Abstract
Using size exclusion chromatography, the nicotinamide adenine dinucleotide malic enzyme purified to near homogeneity from leaves of Crassula argentea was found to exist in at least three aggregational states (dimer, tetramer, and octamer). These forms differ in their apparent kinetic characteristics in initial rate assays, but all display similar characteristics at the steady state. The presence of 50 millimolar malate during chromatography causes a shift in favor of the smaller forms with the tetramer predominating. The native enzyme, when diluted 1/1000 and incubated 18 hours in buffer of high ionic strength, changes its steady state kinetic parameters to ones which indicate a low activity and low affinity for malate. When 50 millimolar malate or 50 micromolar coenzyme A are present the loss of activity and increase in Km is reduced. When both malate and coenzyme A are present the effects in minimizing the change in kinetic characteristics are additive.
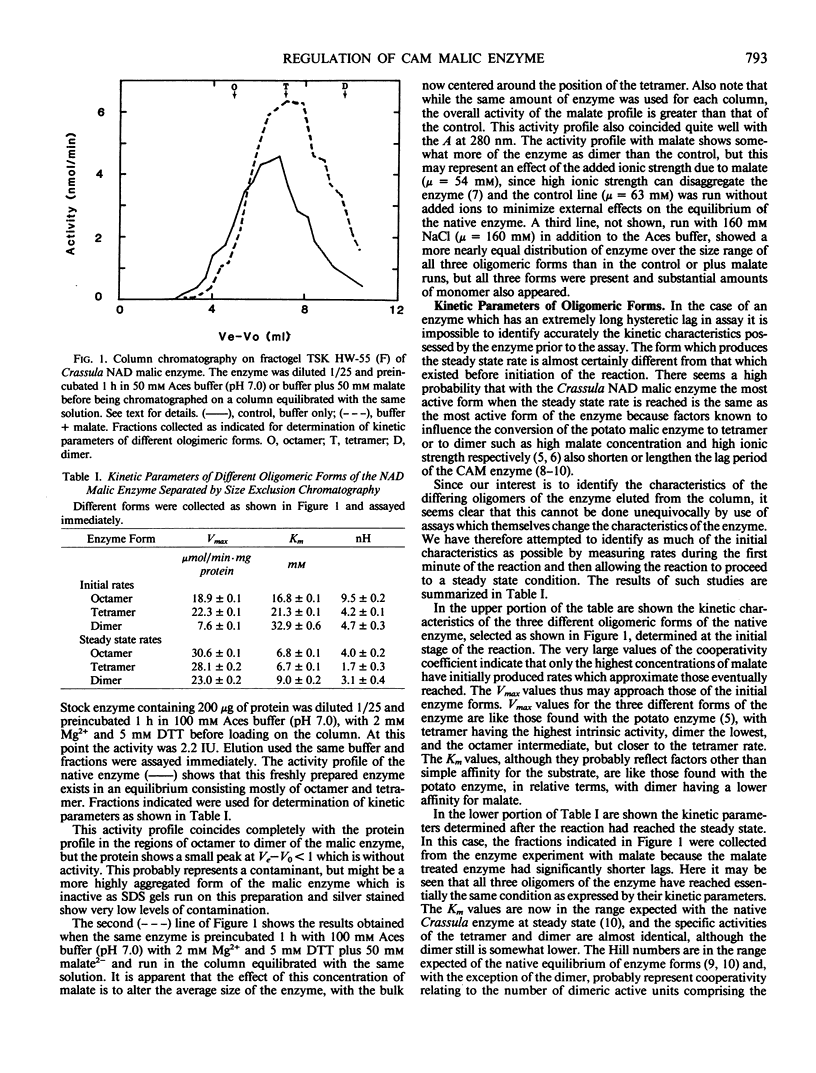
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Grover S. D., Canellas P. F., Wedding R. T. Purification of NAD malic enzyme from potato and investigation of some physical and kinetic properties. Arch Biochem Biophys. 1981 Jul;209(2):396–407. doi: 10.1016/0003-9861(81)90297-6. [DOI] [PubMed] [Google Scholar]
- Grover S. D., Wedding R. T. Kinetic Ramifications of the Association-Dissociation Behavior of NAD Malic Enzyme : A Possible Regulatory Mechanism. Plant Physiol. 1982 Oct;70(4):1169–1172. doi: 10.1104/pp.70.4.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover S. D., Wedding R. T. Modulation of the activity of NAD malic enzyme from solanum tuberosum by changes in oligomeric state. Arch Biochem Biophys. 1984 Nov 1;234(2):418–425. doi: 10.1016/0003-9861(84)90288-1. [DOI] [PubMed] [Google Scholar]
- Wedding R. T., Black M. K. Physical and Kinetic Properties and Regulation of the NAD Malic Enzyme Purified from Leaves of Crassula argentea. Plant Physiol. 1983 Aug;72(4):1021–1028. doi: 10.1104/pp.72.4.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedding R. T., Canellas P. F., Black M. K. Slow Transients in the Activity of the NAD Malic Enzyme from Crassula. Plant Physiol. 1981 Dec;68(6):1416–1423. doi: 10.1104/pp.68.6.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M. X., Wedding R. T. Regulation of phosphoenolpyruvate carboxylase from Crassula by interconversion of oligomeric forms. Arch Biochem Biophys. 1985 Aug 1;240(2):655–662. doi: 10.1016/0003-9861(85)90073-6. [DOI] [PubMed] [Google Scholar]


