Abstract
An alternative group 2 sigma factor was identified in the nitrogen-fixing, symbiotically competent cyanobacterium Nostoc punctiforme and designated sigH. Transcription of sigH was specifically induced within 1.5 h following exposure of N. punctiforme to its symbiotic plant partner, Anthoceros punctatus. A mutation in sigH resulted in a sixfold-higher initial infection of A. punctatus tissue without a parallel increase in nitrogen-fixing activity.
Sigma factor proteins are responsible for conferring promoter-specific contacts upon the RNA polymerase enzyme of eubacteria, thereby allowing specific genes to be transcribed (11). Comparative sequence analysis has revealed that there are two fundamental families of sigma factors in eubacteria. These are referred to as the ς70 and ς54 families, by virtue of their similarities either to the principal Escherichia coli sigma factor, ς70, or to E. coli ς54, which is responsible for transcription of nitrogen-regulated genes. Within the ς70 family, three classes are recognized on the basis of function and sequence similarity (14). Group 1 comprises the primary, or housekeeping, sigma factors, which are essential for exponential cell growth. Group 2 sigma factors exhibit sequence similarity to the group 1 sigma factors—especially in the regions which may recognize promoter sequences—but are dispensable for exponential cell growth. Group 3 sigma factors, which are the most divergent ones, fall into specialized subgroups and are required for initiating transcription at distinct promoters, such as ones for heat shock, motility, or sporulation genes (14). Specific environmental changes may result in activation of some group 2 or 3 sigma factors, leading to differential gene expression and adaptation to the new environment.
Some filamentous cyanobacteria have multiple cellular developmental alternatives as well as nutritional versatility. Adaptation in response to environmental stimuli in these organisms is presumed to be mediated by distinct regulatory systems, resulting in differential gene expression. However, the mechanisms of differential gene expression in cyanobacteria that are responsible for adaptation to environmental changes or initiation of cellular differentiation are not well understood (6).
Filamentous heterocyst-forming (sites of nitrogen fixation in air) Nostoc punctiforme ATCC 29133 (PCC 73102) is facultatively heterotrophic and regulates transcription of carbon catabolic genes (18). In addition to heterocysts, N. punctiforme differentiates spore-like akinetes in response to phosphate or energy limitation (18) and gliding filaments called hormogonia (2). N. punctiforme can form a symbiotic association, via infection by hormogonium filaments, with pure cultures of the bryophyte hornwort Anthoceros punctatus (9, 15). In symbiosis, two of the developmental alternatives, hormogonium formation and heterocyst formation, are enhanced (2, 3). Although this symbiotic association has been characterized physiologically (15), little is known about the changes in gene expression resulting in the Nostoc sp. adaptation to the symbiotic growth state. Since one way bacteria regulate transcription of specific sets of genes is by alteration of the sigma subunit of RNA polymerase, we have examined the potential role of an alternative sigma factor in N. punctiforme development and symbiotic interaction.
Identification of sigH and ctpH.
The Anabaena sp. strain PCC 7120 group 2 sigma factor gene, sigB (1), hybridized to multiple bands of digested genomic DNA from N. punctiforme. Only one of the hybridizing fragments is characterized here. A strongly hybridizing 2.9-kb EcoRI fragment from cosmid p1G9 of N. punctiforme genomic DNA (5) was subcloned into the EcoRI site of pBluescript KS(+) to yield pSCR213. Sequencing of the 2.9-kb EcoRI fragment in pSCR213 revealed two open reading frames (ORF) (Fig. 1). One ORF, 966 bp long, shows high similarity to sigma factor genes from the ς70 family and was designated sigH. N. punctiforme SigH has a deduced molecular mass of 36,904 Da. The amino acid sequence of SigH is 72, 64, and 59% similar to the alternative sigma factors, SigB and SigC, and the primary sigma factor, SigA, respectively, of Anabaena sp. strain PCC 7120. The similarity of SigH to alternative sigma factors identified in unicellular cyanobacteria is no greater: SigH has 53% similarity to SigE of Synechococcus sp. strain PCC 7002 (12) and 64% similarity to RpoD2 of Synechococcus sp. strain PCC 7942 (19). An amino acid alignment of the SigH, SigB, SigC, and SigA proteins shows a high degree of conservation in regions 1 to 4 and especially in regions 2.4 and 4.2 (Fig. 2) of sigma factors of the ς70 family. The amino acids likely to be responsible for base-specific contacts in the promoter regions by analogy to E. coli ς70-type sigma factors (7, 10) are also found in all four cyanobacterial sigma proteins.
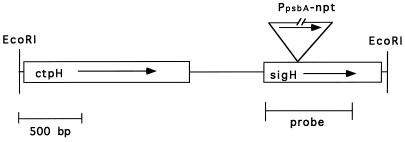
FIG. 1.
Restriction map of the sigH and ctpH genetic region showing the approximate site and orientation of the antibiotic insertion in sigH. PpsbAnpt designates the neomycin phosphotransferase gene with the PpsbA promoter; its size is not drawn to the same scale as the rest of the figure, as indicated by breaks within the gene. Arrows indicate the direction of transcription. The approximate location of the probe used in Northern hybridization is indicated beneath sigH.
FIG. 2.
Amino acid sequence alignment of N. punctiforme ATCC 29133 SigH and Anabaena sp. strain PCC 7120 SigA, SigB, and SigC. Asterisks indicate identical amino acids and dots indicate conserved amino acid changes, as defined in reference 13. Lines drawn above indicate regions 1 to 4 of sigma factors as defined in reference 14. Dashes indicate gaps introduced during the alignment process. Amino acids in bold are those which may make base-specific contacts with promoter sequences by analogy with E. coli sigma factors.
The second ORF, which is 1,272 bp long and is 5′ of sigH, is most similar to the Synechocystis sp. strain PCC 6803 genes (ctp) corresponding to the carboxyl-terminal processing protease as determined by BLAST analysis. N. punctiforme CtpH has a predicted molecular mass of 45,802 Da. It has 59, 50, and 49% amino acid sequence similarity to the CtpC, CtpB, and CtpA proteins, respectively, of Synechocystis sp. strain PCC 6803. Characterization of the N. punctiforme ctpH gene will be reported elsewhere.
Phenotype of the sigH mutant.
An insertion mutation in sigH was constructed by ligation of a neomycin phosphotransferase gene (npt) transcribed by the PpsbA promoter (from plasmid pRL448 [8]) into the unique Esp3I site of sigH (Fig. 1). The direction of transcription of the inserted npt gene coincides with the direction of sigH transcription such that the upstream gene, ctpH, is not affected by antisense transcripts and any cotranscribed downstream genes will now be transcribed from the PpsbA promoter. An ORF with a translational start point 246 bp 3′ of the translational stop site of sigH has been partially sequenced from the cosmid p1G9; the predicted product of this ORF shows similarity to membrane fusion proteins involved in drug resistance and a hypothetical protein from Synechocystis sp. strain PCC 6803. Based on Southern hybridization and PCR amplification (data not shown), this ORF is also adjacent to sigH in the genome of N. punctiforme and is transcribed in the same direction as sigH. However, based on Northern hybridization with an internal PCR-generated probe, this ORF is neither cotranscribed with sigH nor transcribed under steady-state growth or hormogonium induction conditions (data not shown). Thus, the insertion should disrupt only sigH, and the resultant phenotype is unlikely to be a consequence of polar disruption of an undescribed gene adjacent to sigH.
The N. punctiforme sigH mutant, strain UCD 398, had no obvious phenotypic defects under photoautotrophic or heterotrophic culture conditions, with or without added combined nitrogen. Strain UCD 398 formed visibly normal akinetes upon entry into the stationary growth phase, similar to the parental wild-type strain. Since the N. punctiforme sigH product is dispensable for exponential growth and is highly similar at an amino acid level to other ς70 factors, it is by definition a group 2 sigma factor of the ς70 family.
Upon coculture of mutant UCD 398 with A. punctatus, a higher level of infection was observed than with the wild-type strain. Following 2 weeks of coculture, strain UCD 398 formed 1.2 ± 0.2 (mean ± standard error) (n = 19, where n equals the number of replicate experiments) symbiotic colonies per mg (dry weight) of A. punctatus tissue per μg of chlorophyll (Chl) a of the N. punctiforme inoculum. Conversely, in the same coculture period, the wild-type N. punctiforme strain, ATCC 29133, formed 0.21 ± 0.04 (n = 25) symbiotic colonies per mg (dry weight) μg of Chl a inoculum. The sixfold increase in symbiotic colonies over the wild type is similar to the infection frequency observed with mutations in the hrm operon of N. punctiforme (4).
Despite the sixfold increase in the number of symbiotic colonies per unit of A. punctatus tissue, the total amount of nitrogen fixation activity in mutant strain UCD 398-A. punctatus associated tissue remained essentially the same as that of the wild-type association as measured by acetylene reduction after 2 weeks of coculture. The mutant reduced 8.0 ± 3.9 (n = 4) nmol of C2H2 per min per g (fresh weight) of strain UCD 398-A. punctatus associated tissue versus 6.3 ± 1.2 (n = 14) nmol of C2H2 per min per g (fresh weight) of wild-type associated tissue. The proportionally elevated number of colonies decreased as the A. punctatus tissue continued to grow, such that after 9 weeks of coculture both mutant and wild-type reconstituted associations contained about 0.1 symbiotic colony per mg (dry weight) per μg of Chl a. These results are consistent with previous observations that the host plant regulates the level of heterocyst differentiation and/or total nitrogen fixation capability in the associated tissue (9, 15, 17).
Because the phenotype of a sigH mutant is similar to those of strains with mutations of the hrm operon, the potential epistatic relationship between sigH and the hrm operon was examined. A hrmA-luxAB fusion was constructed in the sigH mutant background by recombination following conjugation of the suicide plasmid pSCR9 (4) into strain UCD 398, creating strain UCD 417. Transcription of the hrm operon is induced specifically by an A. punctatus extract containing a hormogonium-repressing factor (4). Strain UCD 417 showed no significant difference in the induction of hrmA-luxAB by plant extract (peak of 1.5 × 106 ± 0.7 × 106 cpm μg of Chl a−1; n = 6) compared with the same reporter construction in the wild-type sigH background (peak of 2.0 × 106 ± 1 × 106 cpm μg of Chl a−1; n = 6). Thus, we conclude that the high-initial-infection phenotypes resulting from a sigH mutation or mutations within the hrm operon reflect dual genetic pathways that are not transcriptionally linked.
Transcription of sigH.
Transcription of sigH under steady-state growth conditions and following exposure to plant factors or environmental shifts was examined by Northern blot analysis. RNA was isolated by the method of Schmidt-Goff and Federspiel (16) and probed with an internal sigH fragment (shown in Fig. 1) generated by PCR with the following primers: 5′ GCTAGTAACACAAACAAAGC 3′ and 5′ TGTGATATAGCTGTTAGAAG 3′.
As expected for a group 2 sigma factor, no sigH transcript was detectable by Northern blotting under normal vegetative growth conditions (time zero [Fig. 3]). However, a 1,250-nucleotide sigH transcript was present within 1.5 h following exposure of N. punctiforme cells to plant culture medium conditioned by incubation of A. punctatus in the absence of combined nitrogen (Fig. 3). Nitrogen starvation of A. punctatus was previously shown to elicit extracellular production of factors (termed hormogonium-inducing factors [HIF]) which enhance the production of the Nostoc sp. hormogonium filaments that subsequently serve as infective units in establishment of the symbiosis (2). The coding region of sigH consists of 966 bp; thus, the transcript size is consistent with that of a monocistronic message. Transcription of sigH appeared to increase slightly at 6 h and then declined. The high-molecular-mass smear in Fig. 3 appears to be characteristic of the sigH probe; it was not evident when this blot was probed with ctpH, but it was evident in RNA samples additionally treated with DNase and probed with sigH (data not shown).
FIG. 3.
Northern blot of RNA isolated from wild-type N. punctiforme cells harvested before (0 h) and 1.5, 6, 12, and 24 h after exposure to plant exudate containing HIF. The size of the sigH transcript appearing to the left is in thousands and was determined relative to RNA standards that were electrophoresed in the same gel.
The expression of sigH was specific for exposure to plant exudate containing HIF; no message was detectable by Northern analysis under any of the other conditions we have tested, including steady-state nitrogen-replete (NH4+) or -limited (N2) growth conditions and transitions between the two conditions; 30-min exposures to high-intensity light (60 W m−2), heat shock (37°C), oxidative stress (120 μM H2O2), and osmotic stress (50 mM fructose); or stationary growth phase (akinetes present in the culture) (data not shown). Consistent with our conclusion that sigH is not involved in transcription of the hrm operon, plant extract containing hormogonium-repressing factor, which induces transcription of the hrm operon (4), did not induce the transcription of sigH as determined by Northern blot analysis (data not shown).
We have now identified a second genetic target in N. punctiforme that responds to chemical signals from A. punctatus. We expect that the identification of genes whose transcription is dependent upon SigH will clarify the nature of its altered response to plant factors and lead to the discovery of additional genes involved in symbiotic interactions.
Nucleotide sequence accession numbers.
Nucleotide sequences have been deposited in the GenBank data base under accession no. AF022822 for sigH and AF022823 for ctpH.
Acknowledgments
We thank F. Wong for confirming the second-strand sequence of sigH and K. Hagen for contributions of acetylene reduction values for the wild-type association. We thank the above as well as T. Hanson and F. F. del Campo for critical reading of the manuscript.
This work was supported by the National Science Foundation (grant IBN 95-14787).
REFERENCES
- 1.Brahamsha B, Haselkorn R. Identification of multiple RNA polymerase sigma factor homologs in the cyanobacterium Anabaena sp. strain PCC 7120: cloning, expression, and inactivation of the sigB and sigC genes. J Bacteriol. 1992;174:7273–7282. doi: 10.1128/jb.174.22.7273-7282.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campbell E L, Meeks J C. Characteristics of hormogonia formation by symbiotic Nostoc spp. in response to the presence of Anthoceros punctatus or its extracellular products. Appl Environ Microbiol. 1989;55:125–131. doi: 10.1128/aem.55.1.125-131.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campbell E L, Meeks J C. Evidence for plant-mediated regulation of nitrogenase expression in the Anthoceros-Nostoc symbiotic association. J Gen Microbiol. 1992;138:473–480. [Google Scholar]
- 4.Cohen M F, Meeks J C. A hormogonium regulating locus, hrmUA, of the cyanobacterium Nostoc punctiforme strain ATCC 29133 and its response to an extract of a symbiotic plant partner Anthoceros punctatus. Mol Plant-Microbe Interact. 1997;10:280–289. doi: 10.1094/MPMI.1997.10.2.280. [DOI] [PubMed] [Google Scholar]
- 5.Cohen M F, Wallis J G, Campbell E L, Meeks J C. Transposon mutagenesis of Nostoc sp. strain ATCC 29133, a filamentous cyanobacterium with multiple cellular differentiation alternatives. Microbiology. 1994;140:3233–3240. doi: 10.1099/13500872-140-12-3233. [DOI] [PubMed] [Google Scholar]
- 6.Curtis S E, Martin J A. The transcriptional apparatus and the regulation of transcription initiation. In: Bryant D A, editor. The molecular biology of cyanobacteria. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 613–639. [Google Scholar]
- 7.Daniels D, Zuber P, Losick R. Two amino acids in an RNA polymerase ς factor involved in the recognition of adjacent base pairs in the −10 region of a cognate promoter. Proc Natl Acad Sci USA. 1990;87:8075–8079. doi: 10.1073/pnas.87.20.8075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elhai J, Wolk C P. A versatile class of positive-selection vectors based on the nonviability of palindrome-containing plasmids that allows cloning into long polylinkers. Gene. 1988;68:119–138. doi: 10.1016/0378-1119(88)90605-1. [DOI] [PubMed] [Google Scholar]
- 9.Enderlin C S, Meeks J C. Pure culture and reconstitution of the Anthoceros-Nostoc symbiotic association. Planta. 1983;158:157–165. doi: 10.1007/BF00397709. [DOI] [PubMed] [Google Scholar]
- 10.Gardella T, Moyle H, Susskind M M. A mutant Escherichia coli ς70 subunit of RNA polymerase with altered promoter specificity. J Mol Biol. 1989;206:579–590. doi: 10.1016/0022-2836(89)90567-6. [DOI] [PubMed] [Google Scholar]
- 11.Gross C A, Lonetto M, Losick R. Bacterial sigma factors. In: Yamamoto K, Mcknight S, editors. Transcriptional regulation. Plainview, N.Y: Cold Spring Harbor Laboratory Press; 1992. pp. 129–176. [Google Scholar]
- 12.Gruber T M, Bryant D A. Characterization of the alternative sigma factors SigD and SigE in Synechococcus sp. strain PCC 7002. SigE is implicated in transcription of post-exponential-phase-specific genes. Arch Microbiol. 1998;169:211–219. doi: 10.1007/s002030050563. [DOI] [PubMed] [Google Scholar]
- 13.Hellman J D, Chamberlin M J. Structure and function of bacterial sigma factors. Annu Rev Biochem. 1988;57:839–872. doi: 10.1146/annurev.bi.57.070188.004203. [DOI] [PubMed] [Google Scholar]
- 14.Lonetto M, Gribskov M, Gross C A. The ς70 family: sequence conservation and evolutionary relationships. J Bacteriol. 1992;174:3843–3849. doi: 10.1128/jb.174.12.3843-3849.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meeks J C. Symbiosis between nitrogen-fixing cyanobacteria and plants. BioScience. 1998;48:266–276. [Google Scholar]
- 16.Schmidt-Goff C M, Federspiel N A. In vivo and in vitro footprinting of a light-regulated promoter in the cyanobacterium Fremyella diplosiphon. J Bacteriol. 1993;175:1806–1813. doi: 10.1128/jb.175.6.1806-1813.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steinberg N A, Meeks J C. Physiological sources of reductant for nitrogen fixation activity in Nostoc sp. strain UCD 7801 in symbiotic association with Anthoceros punctatus. J Bacteriol. 1991;173:7324–7329. doi: 10.1128/jb.173.22.7324-7329.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Summers M L, Wallis J G, Campbell E L, Meeks J C. Genetic evidence of a major role for glucose-6-phosphate dehydrogenase in nitrogen fixation and dark growth of the cyanobacterium Nostoc sp. strain ATCC 29133. J Bacteriol. 1995;177:6184–6194. doi: 10.1128/jb.177.21.6184-6194.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsinoremas N F, Ishiura M, Kondo T, Anderson C R, Tanaka K, Takahashi H, Johnson C H, Golden S S. A sigma factor that modifies the circadian expression of a subset of genes in cyanobacteria. EMBO J. 1996;15:2488–2495. [PMC free article] [PubMed] [Google Scholar]