Abstract
Enterococcus hirae ATCC 9790 grew well in Na+-deficient, low-K+ medium, but growth was inhibited by carbonylcyanide m-chlorophenylhydrazone (CCCP). Growth inhibition and decrease of cellular K+ levels in the presence of CCCP were relieved by the addition of Na+ and a high concentration of K+. In contrast, in the mutant defective in Na+-ATPase or the NtpJ component of the KtrII K+ uptake system, CCCP-induced growth inhibition was rescued by a high concentration of K+ but not of Na+. These transporters are thus indispensable for homeostatis of K+ and Na+ at low proton potential.
Like all other living organisms, bacteria exclude Na+ and accumulate K+ ions. Sodium extrusion is important not only for generation of the inward-directed sodium gradient, which is utilized as the driving force for a variety of secondary Na+-linked systems (5, 18), but also for detoxification of internal sodium. Potassium accumulation is also an important aspect of cell physiology. Potassium, the major intracellular cation, plays a role in charge neutralization of cellular anions. Some enzymes are activated by K+, but all bacteria require K+ for protein synthesis. In bacteria such as Escherichia coli, turgor pressure is maintained in part by regulating intracellular K+ (2). The movements of Na+ and K+ ions are mediated by a variety of transport systems in bacteria (2).
In Enterococcus hirae, two Na+ extrusion systems, NapA Na+/H+ antiporter (8, 22) and vacuolar Na+-translocating ATPase (21), two K+ uptake systems, KtrI (3) and KtrII (10, 15), and one K+ extrusion system are present (12). Characterization of these systems at the molecular level has been initiated for Na+ transport but not for K+ transport. KtrI appears to be similar to the Trk system of E. coli; K+ uptake by this system requires both the electrochemical gradient of protons across the cell membrane (proton potential) and a high-energy phosphate compound, possibly ATP (3). KtrII was discovered as proton potential-independent K+ transport activity (15). KtrII is not constitutive; its activity as well as Na+-ATPase activity is altered in response to medium Na+ concentration (9, 10). We postulate that K+ transport by the KtrII system is linked in some manner with the Na+ gradient generated by vacuolar Na+-ATPase, although the precise mechanism is unknown. The K+ extrusion system is proposed to be a primary K+/H+ exchanger (12).
The proton potential of E. hirae, which lacks the respiratory chain, is generated by proton expulsion via the FoF1, H+-translocating ATPase. The size of proton potential is maximal at acidic external pH but negligible at alkaline external pH; the optimal pH of the H+-ATPase activity is around 6.5 (13). The activities of the NapA antiporter and KtrI are indeed greater at acidic external pHs (11, 15). On the other hand, KtrII and the Na+-ATPase are likely to function to maintain homeostatis of K+ and Na+ under the conditions where KtrI and NapA are inoperative, such as at alkaline pH. Recently, we found that a component of the KtrII K+ uptake system is encoded by the ntpJ gene, a downstream cistron within the vacuolar Na+-ATPase ntp operon (19). Thus, the ntp operon is interesting in that it contains both Na+ and K+ transport genes. Expression of the ntp operon is regulated at the transcriptional level by sodium ions (20), indicating that sodium ions play an important role for physiology of E. hirae in the absence of the proton potential. We show here the importance of the Na+-responsive ntp operon for the growth and potassium accumulation of E. hirae under such a condition.
Experiments with Enterococcus hirae ATCC 9790 and the mutants derived from this strain were done. These mutants were AS25, which is defective in H+-ATPase and proton extrusion (16), JEM2, in which the ntpJ gene is disrupted by insertion of an erythromycin resistance gene (19), and Nak1, a mutant defective in the Na+-ATPase (11). Nak1 is now known to be a nonsense mutant of the ntpA gene encoding the 65-kDa NtpA subunit of Na+-ATPase (14). Cells were grown at 37°C in TrisM medium (7), which consists of (per liter) 10 mmol of K2HPO4; 4.24 ml of acetic acid; 0.6 g of ammonium sulfate; 0.2 g of MgSO4 · 7H2O; 10 mg each of MnCl2 · 4H2O and ferrous ammonium sulfate; 0.6 g of l-glutamic acid; 0.1 g of l-lysine · HCl; 10 mg of l-asparagine · H2O; 5 mg of l-glutamine; 0.2 g each of all other l-amino acids; 30 mg each of adenine, guanine, and uracil; 4 mg of nicotinamide; 1.6 mg each of pantothenic acid, pyridoxamine, and p-aminobenzoic acid; 0.8 mg each of thiamine · HCl and riboflavin; 0.02 mg of biotin; 0.01 mg of folic acid; and 10 g of glucose. The pH was adjusted to 7.5 with Tris. Erythromycin (10 μg/ml) was included in the media for JEM2. The growth of cells was monitored by measuring the optical density at 540 nm (OD540) with a Milton-Roy spectrophotometer (SPECTRONIC 20D+). The growth rates were determined between the optical densities of 0.1 and 0.2. There was no significant change in the medium pH during this period.
The amounts of Na+ and K+ in cells were determined with an atomic absorption spectrophotometer after cells growing in the middle logarithmic phase were collected by filtration (0.45-μm-pore-size filter; Millipore) and washed twice with 2 mM MgSO4 as described previously. Internal water space of 2 μl per mg of dry weight was determined previously (10).
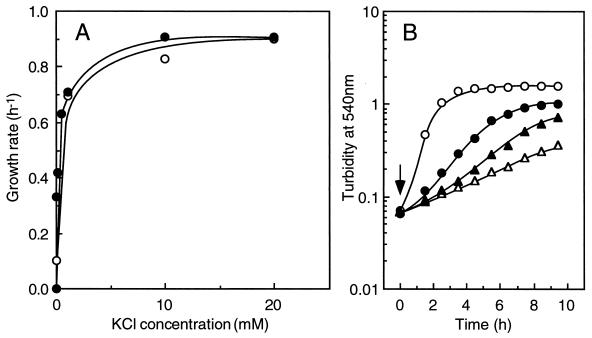
Figure 1A shows that E. hirae ATCC 9790 did not grow in the defined medium deficient in K+ and Na+; this medium contained less than 0.1 mM Na+ and 0.02 mM K+. More than 10 mM K+ was required for the optimal growth rate of 0.9 to 1.0 h−1, as described previously (1). This K+ requirement was not influenced by the presence of sodium ions. Even in the presence of 100 mM NaCl, cells did not grow unless the medium was supplemented with K+, and again, more than 10 mM K+ was required for optimal cell growth. The intracellular K+ concentration of the cells grown in this medium supplemented with 20 mM KCl was 450 mM, establishing a K+ gradient of about 20-fold. The growth of ATCC 9790 in TrisM medium (containing 10 mM K2HPO4) was inhibited by addition of the protonophore carbonylcyanide m-chlorophenylhydrazone (CCCP) (Fig. 1B). We postulate that this inhibition was caused by defective K+ accumulation, since the proton potential-dependent KtrI transport system should be inoperative in the presence of CCCP. Instead, growth in the presence of CCCP was restored by the addition of 200 mM KCl, although the growth rate was not entirely restored to the control level (Fig. 1B). Furthermore, in a complex medium, the addition of 200 mM KCl restored the CCCP-induced growth inhibition nearly completely (6; also data not shown). It is noteworthy that the cell growth in the presence of CCCP was somewhat rescued by NaCl (Fig. 1B), suggesting a possible role of Na+ ions for cell physiology of this bacterium where the proton potential was dissipated.
FIG. 1.
Growth of E. hirae ATCC 9790 in a defined medium. (A) Effect of KCl on growth rates in K+-deficient medium. The medium was prepared by replacing K2HPO4 in TrisM medium with phosphoric acid. Symbols: •, without NaCl; ○, with 100 mM NaCl. (B) Effect of CCCP on cell growth in TrisM medium. CCCP (50 μM), KCl (200 mM), or NaCl (200 mM) was added to the cell culture at an OD540 of 0.06 (indicated by the arrow), and cell growth was monitored by the OD540. Symbols: ○, control; ▵, TrisM plus CCCP; •, TrisM plus CCCP and KCl; ▴, TrisM plus CCCP and NaCl.
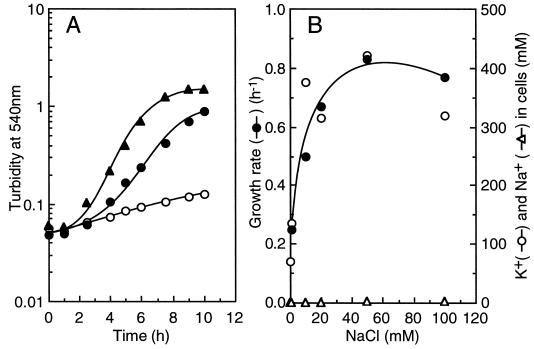
Because multidrug extrusion systems are found in the related streptococcus Lactococcus lactis (17), the effect of the ionophores on the growth of E. hirae must be interpreted with caution. In a second experimental approach, we used mutants defective in specific transport systems. Figure 2A shows the growth of mutant AS25 in TrisM medium in the absence of CCCP. In this mutant, which is defective in the H+-ATPase and proton extrusion, the proton potential is not generated (16). Strain AS25 did not grow well in unsupplemented TrisM medium (Fig. 2A). However, growth was stimulated by the addition of 200 mM NaCl as well as by the addition of KCl. Growth of E. hirae in low-K+ medium when the proton potential is dissipated thus depends upon the presence of Na+ ions. Figure 2B shows the effect of NaCl on the growth rates and the internal K+ levels of AS25. Without the addition of NaCl, the internal K+ concentration was about 60 mM, and the cells did not grow well (Fig. 2A). Addition of 20 mM NaCl was sufficient to increase the internal K+ concentration to its maximal level of about 400 mM and to stimulate growth to optimal rates (Fig. 1B). The intracellular Na+ concentration was maintained at less than 10 mM even when the external Na+ concentration was 100 mM. These results suggest that Na+ becomes essential for potassium accumulation and for the growth of this bacterium when the proton potential is dissipated.
FIG. 2.
Growth of E. hirae AS25 in TrisM medium. (A) Effect of KCl or NaCl on growth. KCl (200 mM) or NaCl (200 mM) was added to the cell culture at an OD540 of 0.05, and cell growth was monitored. Symbols: ○, control; •, TrisM plus KCl; ▴, TrisM plus NaCl. (B) Effect of NaCl on growth rates (•) and the internal levels of K+ (○) and Na+ (▵) of AS25. Cells were harvested at middle logarithmic phase and washed, and the levels of K+ and Na+ were determined with an atomic absorption spectrophotometer.
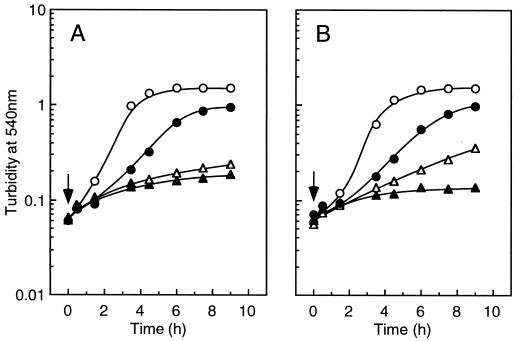
The physiological significance of Na+ circulation in E. hirae is not fully understood. The KtrII K+ transport system is speculated to be linked with Na+ in some manner; a component of the KtrII system is encoded by the ntpJ gene of the Na+-ATPase ntp operon ntpFIKECGABD(H)J (19). The KtrII system is a candidate for Na+-dependent K+ accumulation described above, since (i) the KtrII system is independent of the proton potential and (ii) expression of the ntp operon is regulated by an increase in the internal Na+ concentration as the signal (20). Figure 3 shows the growth of mutants JEM2 and Nak1, which are defective in KtrII and the Na+-ATPase, respectively. In TrisM medium, these mutants grew well, and growth was inhibited by the addition of CCCP, particularly in the case of JEM2 (Fig. 3A). Growth in the presence of CCCP was partially restored by the addition of a high concentration of KCl but not by the addition of NaCl (Fig. 3A). In the case of Nak1, the growth was even inhibited by NaCl (Fig. 3B). It is likely that NtpJ protein is expressed in the Nak1 mutant because (i) Nak1 is a point mutant of the A subunit of the Na+-ATPase and (ii) Western blotting with antiserum against purified Na+-ATPase showed that the ntpD gene product is expressed in Nak1 as well as in ATCC 9790 (14). These results suggest that expression of the ntp operon coding for the KtrII system and vacuolar Na+-ATPase is indispensable for Na+-dependent K+ accumulation and cell growth at low proton potential.
FIG. 3.
Effect of CCCP on the growth of strains JEM2 (A) and Nak1 (B) in TrisM medium. CCCP (50 μM), KCl (200 mM), or NaCl (200 mM) was added to the cell culture at an OD540 of 0.1 (indicated by the arrows). Symbols: ○, control; ▵, TrisM plus CCCP; •, TrisM plus CCCP and KCl; ▴, TrisM plus CCCP and NaCl.
The mechanism of Na+-induced activation of the KtrII system is also unknown. When the cells were made freely permeable to sodium ions by the addition of ionophore, the external Na+ concentration of less than 20 mM was sufficient for full expression of the ntp operon (20). This suggests that sodium ions are simply required as an inducer of expression of the ntpJ gene. However, the growth of Nak1 in TrisM medium in the presence of CCCP was not restored by low concentrations of NaCl (data not shown). This finding suggests that in addition to induction, a Na+-ATPase-generated sodium gradient is also required for the activity of KtrII.
One of the characteristics of enterococci among streptococci is that they are tolerant to high salinity and extremely alkaline pHs (4). These features are closely related and embodied by the expression of a unique ntp operon indispensable for homeostatis of Na+ and K+ ions at limited proton potential.
Acknowledgments
We thank D. Kropf for critical reading of the manuscript.
This work was supported by a grant-in-aid for Scientific Research from the Ministry of Education, Science, Sports and Culture of Japan, and also by the Hamaguchi Biochemistry Foundation and the Salt Science Research Foundation.
REFERENCES
- 1.Abrams A, Smith J B. Increased membrane ATPase and K+ transport rates in Streptococcus faecalis induced by K+ restriction during growth. Biochem Biophys Res Commun. 1971;44:1488–1495. doi: 10.1016/s0006-291x(71)80254-1. [DOI] [PubMed] [Google Scholar]
- 2.Bakker E P, editor. Alkali cation transport systems in prokaryotes. Boca Raton, Fla: CRC Press; 1993. [Google Scholar]
- 3.Bakker E P, Harold F M. Energy coupling to potassium transport in Streptococcus faecalis: interplay of ATP and the protonmotive force. J Biol Chem. 1980;255:433–440. [PubMed] [Google Scholar]
- 4.Hardie J M. Genus Streptococcus. In: Sneath P H A, Mair N S, Sharpe M E, Holt J G, editors. Bergey’s manual of systematic bacteriology. Vol. 2. Baltimore, Md: Williams & Wilkins; 1986. pp. 1043–1071. [Google Scholar]
- 5.Harold F M. Ion currents and physiological functions in microorganisms. Annu Rev Microbiol. 1977;31:181–203. doi: 10.1146/annurev.mi.31.100177.001145. [DOI] [PubMed] [Google Scholar]
- 6.Harold F M, Van Brunt J. Circulation of H+ and K+ across the plasma membrane is not obligatory for bacterial growth. Science. 1977;197:372–373. doi: 10.1126/science.69317. [DOI] [PubMed] [Google Scholar]
- 7.Harold F M, Harold R L, Baarda J R, Abrams A. A genetic defect in retention of potassium by Streptococcus faecalis. Biochemistry. 1967;6:1777–1784. doi: 10.1021/bi00858a028. [DOI] [PubMed] [Google Scholar]
- 8.Kakinuma Y. Sodium/proton antiporter in Streptococcus faecalis. J Bacteriol. 1987;169:3886–3890. doi: 10.1128/jb.169.9.3886-3890.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kakinuma Y. K+ transport in Enterococcus hirae. In: Bakker E P, editor. Alkali cation transport systems in prokaryotes. Boca Raton, Fla: CRC Press; 1993. pp. 277–290. [Google Scholar]
- 10.Kakinuma Y, Harold F M. ATP-driven exchange of Na+ and K+ ions by Streptococcus faecalis. J Biol Chem. 1985;260:2086–2091. [PubMed] [Google Scholar]
- 11.Kakinuma Y, Igarashi K. Mutants of Streptococcus faecalis sensitive to alkaline pH lack Na+-ATPase. J Bacteriol. 1990;172:1732–1735. doi: 10.1128/jb.172.4.1732-1735.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kakinuma Y, Igarashi K. Active potassium extrusion regulated by intracellular pH in Streptococcus faecalis. J Biol Chem. 1988;263:14166–14170. [PubMed] [Google Scholar]
- 13.Kakinuma Y, Igarashi K. Sodium-translocating adenosine triphosphatase in Streptococcus faecalis. J Bioenerg Biomembr. 1989;21:679–692. doi: 10.1007/BF00762686. [DOI] [PubMed] [Google Scholar]
- 14.Kakinuma Y, Igarashi K. Purification and characterization of the catalytic moiety of vacuolar-type Na+-ATPase from Enterococcus hirae. J Biochem (Tokyo) 1994;116:1302–1308. doi: 10.1093/oxfordjournals.jbchem.a124679. [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi H. Second system for potassium transport in Streptococcus faecalis. J Bacteriol. 1982;150:506–511. doi: 10.1128/jb.150.2.506-511.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kobayashi H, Unemoto T. Streptococcus faecalis mutants defective in regulation of cytoplasmic pH. J Bacteriol. 1980;143:1187–1193. doi: 10.1128/jb.143.3.1187-1193.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Konings W N, Lolkema J S, Bolhuis H, van Veen H W, Poolman B, Driessen A J. The role of transport processes in survival of lactic acid bacteria. Energy transduction and multidrug resistance. Antonie Leeuwenhoek. 1997;71:117–128. doi: 10.1023/a:1000143525601. [DOI] [PubMed] [Google Scholar]
- 18.Lanyi J K. The role of Na+ in transport processes of bacterial membranes. Biochim Biophys Acta. 1979;559:377–397. doi: 10.1016/0304-4157(79)90011-x. [DOI] [PubMed] [Google Scholar]
- 19.Murata T, Takase K, Yamato I, Igarashi K, Kakinuma Y. The ntpJ gene in the Enterococcus hirae ntp operon encodes the component of KtrII potassium transport system functionally independent of vacuolar Na+-ATPase. J Biol Chem. 1996;271:10042–10047. doi: 10.1074/jbc.271.17.10042. [DOI] [PubMed] [Google Scholar]
- 20.Murata T, Yamato I, Igarashi K, Kakinuma Y. Intracellular Na+ regulates the transcription of the ntp operon encoding vacuolar-type Na+-translocating ATPase in Enterococcus hirae. J Biol Chem. 1996;271:23661–23666. doi: 10.1074/jbc.271.39.23661. [DOI] [PubMed] [Google Scholar]
- 21.Takase K, Kakinuma S, Yamato I, Konishi K, Igarashi K, Kakinuma Y. Sequencing and characterization of the ntp gene cluster for Na+-translocating ATPase in Enterococcus hirae. J Biol Chem. 1994;269:11037–11044. [PubMed] [Google Scholar]
- 22.Waser M, Hess-Bienz D, Davies K, Solioz M. Cloning and disruption of a putative NaH-antiporter gene of Enterococcus hirae. J Biol Chem. 1992;267:5396–5400. [PubMed] [Google Scholar]