AN, BN, and multiple eating disorders (mED)] compared to healthy controls; 2) to examine the specific impact of disordered eating behaviors on the risk of developing problems in specific DGBI categories; and 3) to compare the total number of DGBI in individuals reporting high current eating disorder symptoms to those with low current eating disorder symptoms, and healthy controls. We hypothesized that DGBI would be more frequently reported in individuals with eating disorders, especially in those with a history of multiple eating disorder diagnoses, compared to controls. We further hypothesized that DGBI would be associated with various disordered eating behaviors, in particular with laxative misuse and purging behavior. Lastly, we hypothesized that those with high current symptoms would have more DGBI compared to those with low current symptoms.
METHOD
Population
Participants represented the initial freeze of the ongoing Binge Eating Genetics Initiative-Sweden project (BEGIN-SE). BEGIN-SE is a large-scale, cross-sectional study collecting genetic, microbiome, and phenotypic data from individuals primarily diagnosed with BN and BED (but also other eating disorders) in Sweden. Cases are identified via the national quality register for eating disorder treatment (Riksät) (Swedish Association of Local Authorities and Regions, 2007). The register began in 1999 and is an internet-based register for individuals being treated for eating disorders throughout Sweden. For individuals in the register who were invited to participate in BEGIN-SE, the eating disorder diagnoses was based on clinician interviews and clinical observation until 2013 when the Structured Eating Disorder Interview (SEDI) (de Man Lapidoth & Birgegård, 2010) was also implemented. Individuals who have received a diagnosis of BN or BED in the register, and are age 18 or older are invited to participate in BEGIN-SE. Individuals who consented to participate in the study completed the ED100K to confirm a current and or lifetime eating disorder diagnosis. The healthy control individuals participating in the study are recruited with help from a professional company from a population sample, and are frequency matched on age and sex and have no prior history, or family history, of eating disorders. In addition, the controls cannot currently be taking any medication for any other psychiatric illness. Interviewers call eligible individuals (based on matching criteria), describe the study, and if the individuals are interested in participating, the interviewer conducts an initial screening to determine personal eligibility. Controls who screen eligible are then contacted by data collectors from BEGIN-SE to officially be enrolled in the study. General exclusion criteria for all participation in the BEGIN-SE study are having had weight reduction surgery, having any form of inflammatory bowel disease (e.g., ulcerative colitis), current antibiotic or probiotic treatment (because of the microbiome component), hormonal replacement therapy, being pregnant, or being currently breastfeeding. The BEGIN-SE study was approved by the Regional Ethical Review Board in Stockholm, Sweden. All participants electronically sign informed consent before entering the study. The first freeze sample included 765 cases and 1240 controls, and the included participants were recruited between December 2017 and February 2020. Of the potentially eligible individuals in the Riksät register, 27% consented to participate in the study. From the register, 4772 potential cases were invited to take part in the BEGIN-SE study via either phone or email, and out of the invited, 1309 consented to participate (27%). We included all cases with complete data on the included questionnaires.
Measures
Participants in BEGIN-SE complete a battery of validated, online questionnaires (see below), and self-report measures of weight, height, age, and sex.
The ED100K (Thornton et al., 2018) is a self-report questionnaire that captures information on lifetime history of eating disorders and disordered eating behavior. Algorithms to classify the participants into AN, BN, and BED were based on DSM-5 diagnostic criteria. We categorized cases into groups who reported having had exclusively one diagnosis in their lifetime [AN (~40%) and BN (~25%)], as well as mED group (~35%) comprising participants who reported having had more than one eating disorder diagnosis (AN, BN, and/or BED) in their lifetime. Only 5 individuals reported having had exclusively BED and we did therefore exclude these individuals from the analysis. From the ED100K, we also collected information about current, lifetime lowest, and lifetime highest BMI. The ED100K has been validated and shows high agreement with diagnosis determined by the Structured Clinical Interview SCID-Eating Disorders Module (Thornton et al., 2018).
The Eating Disorders Examination Questionnaire (EDE-Q) (Fairburn & Beglin, 1994) assesses symptoms of eating disorders over the past 28 days. Participants reported on frequency of binge-eating, purging, laxative misuse, and fasting. To analyze the second aim of the study, the behaviors were coded into binary variables, those reporting binge-eating, purging, and laxative misuse ≥4 times in the past 28 days (i.e., ≥ once per week), as well as those reporting fasting ≥6 days in the past 28 days, were coded as having the behavior, those reporting a lower frequency were coded as not having the behavior.
The EDE-Q also yields 4 subscale scores, and a global score. To analyze the third aim of the study, we used the Swedish norms for the EDE-Q to categorize the cases by applying a cut-off of 2.76 EDE-Q for the global score (Ekeroth & Birgegård, 2014; Welch, Birgegård, Parling, & Ghaderi, 2011). This cut-off was used to identify individuals who were currently having high levels of eating disorder symptoms versus individuals who were currently having low levels of symptoms.
Test-retest reliability of the EDE-Q has been shown to be good, ranging from 0.66–0.94 for the individual subscales and from 0.51–0.92 for the key eating disorder behavior items (Luce & Crowther, 1999; Reas, Grilo & Masheb, 2006). Studies of validity comparing the EDE-Q with its interview equivalent (the Eating Disorder Examination) have generally demonstrated good agreement between the measures (Fairburn & Beglin, 1994).
All participants in BEGIN-SE completed the ROME III questionnaire (Drossman, 2006), which is a screening tool for DGBI. There are 6 DGBI categories (A-F), and each category includes several individual DGBI. The ROME III addresses symptoms during the past 3 months. We included all individual DGBI; however, we acknowledge that some disorders require clinical evaluation or laboratory tests to confirm the diagnosis. The ROME III has been validated and show high specificity (ranging from 87.8–100% depending on DGBI category), and test-retest agreement (0.82–0.98) (Drossman, 2006).
All study participants self-reported weight and height. Weight reported online and objectively reported weight are highly correlated; online reporting is a valid method of data collection in both the general population (Bonn, Trolle Lagerros, & Balter, 2013) and in individuals with eating disorders (Doll & Fairburn, 1998; White, Masheb, & Grilo, 2010; Wolfe, Kelly-Weeder, Malcom, & McKenery, 2013).
Statistical analysis
All statistical analyses were performed using statistical software R, version 3.6.2. (R Core Team, 2017). Data are presented as means and standard deviations, or frequencies and percentages, as appropriate. Descriptive demographic details of the full sample and by eating disorder group are reported. We calculated the prevalence of the general DGBI categories, of each individual DGBI, and the mean number of total DGBI diagnoses across eating disorder groups and for controls. We compared eating disorder groups and controls on proportions (DGBI categories) using χ2 tests, and on continuous variables (e.g., descriptive, mean number of DGBI diagnoses) using ANOVA with post-hoc tests (Tukey’s HSD).
We used logistic regression to evaluate the association between different disordered eating behaviors (binge eating, purging [self-induced vomiting], laxative misuse, and fasting), independent of eating disorder groups with general DGBI categories and individual DGBI, where disordered eating behavior was the exposure and DGBI was the outcome in all models. For functional gastroduodenal disorders (category B), estimates are provided with and without the inclusion of the cyclic vomiting symptom, given that this could be conflated with self-induced vomiting associated with eating disorders. We present odds ratios (OR) and 95% confidence intervals (CI) for the crude model as well as for the model adjusted for age, sex, and BMI.
Finally, we compared the mean number of DGBI in each of the eating disorder groups by symptom status (i.e., high or low current symptom levels in the past 28 days, as measured by the EDE-Q global score) by using a nonparametric Kruskal-Wallis test, followed by Dunn post hoc method. False discovery rate (FDR) procedure was used to correct for multiple comparisons, q-values (i.e., p-values adjusted by FDR) are presented (Benjamini, Drai, Elmer, Kafkafi, & Golani, 2001; Storey, 2002).
RESULTS
Prevalence of DGBI in eating disorders
Table 1 presents the descriptive details of cases and controls. In comparisons between all cases combined versus controls, participants in the control group were slightly older than the cases (q<0.001). Also, there were more men in the case group than the control group (q<0.001); however, the number of men was small in both groups. Cases had lower mean current as well as lifetime lowest BMI values compared with controls, but no statistically significant difference in mean lifetime highest BMI emerged. Comparing across eating disorders subgroups (AN, BN, and mED) and with controls, as expected, the AN group had significantly lower lifetime highest, lowest, and current BMI compared to all other groups. The BN and mED groups had higher current and lifetime highest BMI compared with controls, and the mED group had also lower lifetime lowest BMI compared with both the BN and the control groups.
Table 1. Descriptive details of the cohort and distribution of diagnoses in the cases.
Cases in each subgroup of eating disorders and sex is presented as proportions (%), age and current, lifetime lowest, and lifetime highest body mass index (BMI) is presented as means and standard deviations (SD) as well as q-values (p-values corrected for multiple comparison using false discovery rate (FDR) procedure).
| Cases | Controls | ||||
|---|---|---|---|---|---|
|
| |||||
| All | AN | BN | mED | ||
|
|
|||||
| N | 765 | 305 | 188 | 267 | 1240 |
| Proportion of cases (%) | - | 39.9 | 24.6 | 34.9 | - |
| Mean age (years) ± SD | 27.8±7.4 | 27.1±7.5 | 27.8±7.0 | 28.5±7.5 | 30.7±7.9† |
| Sex (% female) | 92.2 | 94.1 | 87.2 | 93.3 | 98.9 |
| Mean current BMI (kg/m2) ± SD | 24.1±5.8§ | 21.0± 3.3† | 26.0± 5.6§ | 26.8± 7.2§ | 24.7± 4.1 |
| Mean lifetime lowest BMI (kg/m2) ± SD | 19.0±4.1§ | 16.7± 2.0† | 21.7± 4.5‡ | 19.7± 4.2§ | 21.6± 2.7 |
| Mean lifetime highest BMI (kg/m2) ± SD | 26.8±6.7 | 23.1± 4.4† | 28.9± 6.1§ | 29.9± 7.4§ | 27.3± 4.5 |
AN = anorexia nervosa; BN = bulimia nervosa; mED = multiple ED group.
Significantly different from all other groups at q<0.05.
Significantly different from the control group at q<0.05.
Significantly different from the mED group at q<0.05.
Table 2 presents the prevalences of the general DGBI categories as well as the individual DGBI, across eating disorder groups and in healthy controls: 88.2–95.5% of cases and 79.8% of controls reported having at least one individual DGBI. In terms of overall DGBI burden, experiencing three or more individual DGBI was reported by 34.8–48.7% of individuals in the eating disorder groups compared with 9.1% of individuals in the healthy control group (q<0.05).
Table 2.
Proportion (%) of disorders of gut-brain interaction (DGBI) categories and individual DGBI in eating disorder subgroups and controls.
| DGBI | Cases | Controls | |||
|---|---|---|---|---|---|
|
|
|
||||
| AN (n = 305) | BN (n = 188) | mED (n = 267) | HC (n = 1240) | q<0.05* | |
|
|
|
||||
| A. Functional esophageal disorders | 19.0 | 26.6 | 30.7 | 9.4 | HC<AN<BN/mED |
| A1. Functional heartburn§ | 9.2 | 10.1 | 11.6 | 2.7 | |
| A2. Functional chest pain of presumed esophageal origin§ | 6.2 | 7.4 | 10.5 | 2.7 | |
| A3. Functional dysphagia§ | 2.3 | 4.8 | 9.4 | 2.7 | |
| A4. Globus§ | 2.6 | 6.4 | 4.5 | 1.9 | |
| B. Functional gastroduodenal disorders | 32.1 | 41.5 | 45.7 | 7.5 | HC<AN<BN/mED |
| B1. Functional dyspepsia§ | 24.3 | 23.4 | 31.1 | 5.8 | |
| B1a. Postprandial distress syndrome | 16.7 | 10.1 | 18.0 | 1.0 | |
| B1b. Epigastric pain syndrome | - | - | ¤ | ¤ | |
| B2a/b. Aerophagia§/Unspecified excessive belching | 7.2 | 9.0 | 8.3 | 1.5 | |
| B3a. Chronic idiopathic nausea§ | 7.9 | 5.3 | 9.4 | 1.6 | |
| B3b. Functional vomiting§ | - | - | - | - | |
| B3c. Cyclic vomiting syndrome | 8.5 | 17.6 | 16.9 | ¤ | |
| B4. Rumination syndrome in adults | - | ¤ | ¤ | ¤ | |
| C. Functional bowel disorder | 85.9 | 92.6 | 92.1 | 78.8 | HC<AN<BN/mED |
| C1. Irritable bowel syndrome | 43.9 | 55.3 | 58.8 | 31.0 | |
| C2. Functional bloating | 16.4 | 21.3 | 16.1 | 19.0 | |
| C3. Functional constipation | 35.7 | 36.2 | 40.4 | 18.2 | |
| C4. Functional diarrhea | 2.0 | 3.2 | ¤ | 1.9 | |
| C5. Unspecified functional bowel disorder | 13.1 | 10.1 | 6.4 | 20.7 | |
| D. Functional abdominal pain syndrome | - | - | - | - | |
| E. Functional gallbladder and Sphincter of Oddi (SO) disorders § | - | - | - | - | |
| F. Functional anorectal disorder | 16.4 | 23.4 | 27.3 | 9.0 | HC<AN<BN/mED |
| F1. Functional fecal incontinence§ | 4.6 | 4.8 | 10.1 | 1.9 | |
| F2a. Chronic proctalgia§ | 2.6 | 4.8 | 2.6 | 1.3 | |
| F2b. Proctalgia fugax | 9.8 | 15.4 | 16.5 | 6.1 | |
| F3. Functional defecation disorder§ | ¤ | 3.2 | 2.6 | 0.8 | |
| At least 1 DGBI | 88.2 | 94.7 | 95.5 | 79.8 | HC<AN<BN/mED |
| At least 3 DGBI | 34.8 | 38.8 | 48.7 | 9.1 | HC<AN/BN<mED |
HC = healthy controls, AN = anorexia nervosa, BN = bulimia nervosa, mED = multiple ED group.
Clinical evaluation or laboratory test are required to make the diagnosis.
Less than 5, but more than 0, individuals report having this DGBI.
q-values (p-values corrected for multiple comparison using false discovery rate (FDR) procedure) indicate statistically significant difference between the groups at q<0.05.
A. Functional esophageal disorders
The most commonly reported disorder in group A was functional heartburn, reported by ~10% of all three eating disorder groups compared with 2.7% of controls. The AN group exhibited the lowest overall prevalence of functional esophageal disorders among the eating disorder groups (19.0%), and the mED group the highest (30.7%) with the 9.4% of the healthy control referent group reporting functional esophageal disorders.
B. Functional gastroduodenal disorders
Functional dyspepsia was the most frequently reported disorder in group B reported by 24.3% of individuals with AN, 23.4% of individuals with BN, and 31.1% of individuals in the mED group. AN showed the lowest overall prevalence of functional gastroduodenal disorders (32.1%), whereas BN and mED groups reported higher prevalences (41.5 and 45.7%, respectively)—all eating disorder groups were significantly elevated relative to the healthy control referent group (7.5%).
C. Functional bowel disorders
Functional bowel disorders were the most frequently reported category overall: 85.9–92.6% fulfilled the criteria for functional bowel disorders across the different eating disorder groups, whereas 78.8% reported these disorders in the healthy control group. The most frequently reported disorder in category C was irritable bowel syndrome (IBS), which was reported in 43.9% of individuals with AN, 55.3% of individuals with BN, and in 58.8% of the individuals in the mED group. IBS was also common in the heathy control group, being reported by 31.0% of individuals. All three eating disorder groups displayed high prevalence of functional constipation as well (AN 35.7%, BN 36.2%, and mED 40.4% versus 18.2% of controls).
D. Functional abdominal pain syndrome
No participants fulfilled all criteria for functional abdominal pain syndrome.
E. Functional gallbladder and Sphincter of Oddi (SO) disorders
No participants fulfilled all criteria for functional abdominal pain syndrome.
F. Functional anorectal disorders
Proctalgia fugax was the most frequently reported anorectal disorder, endorsed by 9.8% of AN, 15.4% of BN, and 16.5% of the mED group (compared to 6.1% of healthy controls). Overall, among the eating disorder groups, the AN group had the lowest prevalence of functional anorectal disorders (16.4%), and the mED diagnosis group the highest (27.3%). All eating disorder groups exhibited significantly higher prevalences of functional anorectal disorders compared to the control group where 9.0% fulfilled the criteria.
Association between DGBI and disordered eating behaviors.
Table 3 and Tables S1–4 (in the Supplementary Material) present the association between specific disordered eating behaviors and general DGBI categories (Table 3) as well as individual DGBI (Table S1–4). In Table 3 the adjusted estimates are presented, and in Tables S1–4 both the crude and adjusted estimates are shown.
Table 3.
Risk of disorders of gut-brain interaction (DGBI) categories according to eating disordered behaviors. Presented are results from logistic regression models: odds ratios (OR) with 95% confidence intervals (CI). The models are adjusted for age, sex, and BMI. Q-values (p-values corrected for multiple comparison by FDR) are presented.
| ED behavior | DGBI | ||||
|---|---|---|---|---|---|
|
| |||||
| A. Functional esophageal disorders | B. Functional gastroduodenal disorders | B. Functional gastroduodenal disorders – without B3c Cyclic vomiting | C. Functional bowel disorders | F. Functional anorectal disorders | |
|
|
|||||
| OR (95%CI) | OR (95%CI) | OR (95%CI) | OR (95%CI) | OR (95%CI) | |
|
| |||||
| Binge eating ≥1/week (n=428) | 2.0 (1.5; 2.7) (q< 0.001) | 3.3 (2.5; 4.3) (q< 0.001) | 2.5 (1.9; 3.3) (q< 0.001) | 2.3 (1.5; 3.7) (q= 0.001) | 2.8 (2.1; 3.9) (q< 0.001) |
| Purging ≥1/week (n=178) | 2.6 (1.9; 3.7) (q< 0.001) | 13.8 (9.6; 20.2) (q< 0.001) | 5.7 (4.2; 7.9) (q< 0.001) | 3.0 (1.7; 5.9) (q< 0.001) | 2.9 (2.0; 4.0) (q< 0.001) |
| Laxative misuse ≥1/week (n=32) | 3.5 (1.7; 7.2) (q= 0.001) | 3.6 (1.8; 7.5) (q< 0.001) | 4.4 (2.1; 9.1) (q< 0.001) | 6.3 (1.3; 112.1) (q= 0.094) | 3.9 (1.9; 8.1) (q< 0.001) |
| Fasting ≥6 days/month (n=239) | 2.1 (1.5; 2.8) (q< 0.001) | 4.2 (3.2; 5.6) (q< 0.001) | 3.5 (2.6; 4.6) (q= 0.094) | 1.5 (1.0; 2.4) (q= 0.069) | 2.3 (1.7; 3.2) (q< 0.001) |
Binge eating
Binge eating was associated with all DGBI general categories. The highest OR was between binge eating and the functional gastroduodenal disorders category (Category B) (adjusted OR 3.3, 95% CI 2.5–4.3). Among individual DGBI, the highest ORs were with cyclic vomiting syndrome (adjusted OR 5.4 95% CI: 3.5–8.3) and chronic proctalgia (adjusted OR 4.5 95% CI: 2.1–10.3) in the adjusted models (Table S1).
Purging
Purging was significantly associated with all DGBI categories (Table 3). The highest OR was observed for functional gastroduodenal disorders, both when including and excluding cyclic vomiting syndrome from the general DGBI category (adjusted OR 13.8, 95% CI 9.6–20.2 and adjusted OR 5.7, 95% CI 4.2–7.9). In terms of individual DGBIs (Table S2), strong positive associations were observed with cyclic vomiting syndrome (adjusted OR 42.7, 95% CI 28.8–63.9), functional dyspepsia (adjusted OR 6.9, 95% CI 5.0–9.6), and postprandial distress syndrome (adjusted OR 7.9, 95% CI 5.4–11.5).
Laxative misuse
Laxative misuse was significantly associated with all general DGBI categories, except functional bowel disorders, and the strongest association was with functional gastroduodenal disorders – without cyclic vomiting (adjusted OR 4.4, 95% CI 2.1–9.1) (Table 3). Cyclic vomiting syndrome was also associated with laxative misuse (adjusted OR 6.4, 95% CI 2.7–13.9) (Table S3). Laxative misuse was associated with functional fecal incontinence (adjusted OR 5.1, 95% CI 1.7–12.6) and functional heartburn (adjusted OR 6.7, 95% CI 2.8–14.8) (Table S3). In all models for laxative misuse the confidence intervals were wide, most likely due to a small number of individuals reporting laxative misuse (N=32).
Fasting
Fasting was significantly associated with three of the general DGBI categories. The strongest association was with functional gastroduodenal disorders (adjusted OR 4.2, 95% CI 3.2–5.6) (Table 3). Fasting was positively associated with several different individual DGBI, with cyclic vomiting syndrome (adjusted OR 7.2, 95% CI 4.9–10.4), postprandial distress syndrome (adjusted OR 6.7, 95% CI 4.7–9.5), and functional dyspepsia (adjusted OR 3.6, 95% CI 2.6–4.8) having the strongest associations (Table S4).
DGBI in individuals low and high current eating disorder symptoms
In all three eating disorder groups (AN, BN, and mED), individuals presenting with higher eating disorder symptom scores in the past 28 days (meaning EDE-Q total score >2.76), reported a higher mean number of individual DGBI, compared to the individuals who reported having fewer current symptoms (EDE-Q total score <2.76), all differences were statistically significant (q<0.001) (Figure 1). All cases, regardless of symptom status, had a higher mean number of individual DGBI compared to controls (q<0.001), except for the BN group with low current symptoms who did not have statistically significant more DGBI compared to controls (q=0.08).
Figure 1. Mean number of total disorders of gut-brain interaction (DGBI) diagnosis in cases with low and high current eating disorder symptoms and healthy controls.
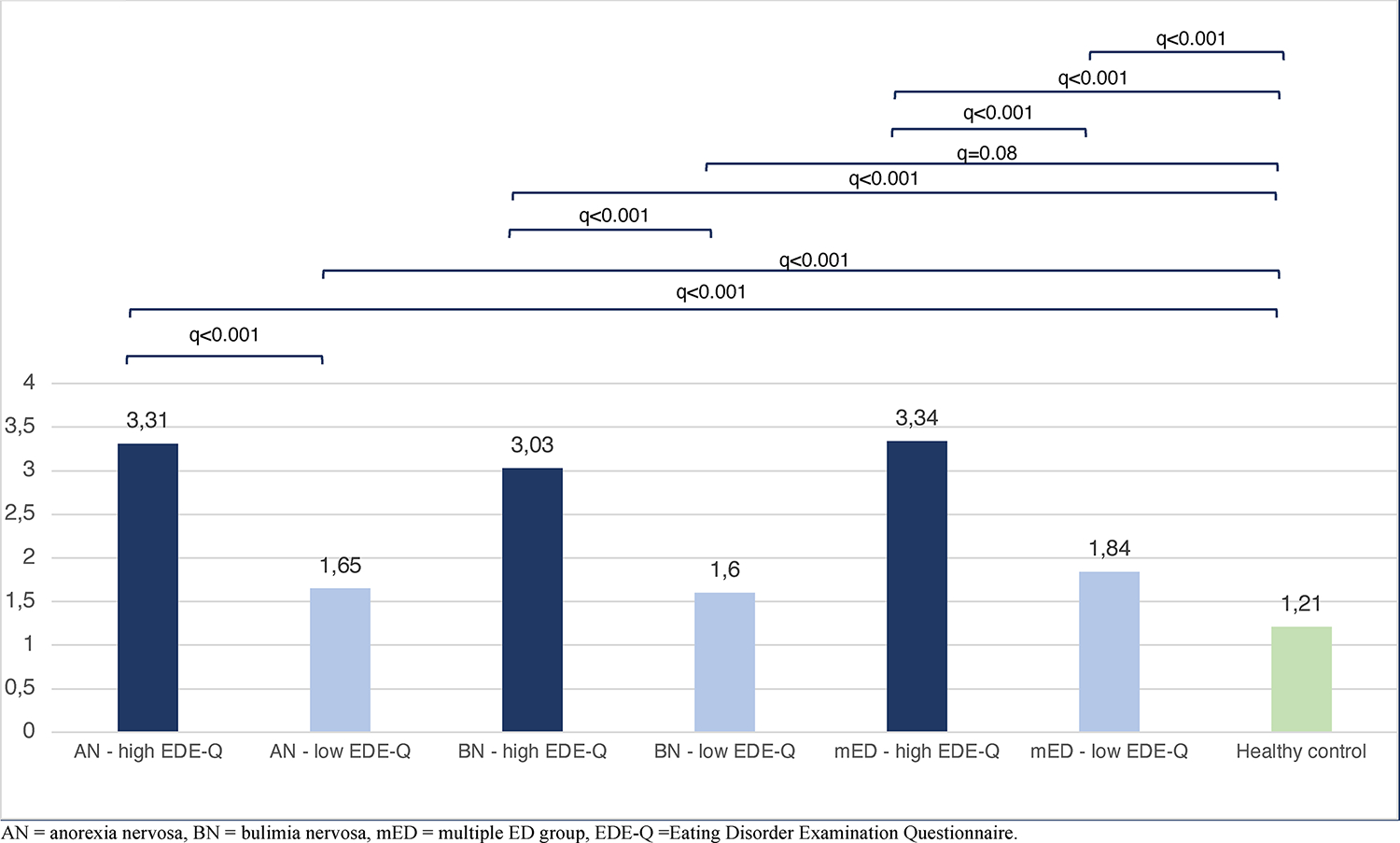
The AN, BN, and mED groups with high current symptoms in the past 28 days had significantly higher mean number of DGBI compared to the AN, BN, and mED groups with low current symptoms, and to the control group. All eating disorder groups (regardless of symptom status) had statistically higher mean number of DGBI compared to controls, except for the BN low symptoms group which did not significantly differ from the controls (q=0.081).
DISCUSSION
In this large case-control study, self-report DGBI were widespread in individuals with eating disorders with one third up to almost half of the eating disorder sample reporting three or more specific DGBIs. Although individuals with lower levels of current symptoms reported fewer DGBIs than individuals with higher symptom levels, they were still elevated above the healthy control referent group, suggesting that gastrointestinal disturbances persist even when eating disorder symptoms are relatively mild.
Prevalence of DGBI in eating disorders
A considerable literature exists on the high prevalence of gastroduodenal disorders in general, and gastric emptying disturbances in particular, in eating disorders (Benini et al., 2004; Boyd et al., 2005; Devlin et al., 2012; Hetterich et al., 2019; Kiss et al., 1990; Wang et al., 2014). Our study confirms previous findings by showing that one third to almost half of participants in the various eating disorder groups report gastroduodenal disorders. Several of the diagnostic criteria for functional gastroduodenal disorders (in particular functional dyspepsia and postprandial fullness) are related to feeling uncomfortably full after, and not being able to finish, a regular-size meal (Drossman, 2006). These particular GI symptom criteria closely interact with dysfunctional eating behaviors, which in turn may contribute to development and maintenance of eating disorders. Furthermore, studies on populations with GI disorders have found prevalent disordered eating behaviors, particularly for those with dietary-controlled GI disorders (Satherley, Howard, & Higgs, 2015). Thus, patients who experience disordered eating behaviors as well as gastroduodenal disorders, may be at higher risk for severe complications as symptoms can be mutually reinforcing. The cross-sectional design of this study cannot disentangle this relationship; however, it will be important for future research to assess temporality in the development of these conditions. In addition, clinicians should be aware of the complicated relationship and intertwined nature of eating disorder and GI symptoms and adopt an integrated approach that attends to both sets symptoms simultaneously in their patients.
In addition to functional gastroduodenal disorders, functional bowel disorders were prevalent in our sample, in particular IBS and constipation. IBS is one of the most frequently reported individual DGBI both in this present study, as well as in previous studies of eating disorders (Dejong, Perkins, Grover, & Schmidt, 2011; Perkins, Keville, Schmidt, & Chalder, 2005; Wang et al., 2014). Binge-eating behavior appears to be an important consideration in IBS, and current results provide further support for the positive association between binge eating and IBS (adjusted OR 2.26). Additionally, a large epidemiological study by Peat et al. (2013) found IBS to be positively associated with binge-eating behavior after adjusting for BMI. A suggested hypothesis explaining this relationship may be that IBS symptoms are often triggered by the intake of foods with a high fat content (Farré & Tack, 2013; Hayes, Fraher, & Quigley, 2014). Concurrently, foods consumed during typical binge-eating episodes have been shown to contain large amounts of fat and carbohydrates (Bartholome, Raymond, Lee, Peterson, & Warren, 2006). Hence, the observed association between binge-eating behavior and IBS might partly be explained by binge-eating episodes contributing to IBS symptoms.
Our results report constipation to be prevalent in all eating disorder groups, with slightly higher prevalence in the mED group compared to both the AN and BN group. This is confirmed in previous research showing constipation to be a widespread GI problem, regardless of eating disorder diagnosis (Chiarioni et al., 2000; Cremonini et al., 2009; Levy, Linde, Feld, Crowell, & Jeffery, 2005; Sileri et al., 2014). There are several potential explanations presented for constipation in eating disorders; restraint and fasting in AN may lead to a reflex disturbance of the colon (Chiarioni et al., 2000; Chun, Sokol, Kaye, Hutson, & Wald, 1997), in addition, longer transit time of the entire gastrointestinal system due poor nutrition in general has also been proposed for both AN and BN (Kamal et al., 1991; Zipfel et al., 2006; Perez, Coley, Crandall, Di Lorenzo, & Bravender, 2013; Bluemel et al., 2017). In BN there are several behavioral mechanisms that can lead to constipation, the most obvious being electrolyte disturbances, especially hypokalemia, attributable to self-induced vomiting and leading to constipation (Hetterich et al., 2019). Electrolyte disturbance, as well as dehydration, can also be the consequence of misuse of laxatives, a common compensatory behavior in BN, and cause of constipation (Sato & Fukudo, 2015; Zipfel et al., 2006). Individuals who become functionally dependent on laxatives, can also become constipated upon discontinuation of their use (Bulik, 1992). In individuals with BED it is has been hypothesized that an increased delivery and stool volume can lead to colon discomfort, and as a result lead to constipation (Cremonini et al., 2009). In addition, eating disorders patients with pelvic floor diagnosis have been found to more frequently report constipation, diarrhea, and fecal incontinence compared to eating disorder patients without pelvic floor diagnosis (Silvernale, Kuo, & Staller, 2020).
Eating disorder behaviors associated with DGBI
Fasting was particularly associated with gastroduodenal disorders, especially with postprandial fullness. Similar findings have been shown in earlier studies where Wang et al. found postprandial fullness to be significantly predicted by starvation (Wang et al., 2014). The association can be explained by physiological repercussions of severe food restriction, such as delayed gastric emptying; however, it cannot be determined if fasting leads to delayed gastric emptying, leading to postprandial fullness, or if the causal pathway is in another direction. It could be that eating-related discomfort, e.g. psychological reasons, leads to fasting due to the fear of postprandial symptoms such as fullness and discomfort. In this particular population of individuals with eating disorder, both pathways are plausible.
Also binge eating was associated with gastroduodenal disorders. These results are in line with previous literature where binge-eating episodes have been claimed to impair lower esophageal sphincter activity by counteracting the sphincter ability, resulting in gastric reflux, symptoms of heartburn, acid regurgitation, and dysphagia (Wildi, Tutuian, & Castell, 2004).
DGBI in individuals with high and low current eating disorder symptoms
Our observation of lower prevalence of DGBI in individuals with lower levels of current eating disorder symptoms compared to individuals with high current symptoms aligns (i.e., below and above the EDE-Q threshold) with several treatment studies reporting overall improvement in both subjective and objective GI symptoms after nutritional rehabilitation and/or psychotherapy for AN and BN (Benini et al., 2004; Chami, Andersen, Crowell, Schuster, & Whitehead, 1995; Cuntz et al., 2013; Riedlinger et al., 2020). However, although individuals reporting fewer eating disorder symptoms had fewer DGBI, they were still elevated above the healthy control referent group, suggesting that gastrointestinal disturbances are present even when eating disorder symptoms are mild. This is in line with the study by Boyd et al. who found DGBI to be stable over a 12-month follow-up period, even when eating disorder symptoms in general were reduced (Boyd et al., 2005). Longitudinal studies are essential to be able to further investigate the long-term effects of DGBI after recovery (or remission) from an eating disorder. The size of the sample of cases in the current study does not allow us to test whether symptoms status for specific eating disorder behaviors explain the dissimilarities in the previous literature. However, it will be important for future research to examine if certain subgroups of eating disorders have different impact on DGBI to better understand and treat the persistent GI symptoms in eating disorders.
Limitations
Although the current study provides important insights regarding the relation between DGBI and eating disorders, several limitations should be considered. First, the current study is cross-sectional, and we cannot draw conclusions regarding temporality or causality. Second, our sample of cases was recruited from the national eating disorder quality register in Sweden, meaning they have all sought treatment for their eating disorder. It is unknown the extent to which this represents all cases in the community as only a 50–75% of individuals ever seek treatment for their eating disorder (Mohler-Kuo, Schnyder, Dermota, Wei, & Milos, 2016). Third, we postulate that prolonged exposure to eating disorder symptoms is likely to exacerbate DGBI symptoms; however, our cross-sectional data do not allow us to explore the impact of duration of eating disorder on severity of DGBI. Fourth, a major limitation of the study is that we relied on self-report questionnaires rather than clinical assessment for DGBI, meaning that laboratory or clinician verification of diagnosis was not available. Although the ROME III questionnaire aims to prevent diagnosis being made based on mild symptoms of short duration, our results need to be confirmed in a sample where both self-reported and clinical/laboratory evaluations of gastrointestinal problems have been made. Previous reviews have compared studies with objective and subjective study designs, finding similar gastrointestinal problems in both (Riedlinger et al., 2020). Fifth, our sample did not contain enough individuals reporting an exclusive diagnosis of BED to perform statistical analysis. We could therefore not draw any conclusion regarding potential differences in DGBI in individuals with BED compared to BN and AN. Sixth, the response rate in the study is low (27%), potentially introducing sampling bias and thereby limiting the possibility to generalize the findings to a larger population. However, for studies with similar design as ours (where individuals in a register are invited to participate) the response rate of this study is comparable to (Forsén Mantilla, Clinton, & Birgegård, 2017) or somewhat lower than others (Magnus et al., 2006; Zagai, Lichtenstein, Pedersen, & Magnusson, 2019). Lastly, psychological traits, such as neuroticism and somatization, as well as psychiatric disorders (e.g., anxiety, depression), may increase the likelihood of self-reporting DGBI. Future studies are needed to disassemble the unique effect of psychological features and specific disordered eating behaviors.
Conclusion
The prevalence of DGBI, especially bowel disorders, is in general high in individuals with eating disorders. Across categories of DGBI, individuals with BN or mED have somewhat higher burden of DGBI than those with AN only. However, prevalence of total burden of DGBI is considerably lower in individuals reporting few current eating disorders symptoms, compared to individuals reporting higher levels of active eating disorder symptoms, potentially suggesting that recovery from an eating disorder may reduce the total burden of gastrointestinal complaints, although not to the same level as healthy controls. Longitudinal investigations across the course of illness and recovery are required to verify this observation. Clinicians dealing with both eating disorders and DGBI should be aware of the high cooccurrence of the two classes of disorders and take an integrated approach and prevent severe and chronic complications of this comorbidity.
Supplementary Material
ACKNOWLEDGEMENTS
CMB is supported by NIMH (R01MH120170; R01MH119084; R01MH118278; U01 MH109528); Brain and Behavior Research Foundation Distinguished Investigator Grant; Swedish Research Council (Vetenskapsrådet, award: 538–2013-8864); Lundbeck Foundation (Grant no. R276–2018–4581). This work was supported by the Swedish Research Council (VR Dnr: 538–2013-8864).
Footnotes
CONFLICT OF INTEREST
Dr. Bulik reports: Shire (grant recipient, Scientific Advisory Board member); Idorsia, consultant; Pearson (author, royalty recipient). No other authors have conflicts to disclose.
DATA AVAILABILITY STATEMENT
Data for the current study are available upon request.
At the completion of the study, anonymized data for portions of this investigation will be available by contacting the author within the bounds of Swedish law.
REFERENCES
- Abraham SF, Boyd C, Luscombe G, Hart S, & Russell J (2007). When energy in does not equal energy out: disordered energy control. Eating behaviors, 8(3), 350–356. doi: 10.1016/j.eatbeh.2006.11.010 [DOI] [PubMed] [Google Scholar]
- Bartholome LT, Raymond NC, Lee SS, Peterson CB, & Warren CS (2006). Detailed analysis of binges in obese women with binge eating disorder: Comparisons using multiple methods of data collection. International Journal of Eating Disorders, 39(8), 685–693. doi: 10.1002/eat.20289 [DOI] [PubMed] [Google Scholar]
- Benini L, Todesco T, Dalle Grave R, Deiorio F, Salandini L, & Vantini I (2004). Gastric emptying in patients with restricting and binge/purging subtypes of anorexia nervosa. American Journal of Gastroenterology, 99(8), 1448–1454. doi: 10.1111/j.1572-0241.2004.30246.x [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Drai D, Elmer G, Kafkafi N, & Golani I (2001). Controlling the false discovery rate in behavior genetics research. Behavioural Brain Research, 125(1–2), 279–284. doi: 10.1016/s0166-4328(01)00297-2 [DOI] [PubMed] [Google Scholar]
- Bern EM, & O’Brien RF (2013). Is it an eating disorder, gastrointestinal disorder, or both? Current Opinion in Pediatrics, 25(4), 463–470. doi: 10.1097/MOP.0b013e328362d1ad [DOI] [PubMed] [Google Scholar]
- Bern EM, Woods ER, & Rodriguez L (2016). Gastrointestinal Manifestations of Eating Disorders. Journal of Pediatric Gastroenterology and Nutrition, 63(5), e77–e85. doi: 10.1097/mpg.0000000000001394 [DOI] [PubMed] [Google Scholar]
- Bluemel S, Menne D, Milos G, Goetze O, Fried M, Schwizer W, … Steingoetter A (2017). Relationship of body weight with gastrointestinal motor and sensory function: studies in anorexia nervosa and obesity. BMC Gastroenterology, 17(1):4. doi: 10.1186/s12876-016-0560-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonn SE, Trolle Lagerros Y, & Balter K (2013). How valid are Web-based self-reports of weight? Journal of Medical Internet Research, 15(4), e52. doi: 10.2196/jmir.2393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd C, Abraham S, & Kellow J (2005). Psychological features are important predictors of functional gastrointestinal disorders in patients with eating disorders. Scandinavian Journal of Gastroenterology, 40(8), 929–935. doi: 10.1080/00365520510015836 [DOI] [PubMed] [Google Scholar]
- Boyd C, Abraham S, & Kellow J (2010). Appearance and disappearance of functional gastrointestinal disorders in patients with eating disorders. Neurogastroenterology and Motility, 22(12), 1279–1283. doi: 10.1111/j.1365-2982.2010.01576.x [DOI] [PubMed] [Google Scholar]
- Bulik CM (1992). Abuse of drugs associated with eating disorders. Journal of Substance Abuse Treatment, 4(1), 69–90. doi: 10.1016/0899-3289(92)90029-w [DOI] [PubMed] [Google Scholar]
- Chami TN, Andersen AE, Crowell MD, Schuster MM, & Whitehead WE (1995). Gastrointestinal symptoms in bulimia nervosa: effects of treatment. American Journal of Gastroenterology, 90(1), 88–92. [PubMed] [Google Scholar]
- Chiarioni G, Bassotti G, Monsignori A, Menegotti M, Salandini L, Di Matteo G, . . . Whitehead WE (2000). Anorectal dysfunction in constipated women with anorexia nervosa. Mayo Clinic Proceedings, 75(10), 1015–1019. doi: 10.4065/75.10.1015 [DOI] [PubMed] [Google Scholar]
- Chun AB, Sokol MS, Kaye WH, Hutson WR, & Wald A (1997). Colonic and anorectal function in constipated patients with anorexia nervosa. American Journal of Gastroenterology, 92(10), 1879–1883. [PubMed] [Google Scholar]
- Cremonini F, Camilleri M, Clark MM, Beebe TJ, Locke GR, Zinsmeister AR, . . . Talley NJ (2009). Associations among binge eating behavior patterns and gastrointestinal symptoms: a population-based study. International Journal of Obesity, 33(3), 342–353. doi: 10.1038/ijo.2008.272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuntz U, Enck P, Frühauf E, Lehnert P, Riepl RL, Fichter MM, & Otto B (2013). Cholecystokinin revisited: CCK and the hunger trap in anorexia nervosa. PLoS One, 8(1), e54457. doi: 10.1371/journal.pone.0054457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Man Lapidoth J, & Birgegård A (2010). Validation of the Structured Eating Disorder Interview (SEDI) against the Eating Disorder Examination (EDE). Karolinska Institutet; Stockholm. [Google Scholar]
- Dejong H, Perkins S, Grover M, & Schmidt U (2011). The prevalence of irritable bowel syndrome in outpatients with bulimia nervosa. International Journal of Eating Disorders, 44(7), 661–664. doi: 10.1002/eat.20901 [DOI] [PubMed] [Google Scholar]
- Devlin MJ, Kissileff HR, Zimmerli EJ, Samuels F, Chen BE, Brown AJ, . . . Walsh BT (2012). Gastric emptying and symptoms of bulimia nervosa: effect of a prokinetic agent. Physiology and Behavior, 106(2), 238–242. doi: 10.1016/j.physbeh.2012.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll HA, & Fairburn CG (1998). Heightened accuracy of self-reported weight in bulimia nervosa: a useful cognitive “distortion”. International Journal of Eating Disorders, 24(3), 267–273. doi: [DOI] [PubMed] [Google Scholar]
- Drossman DA (2006). The functional gastrointestinal disorders and the Rome III process. Gastroenterology, 130(5), 1377–1390. doi: 10.1053/j.gastro.2006.03.008 [DOI] [PubMed] [Google Scholar]
- Drossman DA (2006). Rome III: The Functional GI Disorders. Degnon Associate. [Google Scholar]
- Drossman DA (2016). Functional Gastrointestinal Disorders: History, Pathophysiology, Clinical Features and Rome IV. Gastroenterology. doi: 10.1053/j.gastro.2016.02.032 [DOI] [PubMed] [Google Scholar]
- Ekeroth K, & Birgegård A (2014). Evaluating reliable and clinically significant change in eating disorders: comparisons to changes in DSM-IV diagnoses. Psychiatry Research, 216(2), 248–254. doi: 10.1016/j.psychres.2014.02.008 [DOI] [PubMed] [Google Scholar]
- Fairburn CG, & Beglin SJ (1994). Assessment of eating disorders: interview or self-report questionnaire? International Journal of Eating Disorders, 16(4), 363–370. [PubMed] [Google Scholar]
- Farré R, & Tack J (2013). Food and symptom generation in functional gastrointestinal disorders: physiological aspects. American Journal of Gastroenterology, 108(5), 698–706. doi: 10.1038/ajg.2013.24 [DOI] [PubMed] [Google Scholar]
- Forsén Mantilla E, Clinton D, & Birgegård A (2017). Insidious: The relationship patients have with their eating disorders and its impact on symptoms, duration of illness, and self-image. Psychology and Psychotherapy, 91(3), 302–316. doi: 10.1111/papt.12161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes PA, Fraher MH, & Quigley EM (2014). Irritable bowel syndrome: the role of food in pathogenesis and management. Gastroenterology & Hepatology, 10(3), 164–174. [PMC free article] [PubMed] [Google Scholar]
- Hetterich L, Mack I, Giel KE, Zipfel S, & Stengel A (2019). An update on gastrointestinal disturbances in eating disorders. Molecular and Cellular Endocrinology, 497, 110318. doi: 10.1016/j.mce.2018.10.016 [DOI] [PubMed] [Google Scholar]
- Janssen P (2010). Can eating disorders cause functional gastrointestinal disorders? Neurogastroenterology and Motility, 22(12), 1267–1269. [DOI] [PubMed] [Google Scholar]
- Kamal N, Chami T, Andersen A, Rosell FA, Schuster MM, & Whitehead WE (1991). Delayed gastrointestinal transit times in anorexia nervosa and bulimia nervosa. Gastroenterology, 101(5), 1320–1324. doi: 10.1016/0016-5085(91)90083-w [DOI] [PubMed] [Google Scholar]
- Kessler U, Rekkedal GÅ, Rø Ø, Berentsen B, Steinsvik EK, Lied GA, Danielsen Y (2020). Association between gastrointestinal complaints and psychopathology in patients with anorexia nervosa. International Journal of Eating Disorders, 53(5), 532–536. doi: 10.1002/eat.23243 [DOI] [PubMed] [Google Scholar]
- Kiss A, Bergmann H, Abatzi TA, Schneider C, Wiesnagrotzki S, Höbart J, . . . et al. (1990). Oesophageal and gastric motor activity in patients with bulimia nervosa. Gut, 31(3), 259–265. doi: 10.1136/gut.31.3.259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Lee AM, Ngai E, Lee DT, & Wing YK (2001). Rationales for Food Refusal in Chinese Patients with Anorexia Nervosa. International Journal of Eating Disorders, 29(2), 224–229. [DOI] [PubMed] [Google Scholar]
- Levy RL, Linde JA, Feld KA, Crowell MD, & Jeffery RW (2005). The association of gastrointestinal symptoms with weight, diet, and exercise in weight-loss program participants. Clinical Gastroenterology and Hepatology, 3(10), 992–996. doi: 10.1016/s1542-3565(05)00696-8 [DOI] [PubMed] [Google Scholar]
- Luce KH, & Crowther JH (1999). The reliability of the Eating Disorder Examination-Self-Report Questionnaire Version (EDE-Q). The International journal of eating disorders, 25(3), 349–351. doi.org/ [DOI] [PubMed] [Google Scholar]
- Magnus P, Irgens LM, Haug K, Nystad W, Skjaerven R, Stoltenberg C; MoBa Study Group. (2006). Cohort profile: the Norwegian Mother and Child Cohort Study (MoBa). International Journal of Epidemiology, 35(5), 1146–1150. doi: 10.1093/ije/dyl170 [DOI] [PubMed] [Google Scholar]
- Mohler-Kuo M, Schnyder U, Dermota P, Wei W, & Milos G (2016). The prevalence, correlates, and help-seeking of eating disorders in Switzerland. Psychological Medicine, 46(13), 2749–2758. doi: 10.1017/s0033291716001136 [DOI] [PubMed] [Google Scholar]
- Peat CM, Huang L, Thornton LM, Von Holle AF, Trace SE, Lichtenstein P, . . . Bulik CM (2013). Binge eating, body mass index, and gastrointestinal symptoms. Journal of Psychosomatic Research, 75(5), 456–461. doi: 10.1016/j.jpsychores.2013.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez ME, Coley B, Crandall W, Di Lorenzo C, Bravender T (2013). Effect of nutritional rehabilitation on gastric motility and somatization in adolescents with anorexia. The Journal of Pediatrics, 163(3), 867–872. doi: 10.1016/j.jpeds.2013.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins SJ, Keville S, Schmidt U, & Chalder T (2005). Eating disorders and irritable bowel syndrome: is there a link? J Psychosom Res, 59(2), 57–64. doi: 10.1016/j.jpsychores.2004.04.375 [DOI] [PubMed] [Google Scholar]
- R Core Team. (2017). R: A language and environment for statistical computing. Vienna, Austria.: R Foundation for Statistical Computing. [Google Scholar]
- Reas DL, Grilo CM, & Masheb RM (2006). Reliability of the Eating Disorder Examination-Questionnaire in patients with binge eating disorder. Behaviour research and therapy, 44(1), 43–51. doi.org/ 10.1016/j.brat.2005.01.004 [DOI] [PubMed] [Google Scholar]
- Riedlinger C, Schmidt G, Weiland A, Stengel A, Giel KE, Zipfel S, . . . Mack I (2020). Which Symptoms, Complaints and Complications of the Gastrointestinal Tract Occur in Patients With Eating Disorders? A Systematic Review and Quantitative Analysis. Frontiers in Psychiatry, 11, 195. doi: 10.3389/fpsyt.2020.00195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satherley R, Howard R, & Higgs S (2015). Disordered eating practices in gastrointestinal disorders. Appetite, 84, 240–250. doi: 10.1016/j.appet.2014.10.006 [DOI] [PubMed] [Google Scholar]
- Sato Y, & Fukudo S (2015). Gastrointestinal symptoms and disorders in patients with eating disorders. Journal of Clinical Gastroenterology, 8(5), 255–263. doi: 10.1007/s12328-015-0611-x [DOI] [PubMed] [Google Scholar]
- Sileri P, Franceschilli L, De Lorenzo A, Mezzani B, Todisco P, Giorgi F, . . . Jacoangeli F (2014). Defecatory disorders in anorexia nervosa: a clinical study. Techniques in Coloproctology, 18(5), 439–444. doi: 10.1007/s10151-013-1068-x [DOI] [PubMed] [Google Scholar]
- Silvernale CJ, Kuo B, Staller K (2020). Sa1678 Pelvic floor prolapse associated with GI-specific healthcare utilization and anorexia nervosa in an eating disorder patient cohort. Gastroenterology. 158(6), S-379. doi: 10.1016/S0016-5085(20)31642-5 [DOI] [Google Scholar]
- Storey John D. (2002). A direct approach to false discovery rates. Journal of the Royal Statistical Society Series B (Statistical Methodology), 64, 479–498. doi: 10.1111/1467-9868.00346 [DOI] [Google Scholar]
- Swedish Association of Local Authorities and Regions. (2007). National Healthcare Quality Registries in Sweden. [Google Scholar]
- Thornton LM, Munn-Chernoff MA, Baker JH, Jureus A, Parker R, Henders AK, . . . Bulik CM (2018). The Anorexia Nervosa Genetics Initiative (ANGI): Overview and methods. Contemporary Clinical Trials, 74, 61–69. doi: 10.1016/j.cct.2018.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Luscombe GM, Boyd C, Kellow J, & Abraham S (2014). Functional gastrointestinal disorders in eating disorder patients: altered distribution and predictors using ROME III compared to ROME II criteria. World Journal of Gastroenterology, 20(43), 16293–16299. doi: 10.3748/wjg.v20.i43.16293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch E, Birgegård A, Parling T, & Ghaderi A (2011). Eating disorder examination questionnaire and clinical impairment assessment questionnaire: general population and clinical norms for young adult women in Sweden. Behaviour Research and Therapy, 49(2), 85–91. doi: 10.1016/j.brat.2010.10.010 [DOI] [PubMed] [Google Scholar]
- White MA, Masheb RM, & Grilo CM (2010). Accuracy of self-reported weight and height in binge eating disorder: misreport is not related to psychological factors. Obesity, 18(6), 1266–1269. doi: 10.1038/oby.2009.347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildi SM, Tutuian R, & Castell DO (2004). The influence of rapid food intake on postprandial reflux: studies in healthy volunteers. The American Journal of Gastroenterology, 99(9), 1645–1651. doi: 10.1111/j.1572-0241.2004.30273.x [DOI] [PubMed] [Google Scholar]
- Winstead NS, & Willard SG (2006). Gastrointestinal complaints in patients with eating disorders. Journal of Clinical Gastroenterology, 40(8), 678–682. doi: 10.1097/00004836-200609000-00003 [DOI] [PubMed] [Google Scholar]
- Wolfe BE, Kelly-Weeder S, Malcom AW, & McKenery M (2013). Accuracy of self-reported body weight and height in remitted anorexia nervosa. Journal of the American Psychiatric Nurses Association, 19(2), 66–70. doi: 10.1177/1078390313481062 [DOI] [PubMed] [Google Scholar]
- Zagai U, Lichtenstein P, Pedersen NL, & Magnusson PKE (2019). The Swedish Twin Registry: Content and Management as a Research Infrastructure. Twin Research and Human Genetics, 22(6), 672–680. doi: 10.1017/thg.2019.99 [DOI] [PubMed] [Google Scholar]
- Zipfel S, Sammet I, Rapps N, Herzog W, Herpertz S, & Martens U (2006). Gastrointestinal disturbances in eating disorders: clinical and neurobiological aspects. Autonomic Neuroscience: Basic and Clinical, 129(1–2), 99–106. doi: 10.1016/j.autneu.2006.07.023 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data for the current study are available upon request.
At the completion of the study, anonymized data for portions of this investigation will be available by contacting the author within the bounds of Swedish law.


