Abstract
Background:
Paliperidone palmitate 6-monthly (PP6M) is the first long-acting antipsychotic injectable (LAI) to allow for only two medication administrations per year, though there is presently limited insight into its effectiveness and potential added value in real clinical practice conditions.
Objectives:
To present our ongoing study and draw its preliminary data on patient characteristics initiating PP6M and adherence during the first year of treatment.
Methods:
The paliperidone 2 per year (P2Y) study is a 4-year, multicentre, prospective mirror-image pragmatic study taking place at over 20 different sites in Europe. The mirror period covers 2 years either side of the PP6M LAI initiation. Retrospective data for the previous 2 years are collected for each patient from the electronic health records. Prospective data are recorded at baseline, 6, 12, 18 and 24 months of drug administration and also cover information on concomitant psychiatric medication, relapses, hospital admissions, side effects, discontinuation and its reasons. Meanwhile, here we present preliminary data from the P2Y study at basal and 6-month period (first and second PP6M administration).
Results:
At the point of PP6M initiation, the most frequent diagnosis was schizophrenia (69%), the clinical global impression scale mean score was 3.5 (moderately markedly ill) and the rate of previous hospital admissions per patient and year was 0.21. PP6M was initiated after a median of 3–4 years on previous treatment: 146 (73%) from paliperidone palmitate 3-monthly, 37 (19%) from paliperidone palmitate 1-monthly and 17 (9%) from other antipsychotics. The mean dose of the first PP6M was 1098.9 mg. The retention rate at 6 months and 1 year of treatment on PP6M in our cohort was 94%.
Conclusion:
Patient and clinician preference for LAIs with longer dosing intervals was the main reason for PP6M initiation/switching resulting in high treatment persistence. Future data are needed to evaluate the full impact of PP6M in clinical practice.
Keywords: long-acting injectable antipsychotics, paliperidone palmitate 6-monthly, schizophrenia
Introduction
Schizophrenia is a severe mental disorder characterized by positive and negative symptoms, disorganization and cognitive deterioration impairing self-care and interpersonal relationships. 1 The prevalence of schizophrenia is ∼24 M of patients worldwide and is one of the top 15 leading causes of disability worldwide. 2 For its management and treatment, several approaches are important such as social support, psychological therapies and psychoeducation. Nonetheless, it is well established that schizophrenia is produced by altered dopamine and glutamate neurotransmitter levels in the brain 3 and pharmacotherapy, mainly antipsychotics, remains one of the most important cornerstones in the management of schizophrenia in reducing symptoms, maintaining function and improving quality of life.
However, patients with schizophrenia characteristically suffer from anosognosia or a lack of insight into the nature of the symptoms and difficulties associated with the illness. 4 Therefore, effective treatment is often jeopardized by lack of adherence and compliance. Long-acting injectable antipsychotics (LAIs) have been shown to improve adherence by reducing the risk of full and partial compliance and may therefore enhance the collaborative process.5,6 However, LAIs are frequently underused in current practice, with a highly heterogeneous pattern of use among countries and many other barriers to their adoption exist, such as the overestimation of patient adherence, patient refusal or perceived coercion, underfunded or administrative barriers of health providers services. 7
Paliperidone palmitate is a LAI, first made available as a 1-month administration formulation (PP1M) in 2009 and subsequently as 3-month formulation (PP3M) in 2015. Recently, the new 6-month formulation (PP6M) has been marketed and is available in Europe and other Western countries since May 2022. As demonstrated in a clinical trial, the efficacy of PP6M was noninferior to that of PP3M in preventing relapse at 52 weeks in patients with schizophrenia adequately treated with PP1M or PP3M. 8 This study found that 92.5% of patients treated with PP6M and 95% of those treated with PP3M were relapse-free at 12 months. Furthermore, a number of studies showed additional benefits in terms of patient treatment outcomes and satisfaction with less frequent LAIs and particularly with PP3M, the first long-acting treatment allowing for quarterly administrations.9,10 Nonetheless, there is as yet a lack of studies evaluating the effectiveness and potential added value of PP6M in real-world clinical practice. Hence, we aim to present our ongoing study protocol and draw its preliminary data on patients initiating PP6M, including baseline sociodemographic, clinical and treatment characteristics and adherence at 6 months.
Methods
Study design
The paliperidone 2 per year (P2Y) study is a 4-year, multicentre, prospective mirror-image pragmatic study. Enrolment takes place from January 2023 to December 2024, at 20 different sites in Spain, Italy and the UK. The present study does not interfere with patient treatment in any way, and PP6M is prescribed according to clinical need. The mirror period covers 2 years either side of the PP6M LAI initiation. Retrospective data for the previous 2 years are collected for each patient from the electronic health records. PP1M or PP3M was considered as the ‘previous treatment’ only if patients were treated with either for more than 6 months [Figure 1(a) and (b)].
Figure 1.
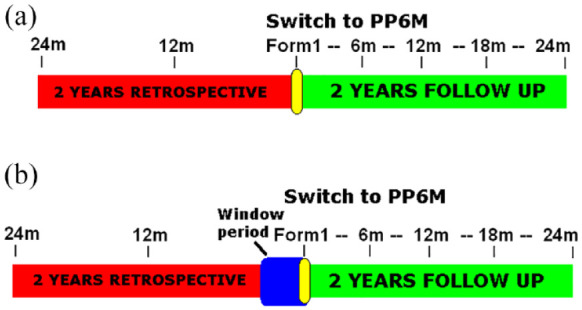
Study timeline desing. (a) for patients previously treated >6 months with paliperidone palmitate 1-monthly (PP1) or paliperidone palmitate 3-monthly (PP3M) prior the switch to paliperidone palmitate 6-monthly (PP6M) and (b) for patients previously treated with other oral or LAI antipsychotics before the switch to PP6M. The window period covers the needed treatment with PP1M (4 months) or PP3M (3 months) before switching to PP6M.
The study is conducted in accordance with International Conference on Harmonization Good Clinical Practice guidelines, the Declaration of Helsinki and approved by the corresponding Ethics Committee (ref. CEI.23-11_PY2_2022). Before study initiation, written informed consent covering retrospective, screening and prospective collecting data was obtained from participants (or their legal representatives, if appropriate). The protocol and consent forms were approved by the respective institutional ethics committee at each site.
Inclusion and exclusion criteria
Participants were eligible for inclusion if they were (1) adults (>18 years), (2) under the care of a mental health service, (3) had a diagnosis of schizophrenia, or other psychiatric disorder such as schizophrenia spectrum disorders (schizoaffective, psychotic and delusional disorders) bipolar or personality disorders, intellectual disability and autism spectrum disorders according to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-V) criteria and (4) initiating PP6M treatment. Patients <18, pregnant women and those without medical records in the past 2 years were excluded. The present study was drawn up following the ‘STrengthening the Reporting of OBservational studies in Epidemiology’ Statement items. 11
Study measures
Study data were collected and managed using REDCap electronic data capture tools hosted at Murcia Health Service. 12 Patients’ baseline characteristics and 2-year retrospective data were extracted from patients’ medical records by the study team at the time of first PP6M injection. Data collected included demographic information such as age, sex, marital status, employment, tobacco and substance use (i.e. alcohol, cannabis, cocaine, heroin or amphetamines), and clinical data such as primary psychiatric and other diagnosis, comorbid substance use disorder, body mass index, cholesterol levels, cardiac QTc interval interval, number of hospital admissions as well as concomitant psychiatric medications, including the use of benzodiazepines, oral antipsychotics, antidepressants, mood stabilizers and anticholinergics.
At each subsequent PP6M injection, the following information was collected: date of injection, PP6M dose received, reason for dose or interval change if applicable, any addition of oral antipsychotics (including treatment indication).
Prospective data are recorded at 6, 12, 18 and 24 months of drug administration and also cover information on concomitant psychiatric medication, relapses, hospital admissions, side effects, discontinuation and its reasons. For the final PP6M administration at 24 months, additional information regarding clinical severity [clinical global impression (CGI) scale endocrine levels and QTc interval] interval as well as patient satisfaction with PP6M will be captured. Meanwhile, here we present preliminary data from the P2Y study at basal and 6-month period (first and second PP6M administration).
Diazepam and haloperidol equivalents
In order to compare the concomitant use of benzodiazepines and oral antipsychotics, we calculate the corresponding daily dose equivalents of diazepam or haloperidol (mg/day) as standards as previously described. 13
All cause treatment discontinuation and side effects
As recommended per manufacturer, the corresponding dose of PP6M can be given 2 weeks before or 3 weeks later than the scheduled date. After this date, it is advised to administer one equivalent dose of PP1M and resume PP6M in 30 days. Nonetheless, we consider treatment discontinuation if the dose of PP6M is not administered within 3 weeks after the expected date for the next dose. The reasons for treatment discontinuation are also collected as follows: no adherence, tolerability, ineffectiveness, all-cause mortality or patient, clinician or family preference of other treatment. The type and prevalence of side effects of PP6M have been reported similar to those of PP3M in a previous clinical trial. 8 Nonetheless, we record the specific side effects if they lead to treatment discontinuation, including weight gain, extrapyramidal effects, insomnia, raised prolactin, QTc prolongation, high blood pressure, sedation, digestive effects, haematological disorders, neuroleptic malignant syndrome or other side effects.
Patient, relatives and psychiatrists satisfaction assessment
We evaluate the satisfaction levels for PP6M antipsychotic formulation on a visual scale from 1 (extremely dissatisfied) to 5 (extremely satisfied) as expressed by the patient, their relatives and their psychiatrist 1 and 2 years after initiating PP6M. Similarly, we also evaluate the experienced effectiveness by the patient, the relatives and the psychiatrists 1 and 2 years after first PP6M administration compared with the previous treatment using a similar visual scale.
Statistical analysis and confounding factors
All analyses were performed using IBM SPSS Statistics version 21.0 (IBM Corp., Armonk, NY, USA). We expressed quantitative variables as means [standard error media] and categorical variables as numbers (percentage). We assessed normality of distributions using histograms and the Shapiro–Wilk test. Sample basal characteristics were analyzed by Student’s t-test, chi-square and univariate analysis. Variables associated in the univariate analysis and variables with statistical trend (p < 0.1) were entered as factors in a multivariate logistic regression model to identify the risk factors or patients characteristics associated with being initiated with PP6M treatment. Differences with a p value <0.05 were considered significant.
Results
Here, we present preliminary data on patients characteristics and predictors of treatment initiation with PP6M as well as adherence at 6 months.
A total of 200 patients were recruited and included in this preliminary analysis from the P2Y study; their basic sociodemographic characteristics are presented in Table 1. Of this initial cohort, 66% were male and 94% Caucasian with a mean age of 50.9 years. At the point of PP6M initiation, the most frequent diagnosis was schizophrenia (69%), 57% lived at home and 43% in a chronic residential care or mental health facility and 92% were unemployed. A majority of patients smoked tobacco (58%), whereas 11–12% of patients consumed alcohol, cannabis or cocaine. In this regard, 15% of patients carried of formal diagnosis of comorbid substance use disorder. Furthermore, the CGI-S mean score was 3.5 (moderately markedly ill) at PP6M initiation (Table 2) while 13% (26) and 17% (34) of patients had at least one psychiatric admission in the first- and second-year, respectively, prior to PP6M initiation with a rate of 0.21 hospital admissions per patient and year. At the time of PP6M initiation, BMI was 28.35 ± 0.82 (kg/m2), total cholesterol 184.95 ± 6.34 (mg/dL), LDL cholesterol 115.94 ± 5.43 (mg/dL), HDL cholesterol 42.67 ± 3.81 (mg/dL) and the QTc interval 391.22 ± 64.53 (ms).
Table 1.
Cohort demographic data.
| n = 200 | |
|---|---|
| Sex (%) | |
| Women | 68 (34) |
| Men | 132 (66) |
| Age (year ± SEM) | 50.9 ± 0.9 |
| Race (%) | |
| Caucasian | 188 (94) |
| Latin American | 5 (3) |
| Black | 7 (3) |
| Living at | |
| Home | 113 (57) |
| Chronic hospital | 87 (43) |
| Employed | |
| No | 184 (92) |
| Yes | 16 (8) |
| Tobacco and drugs (%) | 120 (60) |
| Tobacco | 115 (58) |
| Alcohol | 22 (11) |
| Cannabis | 24 (12) |
| Cocaine | 20 (10) |
| Heroin/opiates | 3 (1) |
| Amphetamines | 3 (1) |
| SUD diagnosis | 28 (15) |
| Mental disorder (%) | |
| Psychosis | 6 (3) |
| Schizoaffective Dis. | 28 (14) |
| Schizophrenia | 137 (69) |
| Delusional Dis. | 4 (2) |
| Bipolar Dis. | 6 (3) |
| Personality Dis. | 8 (4) |
| Autism spectrum Dis. | 1 (1) |
| Intellectual disability | 10 (5) |
Dis., disorder; SEM, standard error media; SUD, substance use disorder.
Table 2.
Cohort clinical data.
| n = 200 | ||
|---|---|---|
| CGI-S score | 3.5 = Moderately markedly | |
| Previous LAI/OAP-Dose | ||
| OAP | 8 (4) | * |
| PP1M | 37 (19) | 171.6 ± 13.8 |
| PP3M | 146 (73) | 539.6 ± 22.9 |
| A1M | 7 (4) | 400 |
| R-LAI | 1 (1) | 300 |
| Z-LAI | 1 (1) | 285 |
| Duration previous treat | 3–4 years | |
| Antipsychotic monotherapy (%) | 78 (39) | |
| Reason for switching to PP6M | ||
| Side effects | 2 (1) | |
| No adherence | 7 (4) | |
| Ineffective | 4 (2) | |
| Patient prefer PP6M | 85 (43) | |
| Family prefer PP6M | 2 (1) | |
| Psychiatrist prefer PP6M | 100 (50) | |
| N benzodiazepines (%) | ||
| 1 | 128 (64) | |
| 2 | 10 (5) | |
| 3 | 5 (3) | |
| N antipsychotics (%) | ||
| 1 | 94 (47) | |
| 2 | 18 (9) | |
| 3 | 10 (5) | |
| Anticholinergics (%) | 32 (16) | |
| Antidepressants (%) | ||
| 1 | 30 (15) | |
| 2 | 6 (3) | |
| Mood stabilizers (%) | ||
| 1 | 34 (17) | |
| 2 | 4 (2) | |
| PP6M initiated at: | ||
| Acute Hospital | 9 (4) | |
| Outpatient | 191 (96) | |
| PP6M initial dose | 1098.9 ± 34.1 | |
A1M, aripiprazole-1-month; LAI, long-acting injectable; CGI, clinical global impression scale; OAP, oral antipsychotic; PP1M, paliperidone palmitate 1-month; PP3M, paliperidone palmitate 3-month; PP6M, paliperidone palmitate 6-month; R-LAI, risperidone-LAI; Z-LAI, zuclopentixol-LAI.
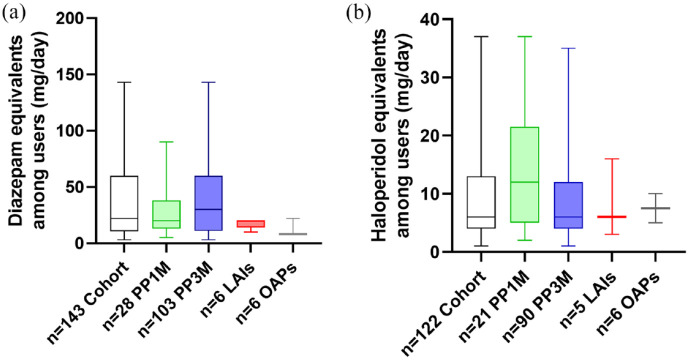
Treatment characteristics are detailed in Table 2. PP6M was initiated after a median of 3–4 years on previous treatment. In total, 146 (73%) patients were switched from PP3M, 37 (19%) directly from PP1M and 17 (9%) were changed over from other LAIs [aripiprazole-LAI, risperidone-LAI (R-LAI) and zuclopenthixol-LAI (Z-LAI)] and oral antipsychotics. Only 78 patients (39%) were previously treated with antipsychotic monotherapy. In this regard, at baseline (before switching to PP6M), 122 (61%) patients were receiving concomitant oral antipsychotics, 143 (72%) of them received benzodiazepines, 32 (16%) of them received anticholinergics, 36 (18%) of them received antidepressants and 38 (19%) patients received mood stabilizers. Moreover, the mean daily doses of benzodiazepines and concomitant oral antipsychotics are shown in Figure 2 as (a) diazepam and (b) haloperidol equivalents. Mean daily dose of diazepam and haloperidol equivalents among users were 36.12 mg (±2.81) and 9.55 mg (±0.69), respectively. No statistical differences were found between groups according to previous treatments (PP1M, PP3M, other LAIs and oral antipsychotics). The reasons for discontinuing the previous treatment and initiating PP6M were mainly patient and clinician preference for a less frequent administration of LAI (n = 85, 43% and n = 100, 50%, respectively). At the time of the first PP6M injection, 182 patients (96%) were treated in a general outpatient psychiatric service, 9 (4%) of them in an acute hospital unit (six of them in an early intervention program) and 9 (7%) of them in an assertive community treatment team. The mean dose of the first PP6M was 1098.9 mg.
Figure 2.
Psychiatric concomitant treatments. Dose of benzodiazepines and antipsychotics showed as (a) diazepam and (b) haloperidol equivalents (mg/ day) among users by long acting injectable (LAIs) or oral antipsychotics (OAPs) groups before switching to paliperidone palmitate 6-monthly (PP6M). Data are expressed as the mean SEM. PP1M = paliperidone palmitate-1-month; PP3M=paliperidone palmitate-3-month, LAIs including aripirazole 1-montlhy, risperidone-LAI and zuclopentixol-LAI; OAPs = oral antipsychotics.
As shown in Tables 2 and 3, patients had been treated with PP1M with a mean dose of 171.6 mg/month with an interval dose between 100 and 300 mg/month. PP6M was initiated at doses of 700 (n = 3 and n = 1 from oral quetiapine), 1000 (n = 9 and n = 3 from other oral and LAIs antipsychotics), 1400 (n = 4 and n = 1 from Z-LAI) and 2000 mg/6-month (n = 5). Similarly, the group of patients treated with PP3M was administered with a mean dose of 539.6 mg/3month interval dose between 263 and 1575 mg/3-month interval. PP6M was initiated at doses of 700 (n = 48), 1000 (n = 65 and n = 1 from R-LAI), 1400 (n = 9), 1700 (n = 18), 2000 (n = 5) and 3000 mg/6-month (n = 1). The dosage switch from PP1M or PP3M to PP6M was made in agreement with the x6.7–7 or x1.9–2 ratios, respectively, recommended by the product monograph for 85% of the patients in both PP1M and PP3M.
Table 3.
Switch to PP6M and dosage.
| n = 200 | n (PP6M administered dose mg) | |
|---|---|---|
| Previous LAI/OAP-Dose mg | ||
| OAP | n = 8 | |
| Olanzapine 15 | 3▸ PP1M 150 | 3 (1000) |
| Quetiapine 300 | 1▸ PP1M 100 | 1 (700) |
| Aripiprazole 15 | 4 ▸PP1M 150 | 4 (1000) |
| PP1M | n = 37 | |
| 100 | 12 | 10 (700), 2 (1000) |
| 150 | 10 | 10 (1000) |
| 200 | 7 | 3 (1000), 4 (1400) |
| 225 | 2 | 1 (1700) 1 (2000) |
| 300 | 6 | 1 (1000), 5 (2000) |
| PP3M | n = 146 | |
| 263 | 8 | 8 (700) |
| 350 | 43 | 40 (700), 3 (1000) |
| 525 | 69 | 61 (1000), 6 (1400), 2 (1700) |
| 740 | 5 | 1 (1000), 3 (1400), 1 (1700) |
| 875 | 5 | 5 (1700) |
| 1050 | 15 | 10 (1700), 5 (2000) |
| 1575 | 1 | 1 (3000) |
| A1M | n = 7 ▸ PP1M 150 | 7 (1000) |
| R-LAI | n = 1 ▸ PP3M 525 | 1 (1000) |
| Z-LAI | n = 1 ▸ PP1M 200 | 1 (1400) |
| Retention rate dose 2 PP6M | 149/158 = 94% | |
A1M, aripiprazole-1-month; LAI, long-acting injectable; OAP, oral antipsychotic; PP1M, paliperidone palmitate 1-month; PP3M, paliperidone palmitate 3-month; PP6M, paliperidone palmitate 6-month; R-LAI, risperidone-LAI; Z-LAI, zuclopentixol-LAI.
Among the 158 patients (94 outpatients and 64 inpatients) who already had an appointment for the next scheduled PP6M administration, 149 of them received the second injection. Therefore, the retention rate and 1 year treatment adherence to PP6M in our cohort was 94%. Among the nine patients who refused the second PP6M injection, all of them were outpatients: one reported side effects, one died for other medical causes, one was lost the follow-up and the other six preferred other treatments (four oral antipsychotics and two the previous PP3M LAI).
Discussion
The P2Y observational, 4-year mirror-image study is being conducted at multiple sites in Europe in order to capture the wider clinical use and application of PP6M. The study aims to describe patient characteristics, reasons for prescribing PP6M, use and changes in concomitant treatments, hospital admissions, as well as level of adherence, discontinuation rates and patient satisfaction up to 24 months after PP6M initiation in real-world clinical practice. Here, we present the baseline patient characteristics and treatment patterns for the first 200 patients initiated on PP6M across multiple European settings for the first time.
Our preliminary findings suggest that PP6M is mostly prescribed in middle-aged male patients diagnosed with schizophrenia or schizophrenia spectrum disorders. Nonetheless, some patients were carrying a primary diagnosis of personality, bipolar or autism spectrum disorders and intellectual disability. In fact, previous studies have shown that paliperidone LAIs formulations is sometimes used for the maintenance treatment of bipolar disorder14,15 as well as for the treatment of personality, autistic disorders or mental retardation to help manage aggression, self-harm or impulsivity.16–19
Furthermore, although the majority of patients are treated with doses that fall within the recommended range, a few were prescribed with higher doses of PP6M and previous treatments of PP1M and PP3M. This is akin to previous reports detailing the occasional use of higher than recommended doses of PP1M and PP3 in clinical practice.20–22 Interestingly, although the cohort of patients presented a moderate severity illness, measured by CGI-score, they required an oral antipsychotic therapy supplementation in most cases. Although high-dose prescribing of antipsychotic medication may often lead to worsening tolerability without necessarily any efficacy gains, recent evidence from meta-analysis advices caution in reducing the dose of antipsychotic during the maintenance treatment phase in schizophrenia below the optimal level used for acute stabilization because it may be associated with an increased risk of both relapse and treatment discontinuation.23,24 Future data from the P2Y study are needed to assess whether patients treated with PP6M need lower doses of concomitant treatments such as antipsychotics or benzodiazepines.
Many patients with schizophrenia and other psychotic disorders often display varying degrees of anosognosia which may impact treatment adherence and the shared decision-making process. Interestingly, in this naturalistic cohort, most patients were previously treated with PP1M and PP3M for long periods of time and 43% indicated their preference for a less frequent injection treatment as the main reason for switching to PP6M. Indeed, evidence shows that patients’ participation in their own treatment could encourage better adherence and improve outcomes.5,25
In this regard, previous data from real-world studies demonstrated retention rates as high as 78% and 87% at 1 year for PP1M and PP3M, respectively.26–28 A 1-year continuation rate of 87% has also been shown for PP6M in a recent clinical trial. 8 In our naturalistic cohort, the retention rate for the second dose of PP6M was even higher (94%) and also for the outpatient subgroup (90%), confirming the experience thus far that LAIs with longer dosing intervals may have favourable treatment persistence. In this regard, a recent systematic review showed the benefits of PP3M in the management of schizophrenia due to improved adherence, decreased risk of relapse (even after several months of treatment discontinuation) and its positive impact on patients’ and caregivers’ satisfaction/quality of life facilitating long-term goals. 29 Therefore, long-term LAI as PP6M could favour the recovery of schizophrenia and improve ill course with a reduced risk of relapse and hospitalizations as well as the risk of isolation and social drift induced by schizophrenia spectrum disorders. Future data and studies are needed to assess the impact of PP6M in clinical practice compared with other LAIs.
Having said that, the extended dose interval administration does not necessarily have to translate to less frequent clinical contact. Importantly, the time saved for administration and the time spent discussing the need of the treatment can be utilized for other therapeutic rehabilitation, psychotherapy or to improve the patient–clinician therapeutic alliance. To this end, there is a growing body of evidence supporting patient satisfaction and acceptance with the use of PP3M and longer-acting treatments in general, leading to improved quality of life and less stigma.30,31
Finally, the mean number of hospitalizations 2 years before PP6M was overall low as most patients had been on either PP1M or PP3M, which has been repeatedly associated with a significant reduction in bed usage.32–34 Moreover, a recent model study in the US concluded that for each 5% of patients with relapsing schizophrenia who switch to PP1M, and subsequently to PP3M/PP6M, the cumulative 3-year cost savings were estimated at $2.0 M with 223 relapses avoided. 35 Nonetheless, further data from this and future studies are needed to establish the potential overall and cost-effectiveness of PP6M in clinical practice.
Conclusion
To the best of our knowledge, the P2Y is the first ongoing mirror-image naturalistic study evaluating the clinical impact of PP6M. We showed that despite PP6M being mainly initiated in patients with schizophrenia, it can on occasions also be used for other indications and/or higher than recommended doses. Patient and clinician preference for LAIs with longer dosing intervals was the main reason for PP6M initiation/switching. The retention rate at 6 months (at the point of the second injection) was 94%. Future data from the P2Y and other studies in clinical practice are needed to evaluate the full impact of PP6M in the long-term management of patients in clinical practice.
Limitations
Given that our inclusion/exclusion criteria were not restrictive, our cohort could provide high external validity and allow for some degree of generalizability across other Western countries. Nonetheless, due to the naturalistic study design, the present report does not allow any causal inference. Moreover, the estimates of concomitant oral medication intake may be inaccurate as it was quantified by using the electronic prescription registry, and it could be over or underestimated due to different prescription trends among the different countries and regions of centres included in the study. Additional factors that could affect the prescription of a LAI are the psychiatrists’ choice and the cost, which were not included and may confound our study results. Also, in the present report, we present very preliminary data at baseline and 6 months after PP6M initiation. Other aspects concerning changes in BMI, cholesterol levels and QTc interval as well as patient, relatives and psychiatrists experience and satisfaction with PP6M will be underscored in the following reports concerning the P2Y study.
Acknowledgments
None.
Footnotes
ORCID iDs: Juan Antonio García-Carmona  https://orcid.org/0000-0003-3938-7698
https://orcid.org/0000-0003-3938-7698
Sofia Pappa  https://orcid.org/0000-0002-6303-1547
https://orcid.org/0000-0002-6303-1547
Contributor Information
Juan Antonio García-Carmona, Department of Neurology, Santa Lucía University Hospital, C/Mezquita s/n 30202, Cartagena, Murcia 30202, Spain; Group of Clinical and Experimental Pharmacology, Institute for Biomedical Research of Murcia (IMIB), Murcia, Spain; Faculty of Pharmacy and Nutrition, San Antonio Catholic University of Murcia (UCAM), Murcia, Spain.
Alba García-Pérez, Centre of Mental Health Molina de Segura, Molina de Segura, Murcia, Spain.
Guillermo Isidro García, Department of Psychiatry, Marqués de Valdecilla University Hospital, Universidad de Cantabria, Santander, Spain; Psychiatry and Mental Health Research Group, Instituto Investigación Sanitaria Valdecilla (IDIVAL), Santander, Spain.
Luis Alberto Forcen-Muñoz, Centre of Mental Health Lorca, Lorca, Murcia, Spain.
Santiago Ovejero García, Fundación Jiménez Díaz University Hospital, Madrid, Spain.
Rocío Sáez Povedano, Department of Psychiatry, General Hospital of Villarrobledo, Villarrobledo, Albacete, Spain.
Ana Luisa González-Galdámez, Department of Psychiatry, Los Arcos-Mar Menor University Hospital, San Javier, Murcia, Spain.
Laura Mata Iturralde, Fundación Jiménez Díaz University Hospital, Madrid, Spain.
Fernando Hernández-Sánchez, Centre of Mental Health Lorca, Lorca, Murcia, Spain.
Mariluz Ramirez Bonilla, Department of Psychiatry, Marqués de Valdecilla University Hospital, Universidad de Cantabria, Santander, Spain; Psychiatry and Mental Health Research Group, Instituto Investigación Sanitaria Valdecilla (IDIVAL), Santander, Spain.
Paloma Fuentes-Pérez, Psychiatry and Mental Health Research Group, Instituto Investigación Sanitaria Valdecilla (IDIVAL), Santander, Spain.
Claudia Ovejas-Catalán, Psychiatry and Mental Health Research Group, Instituto Investigación Sanitaria Valdecilla (IDIVAL), Santander, Spain.
Paula Suárez-Pinilla, Department of Psychiatry, Marqués de Valdecilla University Hospital, Universidad de Cantabria, Santander, Spain; Psychiatry and Mental Health Research Group, Instituto Investigación Sanitaria Valdecilla (IDIVAL), Santander, Spain.
Francisco Valdivia-Muñoz, Department of Psychiatry, Santa Lucía University Hospital, Cartagena, Murcia, Spain; Unit of Assertive Community Treatment, Centre Mental Health Cartagena, Cartagena, Murcia, Spain.
Blanca Fernández Abascal, Department of Psychiatry, Marqués de Valdecilla University Hospital, Universidad de Cantabria, Santander, Spain; Psychiatry and Mental Health Research Group, Instituto Investigación Sanitaria Valdecilla (IDIVAL), Santander, Spain.
Miguel Omaña Colmenares, Department of Psychiatry, Alcanyis General Hospital, Xátiva, Valencia, Spain.
Ángela de Lourdes Martín-Pérez, Department of Psychiatry, Santa Lucía University Hospital, Cartagena, Murcia, Spain.
María Pilar Campos-Navarro, Department of Psychiatry, Santa Lucía University Hospital, Cartagena, Murcia, Spain.
Enrique Baca-García, Fundación Jiménez Díaz University Hospital, Madrid, Spain.
Sergio Benavente-López, Department Psychiatry, Infanta Elena University Hospital, Madrid, Spain.
Alberto Raya Platero, Department of Psychiatry, San Cecilio Clinic Hospital, Granada, Spain.
Miguel Barberán Navalón, Department of Psychiatry, Alcanyis General Hospital, Xátiva, Valencia, Spain.
Sergio Sánchez-Alonso, Fundación Jiménez Díaz University Hospital, Madrid, Spain.
Javier Vázquez-Bourgon, Department of Psychiatry, Marqués de Valdecilla University Hospital, Universidad de Cantabria, Santander, Spain; Psychiatry and Mental Health Research Group, Instituto Investigación Sanitaria Valdecilla (IDIVAL), Santander, Spain; Centro de Investigación Biomédica en Red en Salud Mental (CIBERSAM), Instituto de Salud Carlos III, Sevilla, Spain.
Sofia Pappa, West London National Health System (NHS) Trust, London, UK; Department of Brain Sciences, Imperial College of London, London, UK.
Declarations
Ethics approval and consent to participate: The study was conducted according to the World Medical Association Declaration of Helsinki, and it was approved by the Ethics Research Committee from the coordinating centre, the Santa Lucía University Hospital (ref. CEI.23-11_PY2_2022) in February 2023. Authorization to access patients’ medical records was granted by each site’s Professional Services Directorate. Oral and written information about the study was given, and written informed consent from the patient or their legal guard was obtained to participate in the P2Y study.
Consent for publication: Not applicable.
Author contributions: Juan Antonio García-Carmona: Conceptualization; Formal analysis; Investigation; Methodology; Project administration; Supervision; Writing – original draft; Writing – review & editing.
Alba García-Pérez: Data curation; Investigation; Writing – review & editing.
Guillermo Isidro García: Data curation; Investigation; Writing – review & editing.
Luis Alberto Forcen-Muñoz: Data curation; Investigation; Writing – review & editing.
Santiago Ovejero García: Data curation; Investigation; Writing – review & editing.
Rocío Sáez Povedano: Data curation; Investigation; Methodology; Writing – review & editing.
Ana Luisa González-Galdámez: Data curation; Investigation; Writing – review & editing.
Laura Mata Iturralde: Data curation; Formal analysis; Investigation; Writing – review & editing.
Fernando Hernández-Sánchez: Data curation; Investigation; Methodology; Writing – review & editing.
Mariluz Ramirez Bonilla: Data curation; Investigation; Methodology; Validation; Writing – review & editing.
Paloma Fuentes-Pérez: Data curation; Investigation; Methodology; Writing – review & editing.
Claudia Ovejas-Catalán: Data curation; Investigation; Methodology; Writing – review & editing.
Paula Suárez-Pinilla: Data curation; Investigation; Methodology; Writing – review & editing.
Francisco Valdivia-Muñoz: Data curation; Investigation; Writing – review & editing.
Blanca Fernández Abascal: Data curation; Formal analysis; Investigation; Methodology; Validation; Writing – review & editing.
Miguel Omaña Colmenares: Data curation; Investigation; Writing – review & editing.
Ángela de Lourdes Martín-Pérez: Data curation; Investigation; Methodology; Writing – review & editing.
María Pilar Campos-Navarro: Data curation; Investigation; Methodology; Writing – review & editing.
Enrique Baca-García: Data curation; Investigation; Methodology; Supervision; Writing – review & editing.
Sergio Benavente-López: Data curation; Investigation; Writing – review & editing.
Alberto Raya Platero: Data curation; Investigation; Methodology; Writing – review & editing.
Miguel Barberán Navalón: Data curation; Investigation; Methodology; Supervision; Writing – review & editing.
Sergio Sánchez-Alonso: Conceptualization; Data curation; Investigation; Methodology; Supervision; Writing – review & editing.
Javier Vázquez-Bourgon: Conceptualization; Data curation; Investigation; Methodology; Supervision; Writing – original draft; Writing – review & editing.
Sofia Pappa: Conceptualization; Data curation; Investigation; Methodology; Project administration; Supervision; Validation; Writing – original draft; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
JAGC received speaking honoraria from Janssen-Cilag Inc., advisor honoraria from Teva and research and speaking honoraria from Neuraxpharm, all of them outside the submitted work. Sofia Pappa reports grants and honoraria outside the submitted work. Other authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Availability of data and materials: The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request.
References
- 1. Owen MJ, Sawa A, Mortensen PB. Schizophrenia. Lancet 2016; 388: 86–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017; 390: 1211–1259. Erratum in: Lancet 2017; 390: e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Buck SA, Quincy Erickson-Oberg M, Logan RW, et al. Relevance of interactions between dopamine and glutamate neurotransmission in schizophrenia. Mol Psychiatry 2022; 27: 3583–3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Little JD. In schizophrenia, are lack of capacity and lack of insight more usefully understood as anosognosia? Australas Psychiatry 2021; 29: 346–348. [DOI] [PubMed] [Google Scholar]
- 5. Pappa S, Barnett J, Gomme S, et al. Shared and supported decision making in medication in a mental health setting: how far have we come? Community Ment Health J 2021; 57: 1566–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pappa S, Mason K. Partial compliance with long-acting paliperidone palmitate and impact on hospitalization: a 6-year mirror-image study. Ther Adv Psychopharmacol 2020; 10: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kane JM, McEvoy JP, Correll CU, et al. Controversies surrounding the use of long-acting injectable antipsychotic medications for the treatment of patients with schizophrenia. CNS Drugs 2021; 35: 1189–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Najarian D, Sanga P, Wang S, et al. A randomized, double-blind, multicenter, noninferiority study comparing paliperidone palmitate 6-month versus the 3-month long-acting injectable in patients with schizophrenia. Int J Neuropsychopharmacol 2022; 25: 238–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Di Lorenzo R, Iorio A, Pinelli M, et al. Effectiveness and quality of life with paliperidone palmitate 3-monthly in comparison with other long-acting drugs. Neuropsychiatr Dis Treat 2022; 18: 829–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fernández-Miranda JJ, Díaz-Fernández S, De Berardis D, et al. Paliperidone palmitate every three months (PP3M) 2-year treatment compliance, effectiveness and satisfaction compared with paliperidone palmitate-monthly (PP1M) in people with severe schizophrenia. J Clin Med 2021; 10: 1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007; 370: 1453–1457. [DOI] [PubMed] [Google Scholar]
- 12. Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software partners. J Biomed Inform 2019; 95: 103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Martínez-Andres JA, García-Carmona JA. Clozapine a controversial gold standard antipsychotic for the 21st century: switching to paliperidone palmitate 3-monthly improves the metabolic profile and lowers antipsychotic dose equivalents in a treatment-resistant schizophrenia cohort. Schizophr Res 2019; 212: 234–236. [DOI] [PubMed] [Google Scholar]
- 14. Pacchiarotti I, Tiihonen J, Kotzalidis GD, et al. Long-acting injectable antipsychotics (LAIs) for maintenance treatment of bipolar and schizoaffective disorders: a systematic review. Eur Neuropsychopharmacol 2019; 29: 457–470. [DOI] [PubMed] [Google Scholar]
- 15. Bartoli F, Cavaleri D, Nasti C, et al. Long-acting injectable antipsychotics for the treatment of bipolar disorder: evidence from mirror-image studies. Ther Adv Psychopharmacol 2023; 13: 20451253231163682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Paton C, Crawford MJ, Bhatti SF, et al. The use of psychotropic medication in patients with emotionally unstable personality disorder under the care of UK mental health services. J Clin Psychiatry 2015; 76: e512–e518. [DOI] [PubMed] [Google Scholar]
- 17. García-Carmona JA, Simal-Aguado J, Campos-Navarro MP, et al. Off-label use of second-generation antipsychotics in borderline personality disorder: a comparative real-world study among oral and long-acting injectables in Spain. Int Clin Psychopharmacol 2021; 36: 201–207. [DOI] [PubMed] [Google Scholar]
- 18. García-Carmona JA, Barnett J, Campos-Navarro MP, et al. Comparative effectiveness of long-acting injectable antipsychotics in a large naturalistic cohort across two European centers: Findings from the long-acting injectable antipsychotics collaborative (LAICO) study. Neurosci Appl 2022; 1: 100111. [Google Scholar]
- 19. Simal-Aguado J, Campos-Navarro MP, Valdivia-Muñoz F, et al. Evaluation of risk factors associated to prescription of benzodiazepines and its patterns in a cohort of patients from mental health: a real world study in Spain. Psychopharmacol Bull 2021; 51: 81–93. [PMC free article] [PubMed] [Google Scholar]
- 20. Turkoz I, Daskiran M, Starr HL, et al. Comparing relapse rates in real-world patients with schizophrenia who were adequately versus not adequately treated with paliperidone palmitate once-monthly injections before transitioning to once-every-3-months injections. Neuropsychiatr Dis Treat 2022; 18: 1927–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. García-Carmona JA, Simal-Aguado J, Campos-Navarro MP, et al. Long-acting injectable antipsychotics: analysis of prescription patterns and patient characteristics in mental health from a spanish real-world study. Clin Drug Investig 2020; 40: 459–468. [DOI] [PubMed] [Google Scholar]
- 22. Devrimci-Ozguven H, Atmaca M, Baran Z, et al. Efficacy and safety of paliperidone palmitate treatment in patients with schizophrenia: a real-world multicenter, retrospective, mirror-image study. J Clin Psychopharmacol 2019; 39: 604–610. [DOI] [PubMed] [Google Scholar]
- 23. Højlund M, Kemp AF, Haddad PM, et al. Standard versus reduced dose of antipsychotics for relapse prevention in multi-episode schizophrenia: a systematic review and meta-analysis of randomised controlled trials. Lancet Psychiatry 2021; 8: 471–486. [DOI] [PubMed] [Google Scholar]
- 24. Ostuzzi G, Vita G, Bertolini F, et al. Continuing, reducing, switching, or stopping antipsychotics in individuals with schizophrenia-spectrum disorders who are clinically stable: a systematic review and network meta-analysis. Lancet Psychiatry 2022; 9: 614–624. [DOI] [PubMed] [Google Scholar]
- 25. Pérez-Revuelta JI, González-Sáiz F, Pascual-Paño JM, et al. Shared decision making with schizophrenic patients: a randomized controlled clinical trial with booster sessions (DECIDE Study). Patient Educ Couns 2023; 110: 107656. [DOI] [PubMed] [Google Scholar]
- 26. Li G, Keenan A, Daskiran M, et al. Relapse and treatment adherence in patients with schizophrenia switching from paliperidone palmitate once-monthly to three-monthly formulation: a retrospective health claims database analysis. Patient Prefer Adherence 2021; 15: 2239–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gutiérrez-Rojas L, Sánchez-Alonso S, García Dorado M, et al. Impact of 3-monthly long-acting injectable paliperidone palmitate in schizophrenia: a retrospective, real-world analysis of population-based health records in Spain. CNS Drugs 2022; 36: 517–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pappa S, Barnett J, Mason K. A 10-year observational study of the use, acceptability and effectiveness of long-acting paliperidone palmitate: implications for clinical decision making. CNS Drugs 2023; 37: 107–116. [DOI] [PubMed] [Google Scholar]
- 29. García-Carmona JA, Pappa S. Cumulative clinical experience of the use of paliperidone palmitate 3-monthly long-acting injection in the treatment of schizophrenia: a critical appraisal. Drug Healthc Patient Saf 2023; 15: 113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Barnett J, Pappa S. Switching from monthly to three-monthly long-acting injectable paliperidone: a survey on subjective satisfaction and safety. Patient Prefer Adherence 2023; 17: 1603–1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pungor K, Sanchez P, Pappa S, et al. The patient, investigator, nurse, carer questionnaire (PINC-Q): a cross-sectional, retrospective, non-interventional study exploring the impact of less frequent medication administration with paliperidone palmitate 3-monthly as maintenance treatment for schizophrenia. BMC Psychiatry 2021; 21: 300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pappa S, Mason K, Howard E. Long-term effects of paliperidone palmitate on hospital stay and treatment continuation. Int Clin Psychopharmacol 2019; 34: 305–311. [DOI] [PubMed] [Google Scholar]
- 33. Mason K, Barnett J, Pappa S. Effectiveness of 2-year treatment with aripiprazole long-acting injectable and comparison with paliperidone palmitate. Ther Adv Psychopharmacol 2021; 11: 20451253211029490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wallman P, Clark I, Taylor D. Effect of 3-monthly paliperidone palmitate on hospitalisation in a naturalistic schizophrenia cohort - a five-year mirror image study. J Psychiatr Res 2022; 148: 131–136. [DOI] [PubMed] [Google Scholar]
- 35. Morrison L, Lin D, Benson C, et al. Projecting the economic outcomes of switching patients with schizophrenia from oral atypical antipsychotics to once-monthly, once-every-3-months, and once-every-6-months paliperidone palmitate. J Manag Care Spec Pharm 2023; 29: 161–171. [DOI] [PMC free article] [PubMed] [Google Scholar]