Abstract
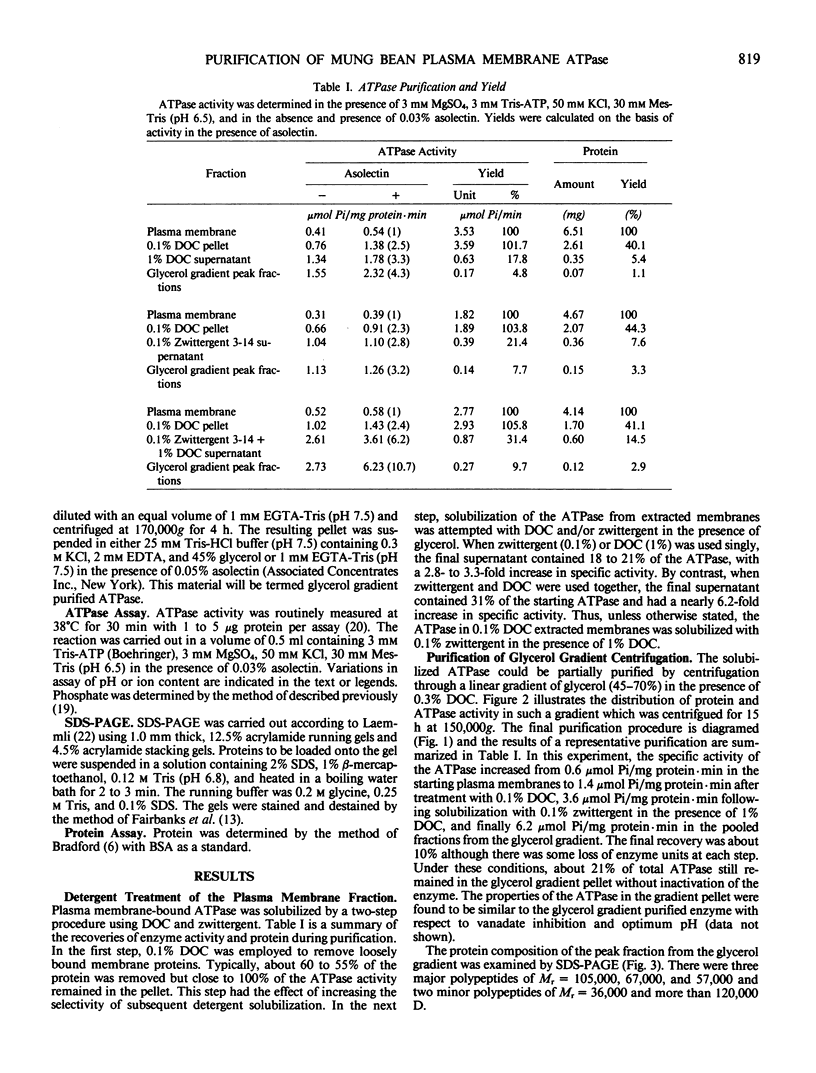
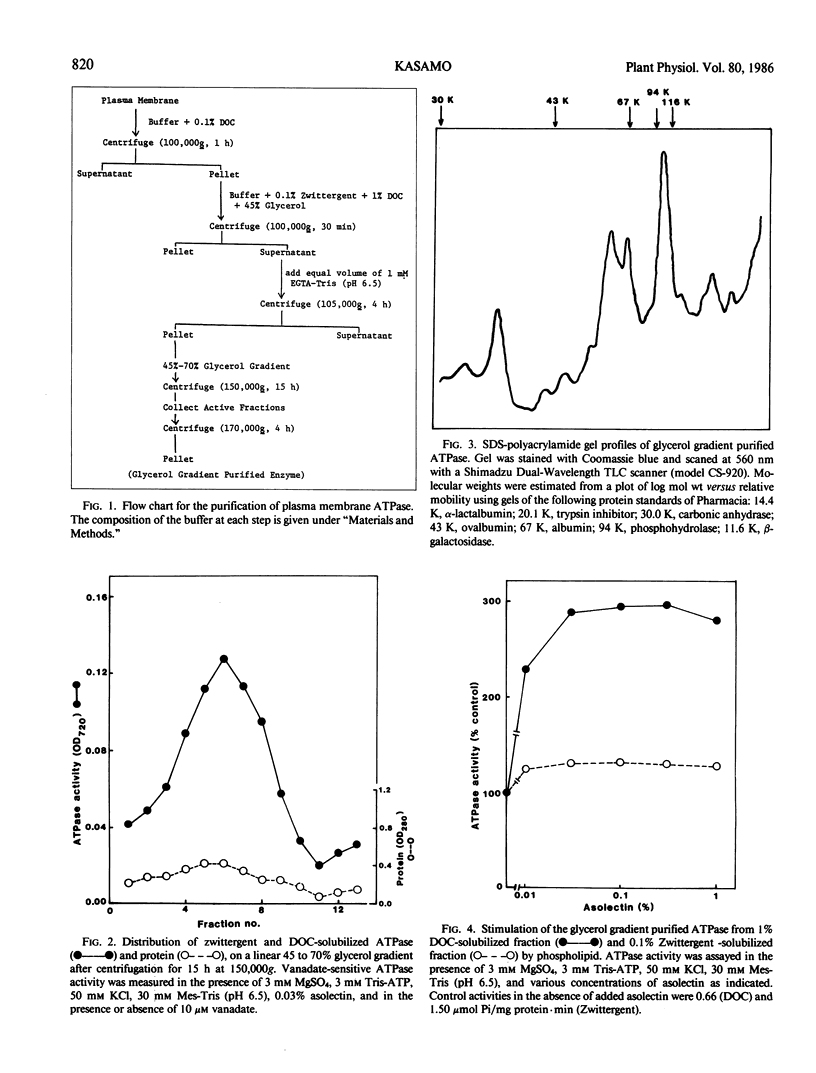
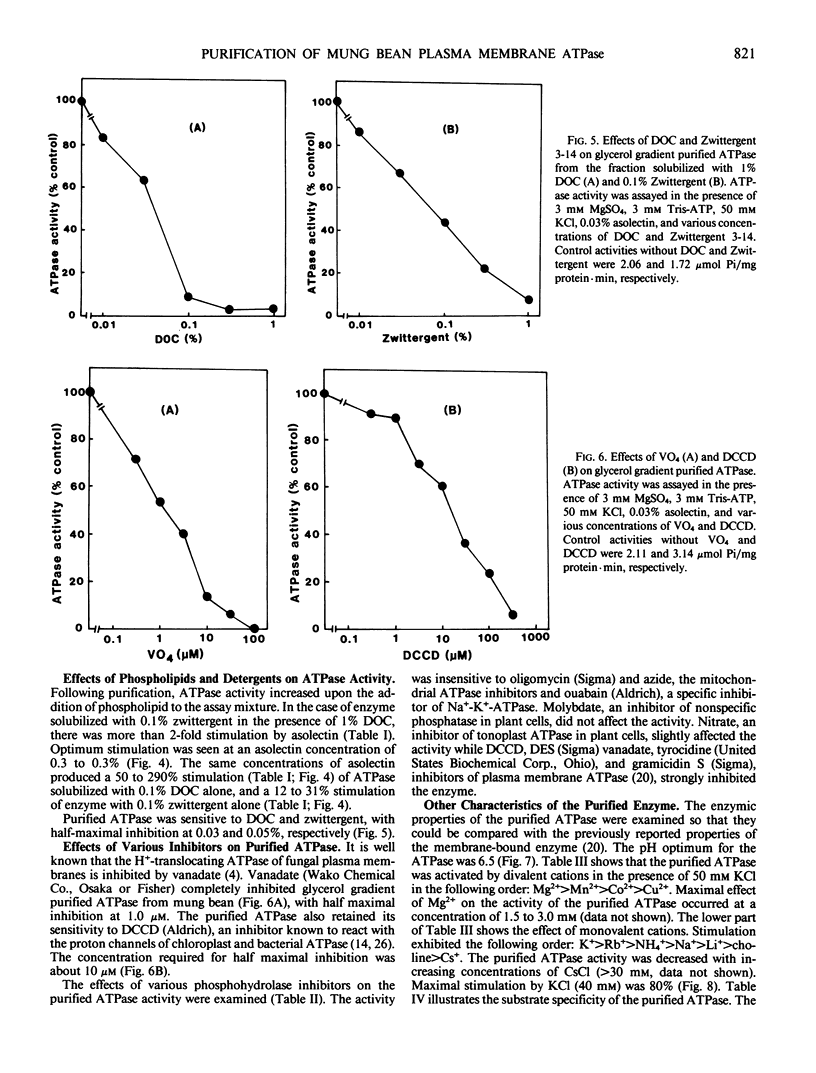
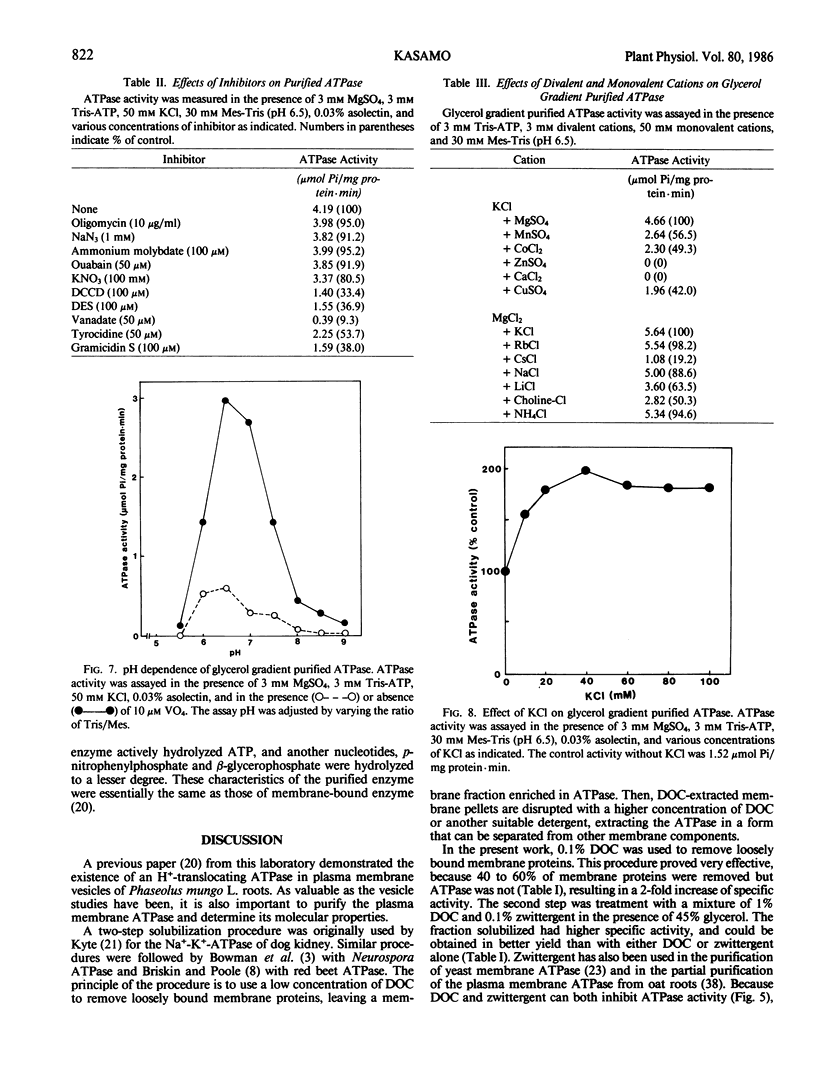
The plasma membrane ATPase of mung bean (Phaseolus mungo L.) roots has been solubilized with a two-step procedure using the anionic detergent, deoxycholate (DOC) and the zwitterionic detergent, zwittergent 3-14 as follows: (a) loosely bound membrane proteins are removed by treatment with 0.1% DOC; (b) The ATPase is solubilized with 0.1% zwittergent in the presence of 1% DOC; (c) the solubilized material is further purified by centrifugation through a glycerol gradient (45-70%). Typically, about 10% of the ATPase activity is recovered, and the specific activity increases about 11-fold. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis shows that the peak fraction from the glycerol gradient contains three major polypeptides of Mr = 105,000, 67,000, and 57,000 daltons. The properties of the purified ATPase are essentially the same as those of membrane-bound ATPase, with respect to pH optimum, substrate specificity, inhibitor sensitivity, and ion stimulation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benson M. J., Tipton C. L. Purification and Characterization of a Cation-stimulated Adenosine Triphosphatase from Corn Roots. Plant Physiol. 1978 Aug;62(2):165–172. doi: 10.1104/pp.62.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman B. J., Blasco F., Slayman C. W. Purification and characterization of the plasma membrane ATPase of Neurospora crassa. J Biol Chem. 1981 Dec 10;256(23):12343–12349. [PubMed] [Google Scholar]
- Bowman B. J., Mainzer S. E., Allen K. E., Slayman C. W. Effects of inhibitors on the plasma membrane and mitochondrial adenosine triphosphatases of Neurospora crassa. Biochim Biophys Acta. 1978 Sep 11;512(1):13–28. doi: 10.1016/0005-2736(78)90214-6. [DOI] [PubMed] [Google Scholar]
- Bowman B. J., Slayman C. W. The effects of vanadate on the plasma membrane ATPase of Neurospora crassa. J Biol Chem. 1979 Apr 25;254(8):2928–2934. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Briskin D. P., Poole R. J. Characterization of the solubilized plasma membrane ATPase of red beet. Plant Physiol. 1984 Sep;76(1):26–30. doi: 10.1104/pp.76.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briskin D. P., Poole R. J. Evidence for a beta-Aspartyl Phosphate Residue in the Phosphorylated Intermediate of the Red Beet Plasma Membrane ATPase. Plant Physiol. 1983 Aug;72(4):1133–1135. doi: 10.1104/pp.72.4.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantley L. C., Jr, Josephson L., Warner R., Yanagisawa M., Lechene C., Guidotti G. Vanadate is a potent (Na,K)-ATPase inhibitor found in ATP derived from muscle. J Biol Chem. 1977 Nov 10;252(21):7421–7423. [PubMed] [Google Scholar]
- Cocucci M., Ballarin-Denti A. Effect of polar lipids on ATPase activity of membrane preparations from germinating radish seeds. Plant Physiol. 1981 Aug;68(2):377–381. doi: 10.1104/pp.68.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont F. M., Leonard R. T. Solubilization and partial purification of the adenosine triphosphatase from a corn root plasma membrane fraction. Plant Physiol. 1980 May;65(5):931–938. doi: 10.1104/pp.65.5.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Fillingame R. H. Purification of the carbodiimide-reactive protein component of the ATP energy-transducing system of Escherichia coli. J Biol Chem. 1976 Nov 10;251(21):6630–6637. [PubMed] [Google Scholar]
- Gallagher S. R., Leonard R. T. Effect of vanadate, molybdate, and azide on membrane-associated ATPase and soluble phosphatase activities of corn roots. Plant Physiol. 1982 Nov;70(5):1335–1340. doi: 10.1104/pp.70.5.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings D. F., Reynolds J. A. Molecular weight of (Na+,K+)ATPase from shark rectal gland. Biochemistry. 1979 Mar 6;18(5):817–821. doi: 10.1021/bi00572a012. [DOI] [PubMed] [Google Scholar]
- Helenius A., Simons K. Solubilization of membranes by detergents. Biochim Biophys Acta. 1975 Mar 25;415(1):29–79. doi: 10.1016/0304-4157(75)90016-7. [DOI] [PubMed] [Google Scholar]
- Imbrie C. W., Murphy T. M. Solubilization and partial purification of ATPase from a rose cell plasma membrane fraction. Plant Physiol. 1984 Mar;74(3):611–616. doi: 10.1104/pp.74.3.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J. Purification of the sodium- and potassium-dependent adenosine triphosphatase from canine renal medulla. J Biol Chem. 1971 Jul 10;246(13):4157–4165. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Malpartida F., Serrano R. Purification of the yeast plasma membrane ATPase solubilized with a novel zwitterionic detergent. FEBS Lett. 1980 Feb 25;111(1):69–72. doi: 10.1016/0014-5793(80)80763-0. [DOI] [PubMed] [Google Scholar]
- Manolson M. F., Rea P. A., Poole R. J. Identification of 3-O-(4-benzoyl)benzoyladenosine 5'-triphosphate- and N,N'-dicyclohexylcarbodiimide-binding subunits of a higher plant H+-translocating tonoplast ATPase. J Biol Chem. 1985 Oct 5;260(22):12273–12279. [PubMed] [Google Scholar]
- Nelson N., Eytan E., Notsani B. E., Sigrist H., Sigrist-Nelson K., Gitler C. Isolation of a chloroplast N,N'-dicyclohexylcarbodiimide-binding proteolipid, active in proton translocation. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2375–2378. doi: 10.1073/pnas.74.6.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson G. L., Hokin L. E. Molecular weight and stoichiometry of the sodium- and potassium-activated adenosine triphosphatase subunits. J Biol Chem. 1981 Apr 25;256(8):3751–3761. [PubMed] [Google Scholar]
- Serrano R. Purification of the proton pumping ATPase from plant plasma membranes. Biochem Biophys Res Commun. 1984 Jun 15;121(2):735–740. doi: 10.1016/0006-291x(84)90243-2. [DOI] [PubMed] [Google Scholar]
- Stout R. G., Cleland R. E. Partial characterization of fusicoccin binding to receptor sites on oat root membranes. Plant Physiol. 1980 Sep;66(3):353–359. doi: 10.1104/pp.66.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sze H., Churchill K. A. Mg/KCl-ATPase of plant plasma membranes is an electrogenic pump. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5578–5582. doi: 10.1073/pnas.78.9.5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzagoloff A., Meagher P. Assembly of the mitochondrial membrane system. V. Properties of a dispersed preparation of the rutamycin-sensitive adenosine triphosphatase of yeast mitochondria. J Biol Chem. 1971 Dec 10;246(23):7328–7336. [PubMed] [Google Scholar]
- Vara F., Serrano R. Partial purification and properties of the proton-translocating ATPase of plant plasma membranes. J Biol Chem. 1982 Nov 10;257(21):12826–12830. [PubMed] [Google Scholar]
- Vara F., Serrano R. Phosphorylated intermediate of the ATPase of plant plasma membranes. J Biol Chem. 1983 May 10;258(9):5334–5336. [PubMed] [Google Scholar]
- Walderhaug M. O., Post R. L., Saccomani G., Leonard R. T., Briskin D. P. Structural relatedness of three ion-transport adenosine triphosphatases around their active sites of phosphorylation. J Biol Chem. 1985 Mar 25;260(6):3852–3859. [PubMed] [Google Scholar]


